What is Cancer?
Richard M. Fleming1*, Matthew R. Fleming1, Tapan K. Chaudhuri2, William C. Dooley3
1FHHI-OmnificImaging-Camelot, El Segundo, CA, USA
2Eastern Virginia Medical School, Norfolk, VA, USA
3Oklahoma University Health Science Center, Oklahoma City, Oklahoma
Received Date: September 29, 2019; Accepted Date: October 09, 2019; Published Date: October 18, 2019
*Corresponding author: Richard M. Fleming, FHHI-OmnificImaging-Camelot, El Segundo, CA, USA. Email: drrichardmfleming@gmail.com
Citation: Fleming RM, Fleming MR, Chaudhuri TK, Dooley WC (2019) What is Cancer? Curr Res in Onco: CRIO-108.
Key words: Cancer; FMTVDM; Metabolism; Regional Blood Flow; Transitional Changes
In 2019, it is estimated that 1.8 million Americans will be diagnosed with cancer, including 268,600 women and 2,670 men with breast cancer, the most commonly diagnosed type of cancer [1].
To understand something, you must first be able to define what it is. To understand what something is, you must also be able to understand what it isn’t. Cancer is not a calcium deposit. There are a variety of reasons why calcium can deposit anywhere in the body. While it is not normal to see deposits of calcium outside of bone, the presence of calcium does not automatically mean the patient has cancer.
Cancer is also not a collection of diseases – but rather – one disease, or at least one type of disease process. Cancer, as it turns out, is in fact, the transition of normal tissue, whatever that tissue is (breast, lung, prostate, pancreas, etc.), and the individual cells making up that tissue, functioning for the good of the organ, to cells which are autonomous – functioning for their own good. These cells have inter alia increased metabolic activity, increased mitochondrial activity, increased cellular division, and contact inhibition.
For decades the medical sciences have focused on the ability to find cancers, by our ability to find something abnormal, either by (A) a visual qualitative imaging test (x- rays like mammograms [2, 3], CTs [4], MRI [5], etc.), or by (B) an abnormally elevated blood test – which does not actually tell us if cancer is present any more than a calcium deposit does-resulting most likely from an increased protein or molecular compound being released from cells at higher than expected levels and thus measured at higher than normal levels in the blood.
We have also come to depend upon (C) biomarkers (e.g. BRCA1/2) suggestive of the potential for cellular and tissue change – but not determinant of change. Finally, we have come to depend upon our ability to diagnose cancer based upon or ability to find something (D) microscopically – including biomarkers (HER2, ER+, PR+) and abnormal cellular details as noted above. However, even this gold standard requires human qualitative interpretation with associated errors [6-8].
The recent development of algorithms [9] combining these approaches with sensitivity and specificity problems, into an effort to better detect cancer fails to understand these limitations and thus provides a false sense of security in the absence of actual quantifiable measurement of tissue changes [10, 11].
The implication of this approach to understanding and finding cancer has been an all-or-nothing classification – which may reflect the diagnostic related group (DRG) approach to the disease - but not reality. The DRG approach is you either have cancer or you don’t. This perspective implies, even if it is not believed, that the transition from normal to cancer is an overnight phenomenon. A serious reflection of this approach demonstrates a flaw in both the approach to understanding cancer and our ability to adequately care for patients and has led to missed opportunities to take a more proactive patient-oriented approach–focusing more on the satisfaction of CMS, Insurance Companies and Big Pharma, than the patient [12].
Cancer: Changes in regional blood flow and metabolism.
The changes that occur as normal cells respond to environmental insults resulting in a series of possible transformative changes, which may or may not result in cancer, can be measured from beginning to end as shown in (Figure 1) [13]. Rather than thinking about patients as a collection of diseases, a model promulgated by DRG and current procedural terminology (CPT) codes, the physician goal, pursuant to the Hippocratic Oath and the Declaration of Geneva, is to think in terms of the health of the patient [12] – viz. the patient’s health-spectrum.
