Publication Information
ISSN 2691-8803
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
The Status of Surface Water at Three Selected Areas of Coastal Guyana and Removal of Contaminants Using a Suitable Natural Adsorbent
Raymond Jagessar1*, Dawnel Monplaisir2
1Department of Chemistry, Faculty of Natural Sciences, University of Guyana
2Final Year Biology students, Faculty of Natural Sciences
Received Date: March 24, 2024; Accepted Date: March 29, 2024; Published Date: April 10, 2024;
*Corresponding author: Raymond Jagessar, Department of Chemistry, Faculty of Natural Sciences, University of Guyana; Email: raymond.jagessar@uog.edu.gy
Citation: Jagessar R, Monplaisir D (2024); The Status of Surface Water at Three Selected Areas of Coastal Guyana and Removal of Contaminants Using a Suitable Adsorbent, Enviro Sci Poll Res and Mang: ESPRM-142
DOI: 10.37722/ESPRAM.2024202
Abstract
The surface water at Atlantic Gardens, Block X Liliendaal, Pattensen, Turkeyen, Guyana was tested for the presence of heavy metal cations and anions, electrical conductivity, EC, turbidity and salinity in the presence and absence of an adsorbent, ground coconut midrib in its uncarbonized state. Metal Cations tested for were: Fe3+ and Al3+, Anions tested for were PO43- and NO3-. Other parameters evaluated was chlorides. The adsorbent, prior to be used was ground, extracted with hexane and subjected to drying. The coconut midrib in its uncarbonised state, was effective in extracting Fe, Cl- , Al3+, PO43- and NO3-. Salinity also decreased. For example, the status of the surface water prior to the use of the adsorbent was: Al3+: 0.689mg/L; PO43-: 0.90mg/L; NO3-: 10.18mg/L; Salinity; 0.01mg/L. For the water treated with the absorbent, the respective concentrations were: Fe: 0.01mg/L; Cl-: 0.00mg/L; Al: 0.20mg/L; PO43-: 0.40mg/L and NO3-: 4.56mg/L. Salinity was registered at 0.0 mg/L. Thus, the adsorbent in its uncarbonized state, was effective in removing cationic and anionic pollutants from the selected surface water.
Keywords: Surface water, cations, anions, adsorbent, ground coconut midrib
Introduction
Water is a universal solvent that sustains all life forms. Much of the current concern with regards to environmental quality is focused on water, because of its importance in maintaining human health and health of the ecosystem. Surface water is water on the surface of the planet, such as in a stream, river, lake, wetland, or ocean. It can be contrasted with groundwater and atmospheric water 1-7. Providing sufficient quantities of high quality water to satisfy our domestic, industrial and agricultural needs is an ongoing global problem. Increasing population size, climate change and pollution will only exacerbate the global status. There is no physical shortage of water on the planet earth as it covers 70% of the globe. However, 97% of the world water is saline and is thus non-drinkable, 2% is locked in glaciers and polar ice caps, resulting in 1% to meet humanity needs7. Guyana water need continual monitoring to assess the concentration of toxic elements8. Surface water plays a very vital role in economics and the functioning of ecosystems 9. In Guyana, surface water is primarily used for agricultural, industrial and commercial purposes. Pollution of surface water, due to industrial effluents and municipal waste in water bodies is a major concern in Georgetown, Linden and many other regions in Guyana.
Surface water is usually rain water that collects in surface water bodies, like oceans, lakes, or streams. Another source of surface water is groundwater that discharges to the surface from springs. Guyana has abundant surface and ground water supplies near all populated centers. Both surface and ground water resources are relied upon for water supply requirements. Heavy amounts of precipitation provide high amounts of surface runoff and ground water recharge. Most of the domestic water supply comes from ground water resources, while most of the water supply for agriculture (such as, sugarcane and rice) and industry comes from surface water.
Surface water pollution occurs when hazardous substances come into contact and either dissolve or physically mix with the water [10-11]. Contamination of surface water can occur when hazardous substances are discharged directly from an outfall pipe or channel or when they receive contaminated storm water runoff. On the other hand, direct discharges can come from industrial sources or from certain older sewer systems that overflow during wet weather. Storm water runoff becomes contaminated when rain water comes into contact with contaminated soil and either dissolves the contamination or carries contaminated soil particles. Surface water can also be contaminated when contaminated groundwater reaches the surface through a spring, or when contaminants in the air are deposited on the surface water. Contaminated soil particles carried by storm water runoff or contaminants from the air can sink to the bottom of a surface water body, mix with the sediment, and remain [12].
