Publication Information
ISSN: 2641-6859
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Whole Blood Selenium Level and Selenium Supplementation in Elite Sports: Effect on Thyroid Metabolism (Performance Fatigue) and Cellular Immune Function (Frequency of Infection)
Klaus Erpenbach1*, Max C. Erpenbach1, Wolfgang Mayer2, Uwe Hoffmann3, Stefan Mücke1
1Institut für medizinische Leistungsoptimierung und Trainingssteuerung, Marienstraße 1, 50374 Erftstadt, Germany
2Lab4more GmbH Bavariahaus, Augustenstraße 10, 80333 München, Germany
3Deutsche Sporthochschule Köln, Am Sportpark Müngersdorf 6, 50933 Köln, Germany
Received Date: September 27, 2021; Accepted Date: October 05, 2021; Published Date: October 15, 2021
*Corresponding author: Erpenbach K, Institut für medizinische Leistungsoptimierung und Trainingssteuerung, Marienstraße 1, 50374 Erftstadt, Germany. Email: info@im-lot.org
Citation: Erpenbach K, Erpenbach MC, Mayer W, Hoffmann U, Mücke S (2021) Whole Blood Selenium Level and Selenium Supplementation in Elite Sports: Effect on Thyroid Metabolism (Performance Fatigue) and Cellular Immune Function (Frequency of Infection). Adv Ortho and Sprts Med: AOASM-152.
DOI: 10.37722/AOASM.2021503
Abstract
Summary: Recurrent and serious infections, muscle damage and training or competition induced fatigue are the most common symptoms in elite sport demolishing optimal training results or prohibiting competition. Are selenium deficiency responsible for these symptoms in elite sport and will daily supplementation prevent them.
Methods: In 107 elite athletes [male: 49 – female: 58 / soccer: 20 – field hockey: 60 – Olympics: 18 – tennis: 5 – motorsports (DTM-Formula1): 4] whole blood selenium was determined. In all elite athletes the symptoms performance fatigue and recurrent infections were correlated. 24 elite athletes, getting daily supplementation of 200µg selenomethionine for 3 months, whole blood selenium, thyreotropine (TSH), free trijodthyronine (fT3), free thyroxin (fT4) and WBC before and after supplementation were determined and the symptoms infections and performance fatigue were correlated. A Spearman-ranking coefficient of correlation, a chi-quadrat-test (²-Test) by Pearson and an independent t-test were used. p<0,05 was supposed to be significant, p<0,01 highly significant.
Results: In 57% of all elite athletes (N=61/107) a whole-blood-selenium-deficiency (< 121 µg/l) was established, in average 117 ± 29.08 µg/l. In cases of young national player (U16/U18) whole blood selenium compared to national A player were poorly supplied [106.47 ± 29.71 µg/l vs. 123.23 ± 32.9 µg/l (p=n.s)]. Comparing the settings of selenium > 145µg/l vs. < 145µg/l in 61% performance fatigue [107.8 ± 25.53 µg/l vs. 177.8 ± 30.74 µg/l, OR=0.39, p=0.091] were less complained in the higher group while comparing the settings of selenium < 145µg/l vs. > 145µg/l infections were 1.75times more frequent [109.4 ± 20.77 µg/l vs. 164 ± 11.53 µg/l, OR=1.75, p=0.27] in the lower group. Daily substitution of 200µg selenomethionine improved whole blood selenium significantly (127.50 ± 16.52 µg/l before vs. 176.29 ± 18.01 µg/l after treatment, p=0.0001). WBC (6.72 ± 1.42 per nL before vs. 4.82 ± 0.73 per nL after supplementation, p=0.0001) and thyreotropine (1.88 ± 0.68 µU/ml before vs. 1.27 ± 0.51 µU/ml after supplementation, p=0.003) decreased significantly after 3 months of daily substitution with 200µg selenomethio-nine, while free trijodthyronine (3.11 ± 0.73 pg/ml before vs. 3.65 ± 0.74 pg/ml after supplementation, p=0.006) improved significantly. Female athletes significantly were more worse supplied with free trjodthyronine than the male athletes (female: 2.48 ± 0.33 pg/ml vs. male: 3.33 ± 0.71 pg/ml, p=0.006). Athletes recurrently suffered from infections were significantly worse supplied with whole blood selenium than athletes without infections (120.9 μg/l with infections vs. 134.4 μg/l without infections, p=0.051). Per increase in whole blood selenium of 10 μg/l, the TSH decreases significantly by 0.12μU/ml (p=0.009) and fT3 improves by 0.11 pg/ml (p=0.088). The selenium-induced improvement of fT3 leads to a significant reduction in TSH [increase in fT3 of 0.1 pg/ml lowers TSH by 0.2 μU/ml, p=0.009). At the same time, the leukocytes decrease significantly [increase in fT3 of 0.1 pg/ml lowers the leukocytes by 0.38per nL, p=0.002).A significant absolute risk reduction of 45.84% for recurrent infections (13/24 before vs. 2/24 after therapy, p=0.043) and of 58.34% for performance fatigue (16/24 before vs. 2/24 after therapy, p=0.227) were calculated. No toxic side effects were observed.