As explained in detail elsewhere [13], the transitional changes associated with the potential development of cancer is the result of (A) enhanced genetic metabolic activity, which results in (1) the release of chemical mediators responding to the environmental factors – both positive and negative – and (2) augmentation of regional blood flow [14-17].
Nuclear imaging provides the only physiologic tool available for medical imaging of the body. As such, nuclear imaging provides the only true, real time, in vivo method for analyzing physiologic changes occurring within the patient.
While prior nuclear imaging procedures addressed qualitative imaging controls, they did not take into account quantitative imaging controls, leaving the interpretation of diagnostic studies flawed and without the accuracy, consistency and reliability required to (A) measure transitional changes in tissue or (B) the ability to measure the true impact of treatment upon cancer or other diseases such as coronary artery disease [18]. This has only recently been made possible [10, 19].
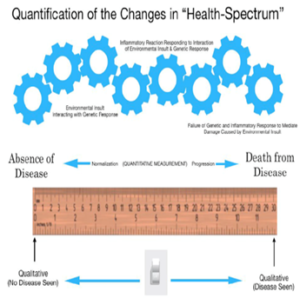
Figure 1: Quantification of changes in the cellular Health-Spectrum [13].Cellular changes resulting from genomic-environmental-treatment (part of the cellular environment) interactions can be measured across the Health-Spectrum.
FMTVDM**: Measuring changes in regional blood flow and metabolism.
Appreciating the importance of being able to measure in vivo changes in physiologic cellular/tissue function, requires an understanding of (A) the equipment being used – in this instance nuclear cameras which measure scintillations - to measure these transitional changes in cells/tissue, (B) the isotope(s) being used, (C) the validity of the imaging/acquisition equipment being used – i.e. the accuracy, consistency and reproducibility – to make the measurements [18], (D) the ability to influence – enhance - the differences in regional blood flow and metabolism present during the transitional tissue changes [10, 18-20], in such a way as to (E) make the test both more meaningful and reliable than what is currently used [21-22]. (Figure 2) provides a demonstration of FMTVDM enhanced and measured changes in regional blood flow and metabolism.
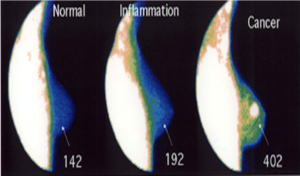
Figure 2: FMTVDM measured changes in metabolism and regional blood flow across the cellular Health-Spectrum [20].
Conclusion
Cancer has now become the number one killer of people worldwide. This fact alone should emphasize the importance of how we approach this global health problem. Cancer is no longer limited to industrialized countries, the poor or even the affluent. Cancer knows no geographic or socioeconomic barriers.
It is impossible to intelligently approach cancer from an absence of knowledge or limited approaches to finding, monitoring and determining the treatment response of a cancer. That which makes cancer, cancer, and that, which makes transitional changes in cells/tissue different from cancer and normal tissue, is what we must be able to measure if we are to address the health risk associated with the number one cause of morbidity and mortality worldwide. We cannot address cancer or its treatment, without the ability to measure these changes [23].
**The Fleming Method for Tissue and Vascular Differentiation and Metabolism (FMTVDM).
Acknowledgment: FMTVDM is issued to first author.
References
- The National Cancer Institute.
- Ekpo EU, Alakhras M, Brennan P (2018) Errors in Mammography Cannot be Solved Through Technology Alone. Asian Pac J Cancer Prev 19: 291-301.
- Miller AB, Wall C, Baines CJ, Sun P, To T, et al. (2014) Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomized screening trial. BMJ; 348:g366. DOI:10.1136/bmj.g366.
- Krishna S, Murray CA, McInnes MD, Chatelain R, Siddaiah M, et al. (2017) CT imaging of solid renal masses: pitfalls and solutions. Clinical Radiology 72: 708-721.
- Rominger M, Berg D, Frauenfelder T, Ramaswamy A, Timmesfeld N (2016)Which factors influence MRI-pathology concordance of tumour size measurements in breast cancer? Eur Radiol 26: 1457-1465.