Subsequently, all levels of an ecosystem will be negatively impacted due to contamination of surface water. This is due to the fact that there will be a great change in the water chemistry. It can impact the health of lower food chain organisms and, consequently, the availability of the food supply up through the food chain. It can also impact the health of wetlands and impair their ability to support healthy ecosystems, control flooding, and filter pollutants from storm water runoff. Contaminated surface water can also affect the health of animals and humans when they drink or bathe in contaminated water or, for aquatic organisms, when they ingest contaminated sediments. One of the major concerns associated with contaminated surface water is the ability of aquatic organisms, like fish, to accumulate and concentrate contaminants such as heavy metals in their bodies. When other animals or humans ingest these organisms, they receive a much higher dose of contamination than they would have if they had been directly exposed to the original source of the contamination.
The most effective approach for cleaning up contaminated surface water is to prevent further discharges from contaminated sources and enable natural biological, chemical, and physical processes to break down the existing contamination. In some surface water bodies where natural processes are not enough to break down the contaminants, other cleanup approaches such as ion exchange, reverse osmosis, precipitation, solvent extraction, membrane technologies, electrochemical treatment, sorption are necessary. Adsorption is by far the most versatile and widely used method and activated carbon is the most commonly used sorbent. However, the use of activated carbon is expensive, so there has been considerable interest in the use of other sorbent materials, particularly biosorbents13. So the technique used is this study was biosorption. A pulverized plant material (coconut fibre from the mid riff of Cocos nucifera) was used to aid in the removal of toxic metal ions from the surface water of selected areas or Mahaica- Berbice administrative region. Mahaica-Berbice was chosen as the study site, since it is well known for its large scale agricultural and industrial activities, which are major sources of surface water, and also due to the fact that surface water to be evaluated have not been previously analyzed.
Coconut fibers contain cellulose, hemi-cellulose and lignin as major composition. These compositions affect the different properties of coconut fibers. The pre-treatment of fibers changes the composition and ultimately changes not only its properties, but also the properties of composites. Sometimes it improves the behavior of fibers, but sometimes its effect is not favorable [14].
The importance of this research project is to give the government and residents of Guyana an understanding of the prevalent and growing issue of water contamination in certain areas of Guyana.
- Also to bring awareness of the status of surface water in these areas and whether or not there is an issue of water contamination.
- Moreover this study gives opportunity for the government of Guyana, academicians and the citizens to understand that this water treatment method may be implemented to improve the physical and chemical quality of water and to make safe, potable water for people living in Guyana by using a less expensive ground up plant material such as coconut midriff.
- This therefore would allow residence to be exposed to cleaner and much healthier water which is beneficial to the government and citizens of Guyana. By being successful this research project methodology can be used on a large scale to purify water and contribute economic value locally, regionally and internationally.
According to an article by (Unicef, 2018), Water contamination is a major ongoing issue in Guyana, especially in areas such as Georgetown and areas along the coast plain. Environmental Protection Agency (EPA) stated that biological and chemical contamination is mostly prevalent along the coast. In addition, farming practices (agricultural practices) along the coast, often change the water chemistry which poses a negative impact on the water quality [15]. Research also shows that inadequate waste management also poses a health risk, mainly in urban areas such as Georgetown. During the 2005 floods, canals were not adequately drained, which was partly due to waste accumulation in them. This contributed to outbreaks of leptospirosis. With water pollution being an issue of major concern in Guyana, immediate action needs to be taken soon to rectify the problem. It has been established that our main contributors of water pollution is indeed due to anthropogenic activities [16].
For example, Jagessar et.al reported the removal of metal ions from three selected surface water on Coastal Guyana, using the peanut shell biomass adsorbent. The water samples were collected from Parika Bushy Park and Vreed En Hoop and stored in water bottles. It was then submitted for physical and chemical analyses using versatile standard methods. These include test for heavy metals cations (Pb, Fe, Zn, Cd, and Al), test for anions (chlorides, sulphates, phosphates) along with the physical parameters (turbidity and conductivity). There was no detection for the toxic lead and cadmium cations at either surface water. The adsorbent was effective in removing Fe2+ at both surface water as there was a decrease in concentration. For example, at Vreed En Hoop surface water, the concentration of Fe2+ decrease from (8.42 ± 2.14 mg/L) to (5.56± 3.42 mg/L), 33.96% reduction, after treatment with the adsorbent. For Al3+ cation, there was a decrease in the concentration of Al3+ from (5.97 ± 0.67mg/L) to (4.20 ± 1.90 mg/L), 29.65%. For the SO42- and Cl- anions, there was a decrease in concentration at the Vreed En Hoop surface water, after treatment with the adsorbent. With SO42-, the concentration decrease from 346 ± 3.15 mg/L to 293 ± 1.77 mg/L, 15.31%, whilst that for chloride, Cl-, decrease from 116 ± 1.75 mg/L to 102 ± 1.70 mg/L, 12.07% reduction. Thus, the peanut shell should find application in the removal of selective cations and anions from surface water [17].
