Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
The Millenary History of the Fig Tree (Ficus carica L.)
Egizia Falistocco*
Department of Agricultural, Food and Environmental Sciences, University of Perugia, Borgo XX Giugno, 06121 Perugia, Italy
Received Date: September 08, 2020; Accepted Date: September 16, 2020; Published Date: September 25, 2020;
*Corresponding author: Egizia Falistocco, Department of Agricultural, Food and Environmental Sciences, University of Perugia, Borgo XX Giugno, 06121 Perugia, Italy. Email: egizia.falistocco@unipg.it
Citation: Falistocco E (2020) The Millenary History of the Fig Tree (Ficus carica L.). Adv in Agri, Horti and Ento: AAHE-130.
Abstract
Ficus carica L. is a classical fruit tree of antiquity, it goes back to the beginning of horticulture in the Mediterranean basin. Domestication produced substantial modifications on the primitive characteristics of the plant, for example by increasing the sugar content and the size of the fruit and determining a gradual shift toward vegetative propagation. However in the wild the spreading of the species is entirely dependent on seed. A very particular feature of this species is the reproductive biology which is regulated by a mechanism of extraordinary complexity based on the mutual symbiosis between the plant and its pollinator wasp Blastophaga psenes. This review provides the description of the intertwined life cycle plant-insect, of the floral biology and of the genetic control determining the different floral forms and the two sexual forms of tree. A part of this review is dedicated to the cytogenetic background of F. carica emphasizing the importance of recent investigations revealing the presence of polyploid cytotypes within wild and cultivated Italian populations. Considerations on the sexual reproduction and the genetic constitution of the fig cultivars are also reported.
Keywords: Blastophaga psenes; Ficus carica; Floral Biology; Pollinator Wasp; Sex Determination; Symbiosis Plant-Insect
The Genus Ficus
General Characteristics
The genus Ficus L., of the Moraceae family, is one of the largest genera of angiosperms including about 800 woody plant species. It is widespread in most of the subtropical and tropical regions of the world exhibiting its highest concentration of species in the Asian-Australian region, with more than 66% of the total species, and its maximum diversity in the Asian mainland and Borneo. The fig tree (Ficus carica L.) is the only member of this genus having a Mediterranean distribution. In the tropical rain forests, the figs are considered as keystone species due to their fundamental role in the ecosystems; with their fruits they provide food to insects, birds and other animals during the entire year [1]. Condit (1064) [2], one of the greatest specialist of this matter, defined Ficus a fantastic member of the plant kingdom because of its extreme diversity of plant forms. It comprises in fact deciduous and evergreen trees, shrubs, climbers and creepers, as well as epiphyte, semi-epiphyte and lithophyte species. Another peculiar feature is the close symbiotic relationship between the plant species and their pollinator wasps offering one of the best known examples of reproductive interdependence plant-insect [3, 4]. The pollinators of the Ficus species are symbiotic wasps of the Agaonidae family of the Hymenoptera order. The symbiosis is highly specific; the majority of species has as pollinator a single specific agaonid wasp. Only in a few cases, a fig species hosts two wasp species or a wasp pollinates a few closely related Ficus species. The evolution of the intertwined life cycle plant-insect gave rise to specialized inflorescences and an extremely complex reproductive biology. The inflorescence of fig species is called syconium, the same term designates also the fruit which, due to its anatomical structure, is considered a false fruit. The syconium is a fleshy branch enlarged in a concave piriform receptacle open to the outside by a narrow orifice denominated ostiole. On its inner surface the syconium bears a multitude of minute unisexual flowers. Depending on the type and combination of these flowers the Ficus species are defined monoecious or gynodioecious [5, 6].