Conclusion: Independent to the type of sports, deficiency of whole blood selenium in elite sports was observed. Daily selenium substitution can significantly reduce performance fatigue by optimizing thyroid function and recurrent infections by optimizing the cellular immune system. Randomized treatment trials over a whole season must show whether performance fatigue or recurrent infections can be avoided by daily and permanent selenium substitution.
Introduction
Micronutrients – vitamins and trace elements – are essential and daily needed to maintain all physiological body functions optimal [1-2]. High training loads, tight game schedules, unbalanced or improper diets, frequent traveling and high psychological stress lead to enormous consumption of these micronutrients and to injuries in 12.9% of cases and acute infections in 9.2% of cases resulting in training absences and competition cancellations [3-6]. In major events such as the Olympics, FIFA World Cup, or IAAF, 9.6-14% of participants miss these events due to injury [5-13]. 5.4-8.9% of participants suffer an acute infectious disease, twice as often in winter as in summer [5-13].
Selenium is an essential trace element, present in plants as selenomethionine and in animals as selenocysteine. Inadequate dietary selenium intake leads to thyroid dysfunction and inflammation (decrease in selenium-dependent deiodinases), increased cell membrane destruction (decrease in selenium-dependent glutathione peroxidases), and recurrent infections, especially viruses (decrease in natural killer cell function CD16/56 and CD16/57) [14, 15]. These biological processes are very relevant to the training and competitive performance of the athletes. Selenium and its organic enzyme systems protect cells and muscle tissue from destruction by exercise- and competition-induced oxidative free radicals [16]. Although no beneficial effects on athletic performance from selenium supplementation have been demonstrated to date, a few studies currently suggest maintaining adequate selenium status in athletes. However, on the one hand, excessive selenium supplementation can be hazardous for the athletes, and on the other hand, selenium deficiency can result in increased exercise-induced oxidative cell stress [17-20].
The aim of this study is to assess the whole blood selenium status of elite athletes of various sports and to analyze possible correlations with the athletes’ complaints of recurrent infections and performance fatigue. Is daily selenium supplementation able to prevent recurrent infections and performance fatigue?
Methods
Participants
107 top athletes from different sports (team sports: field hockey and soccer – individual sports: athletics, tennis, motor sports) were included in the study for performance optimization. All participants gave their written consent to the evaluation. A questionnaire was used to assess performance fatigue and infection frequency. Infection frequency was defined as at least 2 or more viral and/or bacterial infections per year. Performance fatigue was defined as physical exhaustion immediately after training/competition (in the Visual Analog Scale > 5) with persistent morning fatigue after 7 hours of sleep. Whole blood selenium was assessed in all athletes and correlated with symptoms. In addition, 24 elite athletes were substituted with 200µg selenomethionine daily for 3 months, selenium in whole blood as well as serum leukocytes, TSH, fT3 and fT4 were determined before and after supplementation and calculated in relation to symptoms of infections and performance fatigue.