Jagessar and Lord reported the status of surface water at five selected areas of Coastal Guyana: Blairmont, Bath, Bushlot, Belladrum and Mahaicony before and after treatment with a suitable coconut fibre adsorbent. In all cases,the concentration of cations and anions were below the WHO standard. Only at Mahaicony surface water, the concentration of Cl- was above the WHO standards. The adsorbents (coconut fibres) was selective in its removal of Pb2+ at Bushlot, Mahaicony and Belladrum surface water. Also, it showed selectivity for removal of Fe3+ at all cases, whilst, the concentration of Mn2+ remained the same for treated and untreated water. For example, the concentration of Fe2+ in the surface water at Bath for treated and untreated water was (7.31 ± 0.44 mg/L) and (6.88 ± 0.51 mg/l) respectively. It was also shown to reduce the turbidity in all cases, whilst elevating the pH [18].
The suitability of recycled coconut fiber as the filter media for the treatment of waste water has been reported. It has been observed that coconut fiber can be used as an alternative filter media for the removal of pollutants as well as fungus from waste water, as there are large amount of micro-pores with standard surface area existing in coconut fibres [19].
The use and efficiency of activated carbon from coconut shells as the potential cost effective absorbent material in drinking water filter has been noted23. Activation of the coconut shell carbon was carried out by carbonization in the exposure to nitrogen (N2) atmosphere followed by heating with the activating agents for a specific retention period. pH test. Testing of filtered water were conducted using the protocol established by ANSI/NSF Standard 53 (Health Effects of Water Treatment System). The pH value was indicated to increase proportionally to the level of filtering, which has achieved a constant value of 6.41 after eight times of filtering. The activated carbon has been found to remove Methyl Tertiary-butyl Ether (MTBE) to non-detectable level, which is less than 1 part per billion (ppb). The non-detectable level has sufficiently reduced the odour and taste problems [20].
The performance of coconut fiber ash as an alternative low-cost adsorbent to the synthetic adsorbents used in wastewater treatment has been observed [21]. The optimum condition for the adsorption process was investigated, considering the effect of particle size, adsorbent dosage, and contact time of adsorbents of coconut fiber ash. It was found to be effective in the removal of lead (Pb), copper (Cu), and zinc (Zn) metal ions from electroplating wastewater. The adsorbents coconut fiber ash was prepared through activation of carbon at 450º C. The experiments were conducted at varying adsorbent dosages (0.2 g, 0.6 g, and 1 g), particle size (50 to 200 microns), and contact times (40 minutes, 80 minutes, and 120 minutes). The adsorbents show less efficiency in removing Zn metal ions, which is not more than 34% in the case of 1g adsorbent dosage, particle size ranges 100-200 microns, and 120 minute contact time. The maximum removal efficiency of 95.04% and 80% was obtained at the optimum amount (1g) of adsorbent dosage for Pb and Cu respectively. In the case of contact time, it was identified that the optimum condition for maximum removal efficiency is 120 minutes with a 1g adsorbent dosage both for Pb and Cu ions. (Wastewater treatment using coconut fibre ash as an adsorbent for removal of heavy metals [22].
Coconut shells via the production of activated carbon have been used in the treatment processes of ground water to reduce water hardness via the removal of Calcium, Magnesium and Total hardness. The initial values of Calcium, Magnesium and Total Hardness in the raw water sample were 120.24mg/L, 98.29mg/L and 588.00mg/L, respectively which are above the World Health Organization (WHO) standard. Calcium, Magnesium and Total Hardness were removed via the adsorbent at a contact time of 60 minutes with removal efficiencies of 80%, 60.44% and 66.71%, respectively. Also, the optimum dosage occurred at 1.2g for Calcium hardness, 1.5g for Magnesium hardness and Total hardness [23].