The fig tree (Ficus carica L.) The origin and the early history
F. carica is one of the most representative species of the Mediterranean region, not only for its wide diffusion but also for the importance this plant has had since time immemorial for the populations living in these regions. It takes its scientific name from Caria, a region of ancient settlement, noted for its figs and corresponding to the current southwestern part of Turkey. A great number of classical and biblical citations document the interest and consideration of this plant. Significant evidence is, no doubt, the suggestive oath which, fourth century Athenian recruits used to pronounce invoking, as witness of their oath, the gods, the borders of their homeland, grains, barleys, vineyards, olive trees and figs. While maintaining it’s simple and humble nature the fig tree acquired in the classical tradition a sacred aura for its connection to the foundation of Rome. Tito Livio in his “Ab Urbe condita” narrates that the twin brothers Romulus and Remus were abandoned in a makeshift cradle on the Tiber to die, and instead the basket stranded in a little puddle of water where a fig tree stood, here they were found and nursed by a female wolf. Successively this plant was called Ficus Ruminalis. The biblical tradition offers further evidence of the value of this species, beginning from Adam and Eve (Gen 3, 7). In addition, in the Bible the fig tree is included among the seven sacred plants. The consideration for this tree is also attested by some customs such as that of the rabbis who used to study the Scriptures sitting in his shade. Today times have changed, the expression “not worth a dried fig” in the Athens of the fourth century would have been blasphemous.
In ancient times the fig constituted one of the main sources of sustenance for the Mediterranean people being a high energy food for all social strata, not only during the time of fruiting but during the entire year thanks to easy preservation of the fruit by drying. The importance of fig fruits is also demonstrated by the fact that ancient Greeks even forbade their exportation and entrusted to special guardians, the sycophants (συκοϕαντης), the task of preventing smuggling.
Fig domestication goes back probably to 5.000-6.000 B.C. Recent archeological discoveries seems to demonstrate an even much older origin dating back to 11.000 years ago. In this case, fig domestication coincided with the beginning of agriculture. Most probably the domestication of the fig tree took place in the eastern Mediterranean and from there selected forms were brought to other regions, especially the western Mediterranean areas. During the classical epoch, fig trees along with olive trees, grapevines and date palms constituted the main horticultural elements of rain-dependent agriculture in the Mediterranean Basin [7].
Recent History
Today this tree still offers its exquisite fruits which, though no longer fundamental to sustenance as in the past, remains a sought after and much appreciated gastronomic product.
Nowadays the fig is an important crop for many regions with sub-tropical and tropical climates. In temperate zones production is limited and usually the fruit is consumed fresh locally or in dried, canned and preserved form. In those regions with climatic conditions and local traditions not typically favorable for this crop, the fig tree is in any case a very common plant cultivated as a single specimen in orchards and gardens. Domestication induced substantial changes in the primitive characteristics of the fig tree increasing the sugar content and the size of the fruit and determining a gradual shift towards the vegetative propagation. As with many other fruit crops, the discovery of vegetative propagation made fig cultivation possible allowing growers to fix the desired characteristics and thereby contributing to the diffusion of this culture. Instead in the wild the spreading of this species is totally dependent on seed disseminated by birds that voraciously consume its fruit and so fig trees grow everywhere, in heavily populated areas, along the edge of roads and in ruins. With favorable climatic conditions the plants develop grandiose forms creating the most fascinating and typical scenarios of the Mediterranean landscape (Figure 1). The spontaneous populations of F. carica represent a biological resource of extraordinary value which can be exploited for scientific and breeding purposes.

Figure 1: Caprifig growing in the countryside of Monte Romano (Latium, Central Italy).
Reproductive System and Floral Biology
The symbiotic relationship between F. carica and its minuscule pollinating wasp has fascinated biologists since classical times. Aristotle and Theophrastus mentioned the connection between an abundance of wasps and good fruit production in the fig tree (5). Nowadays it is know that the reproductive biology of F. carica is regulated by a mechanism of extraordinary complexity based on the following:
- the elaborate symbiosis with its wasp pollinator Blastophaga psenes;
- three functional floral forms;
- two forms of tree.