- Raab SS, Grzybicki DM, Janosky JE, Zarbo RJ, Meier FA, et al. (2005) Clinical Impact and Frequency of Anatomic Pathology Errors in Cancer Diagnosis. Cancer 104: 2205-2213.
- Raab SS, Grzybicki DM (2010) Quality in cancer diagnosis. CA Cancer J Clin 60: 139-165.
- Tosteson ANA, Yang Q, Nelson HD, Longton G, Soneji SS, et al. (2018) Second opinion strategies in breast pathology: a decision analysis addressing over-treatment, under-treatment, and care costs. Breast Cancer Res Treat 167: 195-203.
- Tucker I. AI cancer detectors. The Guardian 10 June 2018.
- Fleming RM, Dooley WC, Chaudhuri TK (2017) The Development of FMTVDM-BEST Imaging: The Answer for Breast Cancer. Breast Enhanced Scintigraphy Test (BEST): Quantifying the Detection of Breast Cancer and its Treatment. J Nucl Med Radiat Ther 8: 1000350.
- Sheikh A (2018) Evolution of Quantification in Clinical Nuclear Medicine: A Brief Overview of Salient Uses and Upcoming Trends. J Nucl Med Radiat Ther 9: 1000375.
- Fleming RM, Fleming MR, Chaudhuri TK, McKusick A. Ethics in Medicine – The Hippocratic Oath! Acta Scientific Med Sci. 2019: Special Issue 1:08-10. DOI:10.31080/ASMS.2019.S01.0004.
- Fleming RM, Fleming MR (2019) The Importance of Thinking about and Quantifying Disease like Cancer and Heart Disease on a “Health-Spectrum” Continuum. J Compr Cancer Rep 3: 1-3 (Article ID 100011).
- Wright GL, Maroulakou IG, Eldridge J, Liby TL, Sridharan V, et al. (2008) VEGT stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J 22: 3264-3275.
- Siemerink MJ, Klaassen I, Vogels IM, Griffioen AW, Van Noorden CJ, et al. (2012) CD34 marks angiogenic tip cells in human endothelial cell cultures. Angiogenesis 15: 151-63.
- Senchukova MA, Nikitenko NV, Tomchuk ON, Zaitsev NV, Stadnikov AA (2015) Different types of tumor vessels in breast cancer: morphology and clinical value 4:512. DOI: 10.1186/s40064-015-1293-z.
- Luengo-Gil G, Gonzalez-Billalabeitia E, Perez-Henarejos SA, Navarro Manzano E, Chaves-Benito A (2018) Angiogenic role of miR-20a in breast cancer. PLoS ONE 13: e0194638. DOI:10.1371/journal.pone.0194638.
- Fleming RM, Fleming MR, Chaudhuri TK, McKusick A, Dooley WC (2019) Nuclear Imaging: Physician Confusion Over True Quantification and Isotope Redistribution. J Clin Cases Rep 3: 32-42.
- The Fleming Method for Tissue and Vascular Differentiation and Metabolism (FMTVDM) using same state single or sequential quantification comparisons. Patent Number 9566037. Issued 02/14/2017.
- Fleming RM, Dooley WC (2002) Breast Enhanced Scintigraphy Testing (B.E.S.T.) Distinguishes Between Normal, Inflammatory Breast Changes and Breast Cancer. A Prospective Analysis and Comparison with Mammography. Integrative Cancer Therapies 1: 238-245.
- Fleming RM, Fleming MR, Chaudhuri TK, Dooley WC, McKusick A (2019) Letter to the Editor: A response to Hruska’s case study on molecular breast imaging and the need for true tissue quantification. Breast Cancer Res 21:15 doi: 10.1186/s13058-019-1103-6.
- Fleming RM, Fleming MR, Chaudhuri TK, McKusick A, Dooley WC (2019) Statistical demonstration that FMTVDM is superior to mammography. Ann Clin Radiol 2:1012.
- Fleming RM, Fleming MR (2019) Rapid Response Flawed evidence underpins approval of new cancer drugs. BMJ 366:l5399. https://www.bmj.com/content/366/bmj.l5399/rapid-responses