Coconut midrib as plantation waste can be used as raw material for activated charcoal production. In the initial stage, the coconut midrib was dried and homogenized to 2 mesh. After that the coconut midrib was carbonized at temperatures of 425ᵒC, 450ᵒC, and 475ᵒC and a time of 105 minutes, 135 minutes and 165 minutes. In the next stage, the carbonization results are activated with HCl solution to produce activated charcoal. The highest % yield of activated charcoal was obtained at 425ᵒC for 105 minutes at 30.9%. Increased temperature and carbonization time cause reduced yield of activated charcoal from coconut midribs. The results of the initial analysis of jumputan waste indicate a high COD level of 180 mg / L and exceeds the Environmental Quality Standard which is a maximum of 150 mg / L. The maximum adsorption performance of activated charcoal was obtained when the carbonization temperature was 425 ℃ which caused a decrease in COD to 20 mg / L (88.89%). A decrease in BOD which was initially 160 mg / mL was significantly reduced to 16 mg / L (90%) in the same carbonization condition. It shows that the activated carbon from the coconut midrib is able to reduce the levels of COD and BOD of jumputan liquid waste which can be comparable with the Environmental Quality Standards [26]. The use of natural adsorbents to remove heavy metal ions from water, have been finding increasing applications27-28
Guyana is a sovereign state on the northern mainland of South America and is also part of the Caribbean region. Guyana (83,000 square miles) is bordered by the Atlantic Ocean to the north, Brazil to the south and southwest, Suriname to the east and Venezuela to the west [29]. Fig 1.0 is a map of two of the selected areas of coastal Guyana. Its an Amerindian word, meaning land of many waters.
Figure 1: Map of Guyana.
www.worldatlas.com/webimage/countrys/samerica/gy.htm
Thus, the general objectives of the research were to identify and evaluate the status of surface water from three selected areas of coastal Guyana: Block X Liliendaal, Atlantic Gardens and Pattensen Turkeyen of coastal Guyana for selective cations and anions and removal of contaminants, using a suitable ecofriendly alternative adsorbent” in coconut midriff in its uncarbonized state, Fig. 2.0. Also, to identify the optimal percentage level of heavy metals, cations & anions, along with the turbidity and conductivity within the water samples. To the best of knowledge, there is one report of the coconut mibrib in an uncarbonised state as a suitable an adsorbent.

Figure 2: A coconut branch, showing its distinctive midrib
Methodology
Procedure
The water samples were collected in triplicates of two (2) from the three selected areas of Guyana’s Coastland, (Block X Liliendaal, Atlantic Gardens and Pattensen Turkeyen), Six (6) bottles for each site, plus another three (3) bottles for the filtrate of the treated water (3 for treated water and 3 for untreated water and another three to collect the filtrate). The samples were labelled and stored in a cool environment.
Preparation of adsorbent:
5g of adsorbent (coconut midriff) was added to a conical flask with open neck. To this 100ml of hexane was added. It was then stirred and allowed to stand for 24hrs and then filtered into one of the glass bottles. The residue was then treated with another 100ml of hexane, stirred and allowed to stand for another 24 hrs and filtered into the same plastic bottle. The hexane serves to remove the lipophilic content of the adsorbent and at the same time, allowing it to submerge in the filtrate. The residue (treated adsorbent) was then allowed to dry in air/sunlight.
Treatment of water sample
The water samples treated with the dried adsorbent, was stirred for at 2-3 hours and filtered. This was done in triplicates (3 x 3 = 9 water bottles for treated water). The samples were then submitted immediately for analyses at GWI (Guyana Water Inc.) water quality laboratory.
Procedure
For the untreated and treated wáter, the water samples were filtered, using a pore diameter membrane filter. After filtration, the filtrate was transferred to a beaker. 5ml of Conc. H2SO4 and several boiling chips were added. The contents of the beaker was brought to a slow boil and evaporated on a hot plate to the lowest volume (10ml) to initiate precipitation. Heating was continued with concomitant addition of HNO3 until digestion was completed. Drying of the samples was avoided. The flask was washed with water and contents filtered. The filtrate was transferred to a 100ml volumetric flask and make up to the mark. Portions of the solution was then taken for metal ion determinations using Flame Atomic Spectroscopy. For each metal analyzed, appropriate standard solution of known metal concentration in the water with a matrix similar to the sample was prepared. The digested sample was analysed using atomic absorption spectroscopy.
Results
All data obtained from this research project are presented and computed in tables, 1.0-10.0 and graphs, 1.0-6.0. Data were analyzed using standard and scientific calculation of results.