The symbiotic relationship between the plant and the insect is one of obligatory mutualism: they are completely dependent one-another for survival and reproduction, as the fig can be pollinated only by B. psenes and the wasp can reproduce only within the syconia. F. carica represents an excellent example of coevolution. The close relationship between plant and insect resulted in the evolution of an unusual amount of morphological diversity in this tree plant constituting one of the most interesting case of pollination syndrome. The flowers are minuscule and unisexual, they are of three types: staminate flowers, short-styled female flowers and long-styled female flowers (Figure 2 a-c). Male flowers contain a variable number of stamens (4-6) and produce dimorphic pollen which has been classified as triporate spherical and diporate ellipsoidal (Figure 2d). Both types are produced within the same anther and can germinate on a receptive stigma. Female flowers are characterized by a very pronounced dimorphism of style length and a sharp separation of functional role in the breeding system of the species. Both kinds of flowers are single-carpellated with a bifid stigma. The short-styled type serves primarily as the oviposition site for the pollinating wasp, exhibits a style about 0.70 mml long and a nearly globose ovary. In the past this type of flower was referred to as gall but later this term was considered misleading and abandoned. The long-styled flowers have a more or less ovoid ovary and a style of about 1.75 mm, they are not adapted for oviposition by the wasp but function as seed producers, following pollination they develop into fruits of the achene type [8]. On the other hand also the fig wasp has developed specializations which coordinate with those of its host. The most notable specialization is the development, in the female wasp, of an ovipositor which matches the length of the styles. In addition B. psenes exhibits a remarkable sexual dimorphism, the females are black and have well developed wings whereas males are brownish and wingless (Figure 3).
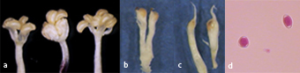
Figure 2: Flowers and pollen types in F. carica, male flowers with 4-6 stamens (a), short-styled female flower (b), long-styled female flower (c), triporate spherical and diporate ellipsoidal pollen grains (d).

Figure 3: Example of female (left) and male (right) of B. psenes.
F. carica is defined a gynodioecious species with a functional dioecious breeding system. It comprises two distinct morphs of tree with separated sexual functions resulting from the combination of the different flowers types.
The fig tree which produces syconia with staminate flowers and short-styled female flowers is called caprifig; it is referred to as the male tree. The fig tree bearing syconia containing only long- styled flowers represents the female plant.
The caprifig produces three types of syconia annually, they are called mamme, profichi and mammoni. An Italian terminology of popular origin which has remained unchanged during time and universally adopted.
The mamme start to develop in autumn, remain dormant in winter and mature in spring. They are located in the sub-terminal part of fruiting shoots and contain exclusively short-styled female flowers. They can be distinguished from the other types of syconia by their violaceous color and flat shape.
The profichi develop in spring in the nodes of the annual shoot growth and are positioned at the top of branches, higher with respect to the position of the mamme (Figure 4). Profichi are the only type of syconia containing, in addition to the short-styled female flowers, male flowers which are strategically situated around the ostiole (Figure 5). The female flowers reach the anthesis several weeks before the male flowers of the same syconium. This marked protogyny prevents the fertilization between pollen and ovules in the same syconium.

Figure 4: Branch of caprifig carrying young profichi, on top, and mamme further down.

Figure 5: The inside of the profico syconium containing short-styled flowers distributed over most of the surface and staminate flowers grouped around the ostiolar canal.
Contrary to the preceding the mammoni crop is not abundant and, in some cases, may be entirely absent.
All the types of syconia produced by the caprifigs have a spongy and stringy consistence and are totally unpalatable.
The female plants are the only producers of edible figs and therefore the only cultivated forms. These plants, commonly referred to as “cultivated or edible figs” or simply “figs” may produce one or two crops of a year depending on the type of cultivar and climatic conditions. The main production of syconia appears in late spring from buds of current growth and matures in late summer (Figure 6). These syconia have acquired no special name since they are present in this period of the year in all female plants both in the wild and in cultivation. Some cultivars have an extra early crop at the beginning of summer which is known as a breba crop. It appears in spring from latent buds on the growth of the previous season.

Figure 6: The interior of syconia of the edible fig at different stages of development, at the early stage (a) and at maturity (b), the inset shows long-styled flowers with achens.