Whole blood selenium (EDTA blood) vSe: Selenium in whole blood was determined by atomic absorption spectrometry (AAS).
Leukocytes: were determined on the hematology system XT-2000i from Sysmex according to the manufacturer’s instructions.
TSH, fT3, fT4: measured on the Abbott Alinity I-Module automated laboratory system according to the manufacturer’s instructions.
Statistical Analysis
Data were statistically analyzed using IBM®SPSS® software 25. To calculate the correlations between the different parameters, the Pearson correlation coefficient (rP) was used for samples with n ≥ 40 and no contradiction to the normal distribution in the Kolmogroff-Smirnoff test (P>0.1). If the conditions were violated, the Spearman correlation coefficient (rSP) was applied. For 2-group mean comparisons, a Levene test was performed to test for variance homogeneity, followed by an independent T test for homogeneous (P>0.1) or inhomogeneous variances with two-sided questioning. Results were considered significant for p ≤ 0.05 and highly significant for p ≤ 0.01.
Results
(Table 1) shows the characteristics of the study population. 44% of athletes complained of performance fatigue and 40% infections. Female athletes had a significantly lower whole blood selenium level (109.8 ± 26.01 vs. 125.4 ± 30.51 µg/l, p<0.005) than their male counterparts. Total population showed a diminished mean level of whole blood selenium 117 ± 29.08 µg/l (norm: 121 – 168 µg/l). Whole blood selenium levels were below 160 µg/l in 93% (100/107) of the athletes, with 56% (60/107) showing selenium deficiency (< 121 µg/l) and 37.4% (40/107) showing inadequate cellular selenium supply (>121-160 µg/l). Table 2 shows the comparison of youth vs. adults. Adolescents are worse supplied with selenium in whole blood compared to adults (106.47 ± 29.71 resp. 111.76 ± 21.31 µg/l vs. 123.23 ± 32.9 resp. 122.24 ± 28.62 µg/l – p=0.104). 62.5% (15/24) of the young national players have concentrations < 121 µg/l and thus compared to the senior national players (41.7% < 121 µg/l selenium) a 2.33-fold higher risk to develop intracellular selenium deficiency (15/24 vs. 10/24, OR = 2.33, 95%CI: 1.23 – 2.12, p=n.s).
In the selenium group comparison (< 145µg/l vs. > 145µg/l whole blood selenium), 61% of athletes in the upper group complained of diminished performance fatigue [107.8 ± 25.53 µg/l vs. 177.8 ± 30.74 µg/l, OR=0.39, p=0.091], while infections were 1.75 times more frequent in the lower group [109.4 ± 20.77 µg/l vs. 164 ± 11.53 µg/l, OR=1.75, p=0.27] (Tab.3). Daily substitution of 200µg selenomethionine for 3 months significantly increased whole blood selenium (127.50 ± 16.52 µg/l before vs. 176.29 ± 18.01 µg/l after supplementation, p=0.0001) (Figure 1). Leukocytes (6.72 ± 1.42 per nL before vs. 4.82 ± 0.73 per nL after supplementation, p=0.0001) as well as TSH (1.88 ± 0.68 µU/ml before vs. 1.27 ± 0.51 µU/ml after supplementation, p=0,003) decreased under 3-month supplementation with 200µg selenomethionine, while free T3 (3.11 ± 0.73 pg/ml before vs. 3.65 ± 0.74 pg/ml after supplementation, p=0.006) increased significantly. Female