Table 1: Showing the parameters (cations) for water analysis for the Atlantic (mg/l)
Location
Iron
Al (mg/L Al3+)
Manganese mg/L
WHO Guidelines
<0.3
<0.200
Untreated Water Atlantic Gardens
0.04
0.375
0.011
Treated Water Atlantic Gardens (Sample A1)
0.00
0.148
0.010
Treated Water Atlantic Gardens (Sample A2)
0.00
0.124
0.010
Treated Water Atlantic Gardens (Sample A3)
0.00
0.100
0.010
Average (Treated water)
0.00 ± 0.00
0.12 ± 0.019
0.010 ± 0.0
% reduction
100
94.78
100
Table 2: Showing the parameters (cations) for water analysis for Block X liliendaal (mg/l) (Sample A1) (Sample A2) (Sample A3)
Location
Iron
Al (mg/L Al3+)
Manganese mg/L
Untreated Water Block X Liliendaal
0.26
0.939
0.035
Treated Water Block X Liliendaal
0.08
0.800
0.0125
Treated Water Block X Liliendaal
0.05
0.700
0.0100
Treated Water Block X Liliendaal
0.05
0.600
0.0100
Average (Treated water)
0.06 ± 0.014
0.7 ± 0.082
0.01 ± 0.0012
% reduction
76.92
25.45
71.43
Table 3: Showing the parameters (cations) for water analysis for the Pattensen surface water (mg/l) (Sample A1) (Sample A2) (Sample A3)
Location
Iron
Al (mg/L Al3+)
Manganese mg/L
WHO Guidelines
<0.3
<0.200
Untreated Water Pattensen Turkeyen
0.03
0.689
0.017
Treated Water Pattensen Turkeyen
0.02
0.345
0.015
Treated Water Pattensen Turkeyen
0.01
0.20
0.013
Treated Water Pattensen Turkeyen
0.01
0.20
0.013
Average (treated water)
0.013 ± 0.004
0.248 ±0.068
0.014± 0.00
% reduction
56.67
64.0
17.65
Table 4: Showing the parameters (anions) for water analysis for the Atlantic Gardens water
Location
Cl-(mg/L)
Phosphate (PO4) (mg/L)
Nitrate (mg/L)
WHO Guidelines
< 11.2
Untreated Water Atlantic Gardens
0.00
0.65
0
Treated Water Atlantic Gardens (Sample A1)
0.01
0.40
1.427
Treated Water Atlantic Gardens (Sample A2)
0.00
0.30
1.320
Treated Water Atlantic Gardens (Sample A3)
0.00
0.20
1.200
Average (Treated) Atlantic Gardens
0.003 ± 0.0047
0.3± 0.082
1.32 ± 0.09
% reduction
53.85
Table 5: Showing the parameters (anions) for water analysis for Block X liliendaal water (Sample A1) (Sample A2) (Sample A3)
Location
Cl-(mg/L)
Phosphate (PO4) (mg/L)
Nitrate (mg/L)
WHO Guidelines
< 11.2
Untreated Water Block X Liliendaal
0.06
0.41
3.542
Treated Water Block X Liliendaal
0.00
0.30
2.257
Treated Water Block X Liliendaal
0.00
0.20
1.200
Treated Water Block X Liliendaal
0.00
0.20
1.200
Average Treated Water Block X Liliendaal
0.0 ± 0.0
0.23 ± 0.047
1.55 ± 0.49
% reduction
43.9
56.24
Table 6: Showing the parameters (anions) for water analysis for the Pattensen Turkeyen water (Sample A1) (Sample A2) (Sample A3) (Sample A3)
Location
Free Chlorine(mg/L)
Phosphate (PO4) (mg/L)
Nitrate (mg/L)
Untreated Water Pattensen Turkeyen
0.00
0.90
10.182
Treated Water Pattensen Turkeyen
0.00
0.60
7.38
Treated Water Pattensen Turkeyen
0.00
0.40
4.56
Treated Water Pattensen Turkeyen
0.00
0.40
4.56
Average Treated Water Pattensen Turkeyen
0.00
0.47
5.5
% reduction
47.78
45.98
Table 7: Table showing the parameters used (pH, Turbidity, Salinity, Total Coliform, E.coli) for Water Analysis for the Atlantic Garden water. (NTU)
Location
pH