The differences between the two sexual forms of trees have been noted since antiquity and differently interpreted. From Aristotle and Theophrastus to the present the female plants being the unique producers of edible figs have been generally referred to as the cultivated form. On the contrary, the male tree, the caprifig, producing spongy, inedible fruit has been considered the wild counterpart of the species. Following this view, the two types of tree have been often described as different varieties and, in the past, even as different species. This misunderstanding derives from the fact that the female plants have long been cultivated in much greater numbers than caprifigs. It is important to specify that both forms are present in the wild where they occur with the same frequency.
The reproductive cycle of F. carica and B. psenes
The reproductive cycle F. carica-B. psenes is a complex mechanism which involves caprifigs, female plants and the pollinator wasp.
The production of the three types of syconia in caprifigs encompasses an annual cycle during which also three generations of wasps take place.
The mamme, profichi and mammoni reach the donor stage in the spring, summer and autumn, respectively, at the same time as the next crop is receptive. In this way the wasps which emerge from one type of syconia enter and lay their eggs in the next.
In autumn the mamme are receptive to oviposition by the wasp. Those syconia which are not visited by the wasps soon cease the development and fall from the tree. Instead the syconia which have been entered and contain in their ovules the egg laid by the wasps remain on the tree and begin developing. The development is stopped by cold weather during the winter and starts again in spring. The syconia ripen in March and April, at this time the male wasp emerges from its enclosing drupelet, cuts the drupelet containing the female and mates with her. Soon after the female leaves the syconium through the ostiole and enter the young profichi. Also in this case the syconia drop when non inhabited by the wasp. The profichi crop matures in June and July. At this time the stamens are completely developed and release the pollen which adheres to the emerging female wasp (Figure 7). The mammoni syconia are receptive in June and July and receive the wasps emerging from the profichi crop. The inhabited mammoni ripen in October and November. At this time the wasps fall out of them and enter the developing mamme. In this way the cycle is completed.

Figure 7: Female wasps emerging from the ostiole and around the profico before flaying out.
The reduction or the complete absence of the mammoni crop could endanger the reproductive cycle of B. psenes, since the mamme are normally receptive one or two months after the bulk of the profichi syconia are at the donor stage. However it has been observed that, in some cases, the caprifig trees mature their profichi as late as the beginning of September. In this way the wasps coming out of profichi may enter the earlier receptive syconia of the mamme. The search for the receptive mamme is facilitated by the fact that these insects are able to fly long distances and therefore cover a large area. The omission of the mammoni crop from the annual cycle of wasp reproduction results in a mixture of two and three generation cycles of annual events [5] (Figure 8). The wasp generation, which establishes the connection between the caprifigs and the female plants is the one emerging from the profichi syconia. Many of the female wasps carrying pollen neglect the very few mammoni syconia available on caprifigs being attracted instead by the numerous young syconia that grow at this time on the female plants. They enter the syconia through the opening, provide for pollination and die without being able to carry out oviposition into the unsuitable long-styled female flowers. This failure is in reality the fundamental event for the completion of fertilization. The pollination of the long-styled flowers of the female plants is called caprification. It is a crucial step in the breeding system of the plant because it assures the development of the fruits, the formation of seeds and therefore the survival and expansion of the species. It is very important also for those cultivars which, like their wild counterpart, require pollination for the production of fruit. Numerous varieties instead are parthenocarpic and their fruits persist and mature without pollination. However, according to what farmers say, all cultivars may benefit from caprification which increases the quality of the fruit. To favour caprification man contrived a simple and rudimentary technique to facilitate the action of the wasps. It consists in taking twigs bearing profichi of spontaneously growing caprifigs or arranging profichi on a type of skewer and suspending them into the crown of the female tree (Figure 9). Caprification is an ancient method practiced in Greek and Roman times and probably earlier. It still survives in some Mediterranean countries, including South Italy and Sicily.