athletes (female: 2.48 ± 0.33 pg/ml vs. male: 3.33 ± 0.71 pg/ml, p=0.006) were significantly lower in free T3 than male athletes (Table 4). Athletes with infections were significantly worse supplied with whole blood selenium than athletes without infections (120.9 µg/l with infections vs. 134.4 µg/l without infections, p=0.051) (Figure 2). Per increase of whole blood selenium by 10 µg/l, TSH significantly decreased by 0.12 µU/ml (p=0.009) (Figure 3a) and fT3 improved by 0.11 pg/ml (p=0.088) (Figure 3b). Selenium-induced improvement of fT3 leads to significant decrease of TSH (increase of fT3 by 0.1 pg/ml decreases TSH by 0.2 µU/ml, p=0.009) [Figure 3c]. At the same time, WBC decrease significantly [increase in fT3 by 0.1 pg/ml decreases WBC by 0.38 per nL, p=0.002) (Figure 3d). A significant absolute risk reduction in athletes for infections of 45.84% (13/24 before vs. 2/24 after supplementation, p=0.043) and for performance fatigue of 58.34% (16/24 before vs 2/24 after supplementation, p=0.227) were calculated (Figure 4). No toxicities were complained. N=24
Selenium
121-168 µg/l
All
117,0
(n=107)
± 29,08
Female
109,8
(n=58)
± 26,01
Male
125,4
(n=49)
± 30,51
difference m/f
p < 0,005
Soccer
134,2
(n=20)
± 26,2
Field hockey
104,2
(n=60)
± 23,49
Athletics
133,1
(n=18)
± 32,12
Tennis
133,8
(n=5)
± 26,93
Motor
128,3
(n=4)
± 20,6
National
121,5
(n=25)
± 31,61
U16
100,1
(n=6)
± 36,25
U18
108,5
(n=15)
±30,12
U21
108,0
(n=6)
± 30,64
Selenium
121-168
µg/l
All
117,00
(n=107)
± 29,08
National player
123,23
Adults
± 32,90
(n=22)
National player
106,47
U16 – U18
± 29,71
(n=24)
other
111,76
U18
± 21,31
(n=20)
other
122,24
adults
± 28,62
(n=41)
Selenium
121-168 µg/l
A
≤ 145
B
> 145
n
All
111,2
97
± SD
± 23,40
All
172,5
10
± SD
± 22,61
Infections
yes (A)
109,4
38
± SD
± 20,77
yes (B)
164,0
4
± SD
±11,53
OD=1,75 – p=0,274
no (A)
112,5
55
± SD
± 24,62
no (B)
176,1
10
± SD
± 29,90
Performance Fatigue
yes (A)
116,1
39
± SD
± 18,17
yes (B)
169,0
9
± SD
± 17,79
OD=0,39 – p=0,091
no (A)
107,8
54
± SD
± 25,53
no (B)
177,8
5
± SD
± 30,74
All
Male N=16
Female N=8
Fatigue
before
Fatigue
after
ARR16/24 (66,67%) 2/24 (8,33%) 58,34%
8/18
(44,44%)
2/18
(11,11%)
33,33%5/6
(83,33%)
0/6
(0%)
100%
Infections
before
Infections
after
ARR13/24 (54,17%) 2/24
(8,33%) 45,84%10/18
(55,55%)
2/18
(11,11%) 44,44%6/6
(100%)
0/6
(0%)
100%
vSelenium
before
vSelenium
after
significance127,50
± 16,52 176,29
± 18,01 p=0,0001129,67
± 16,94
177,44
± 19,14 –121
± 14,57
172,83
±15,08
–
WBC
before
WBC
after
significance6,72 ± 1,42 4,82 ± 0,73 p=0,0001
6,44
± 1,32 4,79
± 0,61 –7,56
± 1,45
4,90
± 1,05
–
TSH
before
TSH
after
significance1,88 ± 0,68 1,27 ± 0,51 p=0,003
1,80 ± 0,63
1,39
± 0,47
–2,10
± 0,82 0,93
± 0,51
–
fT3
before
fT3
after
significance3,11 ± 0,73 3,65 ± 0,74 p=0,006
3,33 * ± 0,71 3,77
± 0,79
–2,48 *
± 0,33 3,30
± 0,41 –
fT4
before
fT4
after
significance1,12 ± 0,22 1,21 ± 0,22 p=0,132
1,17 ± 0,23 1,27 # ± 0,21 –
1,17 ± 0,23 1,27 # ± 0,21 –
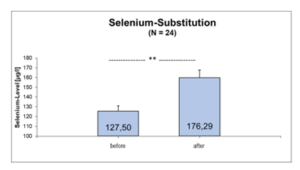
Figure 1: Whole-Blood-Selenium-level before and after daily substitution with 200µg Selenomethionine for 3 month (N=24).