Turbidity
Salinity (ppt)
Total Coliform (CFU)
E.coli (CFU)
WHO Guidelines
6.5-8.5
<5.0
<1
Low 0-25
0
Untreated Water Atlantic Gardens
7.14
2.58
0.01
TNTC
36
Treated Water Atlantic Gardens (Sample A1)
7.00
1.20
0.00
2
0
Treated Water Atlantic Gardens (Sample A2)
6.5
1.00
0.00
1
0
Treated Water Atlantic Gardens (Sample A3)
6.5
0.00
0.00
0
0
Average (Treated water Atlantic gardens
6.67 ǂ0.235
0.73 ǂ0.525
0.00ǂ0.0
1.0ǂ0.8
0.0ǂ0.0
% reduction
6.58
71.71
100
Table 8: Table showing the parameters used (pH, Turbidity, Salinity, Total Coliform, E.coli) for Water Analysis for Liliendaal area (NTU) (Sample A1) (Sample A2) (Sample A3)
Location
pH
Turbidity
Salinity (ppt)
Total Coliform (CFU)
E.coli (CFU)
WHO Guidelines
6.5-8.5
<5.0
<1
Low 0-25
0
Untreated Water Block X Liliendaal
7.00
2.69
0.07
TNTC
0
Treated Water Block X Liliendaal
6.95
1.25
0.06
0
0
Treated Water Block X Liliendaal
6.5
1.10
0.04
0
0
Treated Water Block X Liliendaal
6.5
1.10
0.03
0
0
Average (Treated water block X liliendaal)
6.65 ǂ 0.212
1.15ǂ0.07
0.043ǂ0.012
0.0ǂ0.0
0.0ǂ0.0
% reduction
5
57
38.57
Table 9: Table showing the parameters used (pH, Turbidity, Salinity, Total Coliform, E.coli) for Water Analysis for the three selected area (NTU) Turkeyen (Sample A1) (Sample A2) (Sample A3)
Location
pH
Turbidity
Salinity (ppt)
Total Coliform (CFU)
E.coli (CFU)
WHO Guidelines
6.5-8.5
<5.0
<1
Low 0-25
0
Untreated Pattensen
6.82
4.42
0.01
TNTC
0
Treated Water Pattensen Turkeyen
6.75
4.24
0.01
0
7
Treated Water Pattensen Turkeyen
6.60
2.20
0.00
0
4
Treated Water Pattensen Turkeyen
6.50
2.10
0.00
0
3
Average (Treated Pattensen water)
6.62 ǂ 0.103
2.85 ǂ 0.986
0.003 ǂ 0.00474
0.0 ǂ 0.0
4.67 ǂ 1.69
% reduction
2.93
31.43
70.0
Table 10: ANOVA TESTS
Variable
p-Value
Comment
Anions
Nitrate
0.003711
Significant, reject Null
Chlorine
0.4219
Not significant, accept Null
Phosphate
0.05529
Not significant, accept Null
Cations
Iron
0.01308
Significant
Aluminum
0.00102
Significant
Manganese
0.0002157
Significant
Parameters
pH
0.9658
Not significant
Turbidity
0.03703
Significant
Salinity
0.002331
Significant
Total Coliform
0.125
Not significant
E. coli
0.004571
Significant
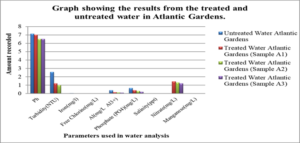
Graphs: Graph #1 shows the results for the treated and untreated water in Atlantic Gardens only
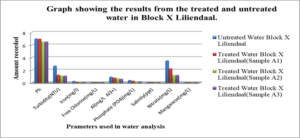
Graph #2 shows the results for the treated and untreated water in Block X Liliendaal only
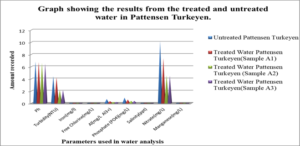
Graph #2 shows the results for the treated and untreated water in Pattensen Turkeyen only
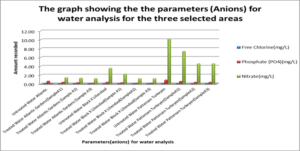
This graph shows the parameters (Anions) which are Chloide, Phosphate and Nitrate for water analysis for the three selected areas.
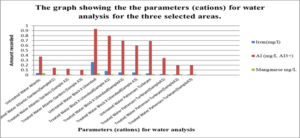
This graph shows the parameters (Cations) which are Iron, Aluminium and Manganese for water analysis for the three selected areas.
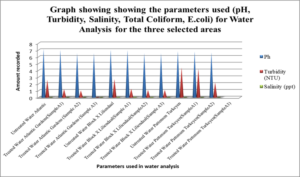
This graph shows the physical parameters such as Ph, Turbidity, Salinity, Total Coliform and E.coli for water analysis for the three selected areas.