** p=0,0001
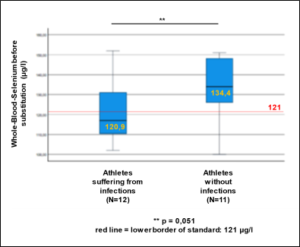
Figure 2: Whole-Blood-Selenium-Level before selenium substitution (n=23).
** p=0,051. Red line = lower standard border.
Figure 3a-d: Bivariate Correlations of Whole-Blood-Selenium (vSe) vs. thyreotropine (TSH) vs. free T3 (fT3) correlations of fT3 vs. TSH vs. WBC.
- a) difference vSe vs. difference TSH,
- b) difference vSe vs. difference fT3,
- c) difference fT3 vs. difference TSH,
- d) difference fT3 vs. difference WBC [p<0,05 = significant, p<0,01 = highly significant].
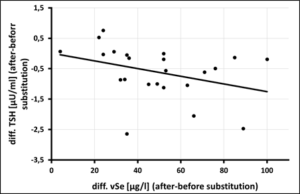 Figure 3a: Correlation of Whole-Blood-Selenium (vSe) [µg/l] vs. thyreotropine (TSH) [µU/ml] after 3 month of selenium substitution (N=24) – (rSP = 0,492, y = -0,0126x + 0,0093 – p = 0,009).
Figure 3a: Correlation of Whole-Blood-Selenium (vSe) [µg/l] vs. thyreotropine (TSH) [µU/ml] after 3 month of selenium substitution (N=24) – (rSP = 0,492, y = -0,0126x + 0,0093 – p = 0,009).
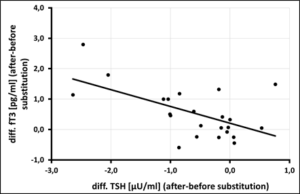
Figure 3b: Correlation of Whole-Blood-Selenium (vSe) [µg/l] vs. free T3 [pg/ml] after 3 month of selenium substitution (N=24) – (rSP = 0,293, y = 0,0111x + 0,0014 – p = 0,088).
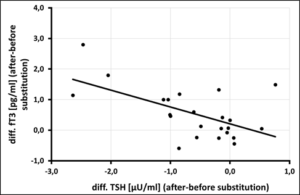
Figure 3c: Correlation of free T3 [pg/ml] vs. thyreotropine (TSH) [µU/ml] after 3 month of selenium substitution (N=24) – (rSP = 0,486, y = -0,5514x + 0, 2074 – p = 0,009).
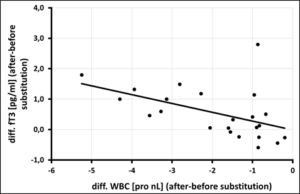
Figure 3d: Correlation of free T3 [pg/ml] vs. WBC [pro nl] after 3 month of selenium substitution (N=24) – (rSP = 0,586, y = -0,2889x – 0,0105 – p = 0,002).
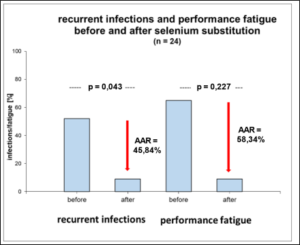
Figure 4: Absolute Risk Reduction (AAR) of recurrent infections and Performance-Fatigue after 3-month of Selenium substitution.
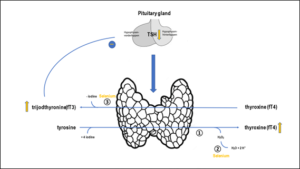
Figure 5: Selenium-dependent thyroid metabolism:
① = Thyreoideaperoxidase (TPO – antioxidatively protected by selenium),
② = Glutathionperoxidase (selenium-dependent) and Thioredoxinreduktase (selenium-dependent),
③ = Dejodasen (selenium-dependet), TSH = thyreotropine (Thyroid-stimulating hormone)
Yellow arrow = increase rsp. decrease – = negative feedback. Optimization of whole blood selenium leads to an increase of the enzyme systems ② + ③, increasing fT3 + fT4, decreasing TSH in the feedback. The increase of the enzyme system ③ decreases H2O2 and therefore the inflammatory activity.
Discussion
The extent of inadequate selenium supply in whole blood (< 121 µg/l) in our study (56%) has not been presented in the international literature so far. Only the average selenium level of the athletes in our study of 117 ± 29.08 µg/l is confirmed in the publication of Lee [21] with 114.33 ± 16.33 µg/l (n=57), whereby anaerobically trained athletes (judoka) were significantly worse supplied with selenium than aerobically trained athletes (107 ± 13 µg/l vs. 122 ± 16 µg/l – p<0.05).
U16/18 national players had a significantly worse supply of whole blood selenium than adult national players (123.23 ± 32.90 µg/l) with an average of 106.47 ± 29.71 µg/l. The proportion of adolescent athletes inadequately supplied with whole blood selenium (< 121 µg/l) in our study is 66.7%, which is significantly higher than in adults. The reason for the significantly poorer whole blood selenium supply in adolescents may be due to the higher requirement at growth age (especially for maintenance of thyroid function). It is not known whether senior national players have a more conscious diet with regard to this parameter. However, they were provided by the association with an electrolyte drink containing selenium as a dietary supplement on the average 80-150 training days per year. Significant associations between inadequate versus adequate selenium level in whole blood in correlation to recurrent infections or performance fatigue could not be demonstrated in our study. However, a significant absolute risk reduction in infections of 46% and in performance fatigue of 58% could be calculated in 24 elite athletes by daily supplementation of 200µg selenium for 12 weeks. Comparative studies on these symptoms are not available in the literature. Performance enhancement has not been demonstrated in the international literature by selenium therapy studies (daily substitution of 180 – 200 µg selenomethionine for 3 – 10 weeks) [17, 22]. Only Tessier, et al. [18] were able to demonstrate a significant increase in glutathione peroxidase activity (protection against oxidative stress) in selenium-substituted athletes versus placebo under sports stress, although cellular selenium levels were not reported.