Discussion
The status of surface water at three selected areas of coastal Guyana: Atlantic Gardens, Lilliendaal Block X and Pattensen, Turkeyen were investigated in the absence and presence of a suitable adsorbent, coconut midrib in its pulverized state. First the untreated water was analysed for cations followed by anions and physical parameters. This was followed by the analyses of the treated water with the suitable adsorbent. Cations tested for were: Iron (Fe3+), Al (Al3+) and manganese (Mn2+). Anions tested for were chloride (Cl-), phosphate (PO43-) and nitrate (NO3-). Physical parameters tested for were: pH, Turbidity, salinity and microbial content such as E.coli (CFU) & Total coliform (CPU). The following were observed:
For the untreated water at Atlantic Gardens, the concentration for the three cation range from: 0.01mgL-1 to 0.375mgL-1, with the highest concentration of 0.375mgL-1 noted for Al3+ and the lowest of 0.04, noted for iron. All these values with the exception of Al range below the WHO standard. For the untreated Liliendaal water, the concentration of the three cations range from 0.26mgL-1 to 0.939mgL-1, with the highest concentration of 0.939 mgL-1 noted for Al3+. For the Pattensen water, the concentration of cation range from 0.03mgL-1 to 0.689mgL-1. For all three selected areas, it seems as though treatment with the adsorbent resulted in a decrease in all of the cations (Fe, Al and Mn) concentration. For Fe, the highest % reduction of 100% in concentration was seen for Atlantic Gardens water. For Al3+the highest % reduction of 94.8% was noted for Atlantic Gardens water. For Mn2+, the highest % reduction of 100% was noted for Atlantic Garden water.
For the untreated Atlantic Gardens water, the concentration of the anions range from 0.00mgL-1 to 0.65mgL-1. For the Cl- anion, there was an increase in concentration from 0.0mgL-1 to 0.003 ± 0.0047mgL-1 . The concentration of PO43- showed a 53.9 % reduction from 0.65 to 0.3 ± 0.082mgL-1 . Nitrate showed an increase in concentration from 0.0 mgL-1 to 1.32 ± 0.094 mgL-1.
For Liliendaal surface water, the concentration of anion range from 0.061mgL-1 to 3.54 mgL-1. There was a decrease in concentration of Cl- from 0.06 mgL-1 to 0.0mgL-1 i.e 100% reduction, whereas both phosphate and nitrate showed % reduction of 43.9% and 56.24% respectively, Table 5.0. For Pattensen, Turkeyen water, the concentration of anion range from 0.0mgL-1 to 10.18 mgL-1. There was no decrease in the concentration of Cl- anion, Table 6.0. However, 47.78 % and 45.98 % reduction in the concentration of PO43- and NO3- were registered respectively.
Table 7, Table 8.0 and Table 9.0 show the pH, Turbidity, salinity, total coliforms (CFU) and E.coli (CFU) profile for the three selected surface water.The pH was shown to decrease for all areas, with the largest reduction of 6.58% recorded for Atlantic Garden waters and the least for Liliendaal Block X water (5%) reduction. Turbidity showed reduction for all three selected surface water areas. Salinity also showed a reduction in concentration in all cases, after been treated with an adsorbent. The highest % reduction of 100% for salinity was noted for Atlantic Gardens water. Results also indicated that the adsorbent also significantly reduced the Total Coliform and E.coli content. This indicates that the pulverized coconut adsorbent has antimicrobial properties.
ANOVA analyses were done to see whether there was any significant differences in the concentration of cations and anions for treated and untreated water. It was found that there was a significant differences in the concentration of nitrates (p=0.003711). Thus, rejecting the Null hypothesis. However, the concentration of chloride (p=0.423) and phosphate (p=0.05529) wasn’t significant. Thus, the Null hypothesis can be accepted. For the cations, the concentration of iron, aluminium and manganese resulted in a P value less than 0.05, hence, there was a significant difference in the concentration before and after treatment with adsorbents. Thus, the Null hypothesis can be rejected. For the physical parameters tested, pH and Total coliform induced a p value greater than 0.05. Hence these weren’t significant. However, Turbidity, salinity and E.coli induced p value less than 0.05 and so were significant. Thus, rejecting the Null hypothesis.
Conclusion
Based on results obtained, it can be concluded that the adsorbent (coconut midriff) is effective for the removal of the three cations: Fe3+, Al3+ and Mn2+ and anion phosphate from all three surface water and removal of nitrate from Block X liliendaal and Pattensen, Turkeyen surface water. The adsorbent was also in effective for the removal of chloride from all three surface water. In terms of physical parameters, the adsorbent was also effective in lowering the pH, turbidity and salinity. Reduction in turbidity and salinity was very significant. Thus, the adsorbent is a promising candidate to be used in water filtration and water remediation, locally, regionally and internationally.