Selenium is an essential trace element for maintaining adequate thyroid function: on the one hand, it serves as a cofactor of the deiodases responsible for deiodination of inactive thyroxine (fT4) into active triiodothyronine (fT3), and on the other hand, it is part of the antioxidant glutathione peroxidases as well as thioredoxin reductases, thus generally protecting cells from oxidative stress – especially working muscle cells and thyroid glands (Figure 5) [23]. There is a lack of data showing an association between cellular selenium deficiency and reduced T3 production by the thyroid glands [24]. In our study, there was a significant association between whole blood selenium deficiency and subclinical hypothyroidism (TSH > 2 µU/ml: 11/24 – p=0.031) in 45.8% of athletes and an fT4 supply ≤ 1 ng/dl (fT4 ≤ 1 ng/dl: 8/24 – p=0.041) detectable in 16.7% of athletes, with female athletes having a higher level of subclinical hypothyroidism [males: 7/18 (38.9%) – females 4/6 (66.7%)] than male athletes. Comparable data are absent in the literature. In 215 male soccer players from the 1st and 2nd Bundesliga the median TSH level was 1.66 µU/ml (95%-CI: 0.66-3.67) whereas the part of athletes with subclinical hypothyroidism (TSH > 2 µU/ml) has not been reported [25]. High training and competition intensities correlate with high TSH levels [26] and are associated with reduced submaximal training performance, dry skin, fatigue, and muscle cramps [27]. Low performance intensity athletes showed significantly lower TSH levels than high intensity athletes (1.69 ± 0.55 µU/ml vs 1.89 ± 0.74 µU/ml – p=0.045) [26]. Correlations between TSH levels and whole blood selenium concentrations in athletes and their impact on performance fatigue and recurrent infections are also missing in the literature.
Optimization of whole blood selenium by daily substitution of 200µg selenomethionine over 3 months to 175 µg/l leads to an increase of free T3 via enhancement of enzyme activities of deiodases and to a decrease in TSH via direct negative feedback. This selenium-dependent increase appears to optimize muscular energy turnover via up-regulation of sarco-endoplasmic reticulum calcium ATPase (SERCA) [28] and to be responsible for the 58.34% improvement in performance fatigue in our study.
Conclusion
Most competitive athletes are on lower limit or even deficient in whole blood selenium. Adolescents U18 show a significantly poorer supply of whole blood selenium than adults. Daily administration of 200µg selenium optimizes cellular selenium levels, improves thyroid function, and lowers leukocyte load, which appears to prevent recurrent infections and performance fatigue. Further randomized studies with higher doses and the goal of raising whole blood selenium into an adequate range of 160 – 200 µg/l over an entire competitive season need to show whether a significant reduction in performance-limiting infections, performance fatigue or muscular injuries can be achieved.
Acknowledgments: Conflict or interest and funding: none declared.
References
- Rodriguez NR; DiMarco NM, Langley S (2009) American College of Sports Medicine position stand. Nutrition and athletic p Med Sci Sports Exerc 41: 709-731.
- Kreider RB, Wilborn CD, Taylor L, Campbell B, Almada AL, et al. (2010) ISSN exercise & sport nutrition review: research & recommendations. J Int Soc Sports Nutr 7: 1-43.
- Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, et al. (2011) Position Part one: immunefunction and exercise. Exerc Immunol Rev 17: 6-63.
- Gleeson M, Pyne DB (2016) Respiratory inflammation and infections in high-performance Immunol Cell Biol 94: 124-131.
- Junge A, Engebretsen L, Mountjoy ML, Alonso JM, Renström PAFH, et al. (2009) Sports injuries during the SummerOlympic Games 2008. Am J Sports Med 37: 2165-172.
- Engebretsen L, Steffen K, Alonso JM, Aubry M, Dvorak J, et al. (2010) Sports injuries and illnesses during theWinter Olympic Games 2010. Br J Sports Med 44: 772-780.
- Engebretsen L, Soligard T, Steffen K, Alonso JM, Aubry M, et al. (2012) Sports injuries and illnesses during theLondon Summer Olympic Games 2012. Br J Sports Med 47: 407-414.
- Soligard T, Steffen K, Palmer-Green D, Aubry M, Grant ME, et al. (2015) Sports injuries and illnesses in the Sochi2014 Olympic Winter Games. Br J Sports 49: 441-447.
- Junge A, Dvorak J, Graf-Baumann T (2004) Football injuries during the World Cup 2002. Am J Sports Med 32:23S-27.
- Dvorak J, Junge A, Grimm K (2007) Medical report from the 2006 FIFA World CupGermany. Br J Sports Med 41: 578-581.
- Alonso JM, Tscholl PM, Engebretsen L, Mountjoy M, Dvorak J, et al. (2010) Occurrence of injuries and illnesses during the 2009 IAAF World Athletics Championships. Br J Sports Med 44: 1100-1105.
- Alonso JM, Edouard P, Fischetto G, Adams B, Depiesse F, et al. (2012) Determination of future prevention strategiesin elite track and field: analysis of Daegu 2011 IAAF championships injuries andillnesses surveillance. Br J Sports Med 46: 505-514.
- Alonso JM, Jacobsson J, Timpka T, Ronsen O, Kajenienne A, et al. (2013) Preparticipation injury complaint is a riskfactor for injury: a prospective study of the Moscow 2013 IAAF championships. Br JSports Med 49: 1118-1124.
- Arthur JR, Nicol F, Beckett GJ (1993) Selenium deficiency, thyroid hormone metabolism, and thyroid hormone deiodinases. In: Am. J. Clinical Nutrition 57: 236-239.
- Krause, W. und P. Oehme (1979) Zur potentiellen Bedeutung von Selenverbindungen für die Medizin. Teil I: Selen als essentielles Spurenelement – Wirkungsmechanismen, Folgen eines Selenmangels. In: Dt. Gesundh.-Wesen 34: 1713-1718.
- Deakin V. Micronutrients. In: Lanham-New SA, Stear SJ, Shirreffs SM, Collins AL, eds: Sport and Exercise Nutrition. 1st Oxford, UK: Wiley-Blackwell; 2011: 66-88
- Heffernan SM, Horner K, DeVito G, Conway GE (2019) The role of mineral and trace element supplementation in exercise and athletic performance: a systemic review. Nutrients 11: 696-722.
- Tessier F, Margaritis I, Richard MJ, Moynot C, Marconnet P (1995) Selenium and training effects on the glutathione system and aerobic performance. Med. Sci. Sports Exerc 27: 390-396.
- Savory LA, Kerr CJ, Whiting P, Finer N, McEneny J, et al. (2012) Selenium supplementation and exercise: Effect on oxidant stress in overweight adults. Obesity 20: 794-801.
- Pingitore A, Lima GP, Mastorci F, Quinones A, Iervarsi G, et al. (2015) Exercise and oxidative Stress: potential effects of antioxidant dietary strategies in sports. Nutrition 31: 916-922.
- Lee O (2018) Assessment of selenium and zinc status in female collegiate athletes. J Nutr Health 51: 121-131.
- Margaritis I, Tessier F, Prou E, Marconnet P, Marini JF (1997) Effects of endurance training on skeletal muscle oxidative capacities with and without selenium supplementation. Trace Elem. Med. Biol 11: 37-43.
- Kohrle J (2015) Selenium and the thyroid. CurrOpin. Endocrinol. Diabetes Obes 22: 392-401.
- 24. Hess SY (2010) The impact of common micronutrient deficiencies on iodine and thyroid metabolism: the evidence from human studies. Best Pract. Res. Clin. Endocrinol. Metab 24: 117-132.
- Meyer T, Meister S (2011) Routine Blood Parameters in Elite Soccer Players. Int J Sports Med 32: 875-881.
- Ciloglu F, Peker I, Pehlivan A, Karacabey K, Ilhan N, et al. (2005) Exercise intensity and its effects on thyroid hormones. Neuro. Endocrinol 26: 830-834.
- Salerno M, Oliviero U, Lettiero T, Guardasole V, Mattiacci DM, et al. (2008) Long-term cardiovascular effects of levothyroxine therapy in young adults with congenital hypothyroidism. J. Clin. Endocrin. Metab 7: 2486-2491.
- Majerczak J, Karasinski J, Zoladz JA (2021) Training induced decrease in oxygen cost of cycling is accompanied by down-regulation of SERCA expression in human vastus lateralis muscle. J PHYSIOL PHARMACOL 59: 589-602.