Acknowledgements: Prof. R. Jagessar thank the department of chemistry and biology for the provision of bench space to conduct the above research
References
- Young RM, Bredehoeft JD. (1972). Digital simulation for solving management problems with conjunctive groundwater and surface water systems from Water Resources Research 8:533-56.
- Eaton AD, Clessicens SL, Greenberg EA. (1995). Standard methods for the Examination of Water and Wastewater, 19th United Book Press Inc. Baltimore, Maryland USA, p. 4-48.
- Eaton AD, AWWA, Chair, et al. (1995) “Standard methods for the examination of Water and Wastewater”, 19th United Book Press Inc. Baltimore, Maryland USA, pp 4-67.
- Booth RL. (1983). Methods for the Chemical analysis of Water and Wastes, 2nd Environmental Monitoring and Support Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati: Ohio 45268; p 352.
- Hach company, Water Analyses handbook, 3rd Loveland Colorado, USA; 1997, p 304-307.
- Radojevic M., Bashkin VN. 1999 “Practical Environmental Analysis”, Royal Society of Chemistry, Cambridge UK.
- Elliot S. Testing the water, Royal Society of Chemistry, RSC, News Magazine, 12 (5), 12-13 (2008).
- Williams N. Guyana Times, a News Magazine, (11), 2010.
- Khublaryan, MG. (1994). Surface Waters: Rivers, streams, lakes and wetlands. Encylopaedia of Life Support Systems.Types and Properties of Water,1
- (2011, August 9). Drinking water contaminants. Retrieved September 13, 2015, from http://www.epa.gov/superfund/students/website/srfcspikl.htm.
- (2013). Total Nitrogen. United States: Environmental Protection Agency.
- Carr GM, Neary JP. Water Quality for Ecosystem and Human Health. 2006. Burlington: United Nations Environment Programme Global Environment Monitoring System / Water Programme. 2008 — 132 p
- Ng J, Cheung W, & McKay G. (2003). Equilibrium studies for the sorption of lead from effluents using chitosan.Chemosphere,52,1021-1030. Retrieved November 12 2015, from Elsevier Science Direct database.
- Hossain M, Nalsy, M, Sobohan M. (2013). Effect of Industrial Pollution on the Spatial Variation of Surface Water Quality. American Journal of Environmental Sciences. 9, 120-129.
- Unicef, (2018). The Impact of CEE (Climate, Environment and Energy) on water, nutrition and health. Retrieved from https://www.unicef.org/lac/media/4671/file/PDF%20Guyana%20climate%20landscape%20analysis%20for%20children.pdf
- Guyana Chronicle. (2015). Water Pollution and EPA’s Regulation. Retrieved from https://guyanachronicle.com/2015/08/08/water-pollution-and-epas-regulation/
- Jagessar RC, Prince C (2021). Status of Surface Water from selected areas of Coastal Guyana and the Removal of Toxic contaminants using a suitable adsorbent. Advances in Earth and Environmental Science. Adv. Earth & Env.Sci. 2 (14), 1-7...
- Jagessar RC, Lord B (2020). Status of Surface Water from Selected Areas of Coastal Guyana and Selective Removal of Pb2+ and Fe2+ using Pulverized Coconut, Environmental Analysis & Ecology Studies, 7 (4), 803-817.
- Islam MT, Islam S, Saifullah I, Datta D, Ahmed A. (2017). Proceedings of the Waste Safe, 5th International Conference on Solid Waste Management in the Developing Countries, 25-27.
- Samdin SM, Peng LH, Marzuki M. (2015). “Investigation of coconut shells activated carbon as the cost effective absorbent in drinking water filter”. Jurnal Teknologi (Sciences & Engineering) 77:22 13-17.
- Jolly, F.A., Abedin, Z., Muyen, Z. (2022). “Wastewater treatment using coconut fibre ash as an adsorbent for removal of heavy metals”. Archives of Agriculture and Environmental Science, 7(2): 192-198.
- ADEWUYI, AS; OLABANJI, TO. (2022). The Use of Coconut-Shell Based Activated Carbon as an Adsorbent in the Treatment of Hard Water. J. Appl. Sci. Environ. Manage. 26 (3) 453-457.
- Nurisman E, Rahmatullah S. (2018). Characteristics of Activated Charcoal from Coconut Midribs in Jumputan Waste Adsorption Process. Conference Paper.
- Sobhanardakani S, Parvizimosaed H, Olyaic E. (2013). “Heavy metals removal from Wastewaters, using organic solid waste-rice husk”, Environ.Sci. Pollution. Res. 20, 5265-5271;
- Amarsinghe, BMWPK, Williams RA. (2007). “Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater”, Chem.Eng. J. 132, 299-309.