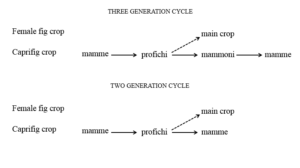
Figure 8: Events in annual cycles of three and two generations of wasps, solid arrows indicate the movement of wasps from donor to receptive syconia, broken arrows indicate the pathways of pollen transfer and fertilization.

Figure 9: Example of caprification practiced by the man.
The conservation of the knowledge of the reproductive system of F. carica may help to avoid the risk of interrupting the symbiotic relationship between fig plants and their pollinator wasps.
Sex Determination
In F. carica sex is determined by two closely linked pairs of alleles which are located in one pair of unidentified chromosomes. They are symbolized as Gg and Aa.
- G, dominant allele for flowers with short-styled pistils;
- g, recessive allele for flowers with long-styled pistils;
- A, dominant allele for the presence of male flowers;
- a, recessive allele for the inhibition of male flowers.
The caprifigs have G and A alleles and their genotypes, theorically, are GA/GA and GA/ga, however the GA/GA combination is highly improbable because homozygous caprifigs would derive from crosses between two caprifigs and these events are extremely rare for two reasons: the first is that the short-styled flowers are almost unfertile and the second is the accentuated protogyny in the profichi syconia. Considering that the female trees have the genotype ga/ga, the crosses which naturally occur are GA/ga x ga/ga, these produce progenies containing male and female plants in the ratio 1:1 [8].
Cytogenetic Background
The cytogenetic background of this large genus is not well known. Information is limited and refers to a minority of the total number. However, it contains useful indications sheding light on some of the evolutionary aspects of the genus. For example, the chromosome counts of Condit [2, 9] confirmed the diploid number 2n=26 as the prevalent one among the fig species, but also revealed a consistent number of polyploid forms. Some species, such as Ficus burkei Miq. Ficus hochstetteri A.Rich. Ficus pretoriae Burtt Davy and Ficus stuhlmannii Warb. And Ficus sonderi Miq. resulted tetraploid with 2n=52 whereas Ficus dusenii Warb., Ficus macrosyce Pittier and Ficus palmeri S.Watson exhibited both 2n=26 and 2n=52 chromosome numbers. Such data suggest that events of polyploidization occurred frequently and played a significant role in the speciation and evolution of this genus.
Regarding the fig tree, it appears that cytogenetic research has until now dedicated little attention to this species. This is strange considering the importance of this fruit tree and its wide diffusion. Chromosome data are very scarce and date back to many decades ago. This gap has been filled only recently following extensive and systematic investigations carried out on Italian populations [10, 11]. These studies provided for the first time a detailed description of the chromosome complement of F. carica realized by karyomorphological and molecular cytogenetic analyses. However, the most significant result of these studies is the identification of triploid (2n=39) and tetraploid (2n=52) cytotypes within wild populations and cultivars. This finding is of remarkable importance because adds a further evidence on the occurrence of polyploidy in the genus Ficus. In addition the high incidence of polyploid cytotypes detected in the examined populations demonstrates that polyploidization in F. carica is e rather widespread phenomenon and not a sporadic event. These results refute previous observations which excluded the presence of natural polyploids within varieties of edible fig [8]. The analysis of pollen produced by some caprifigs consented also a likely hypothesis on the origin of the polyploid plants. The presence of large size pollen grains was considered a reliable cytological evidence of the tendency of caprifigs to produce unreduced gametes, thus suggesting spontaneous sexual polyploidization as the much more possible origin of the polyploidy cytotypes rather than somatic mutations.
The cytogenetic research carried out on F. carica led to interesting considerations on the importance of sexual reproduction and the genetic constitution of the fig cultivars.
The presence of polyploid variants in the fig tree focuses attention on cross fertilization as a source of genetic variability not only in wild populations but also in the cultivated varieties. A fig cultivar is defined as a collection of individuals obtained by vegetative propagation from a wild genotype which was chosen for its agronomic features and introduced into cultivation [12]. Due to the widespread practice of vegetative propagation, the genetic intra-cultivar variability is regarded principally as the result of natural mutations. Keeping in mind that the wild populations reproduce entirely from seed, it is reasonable to suppose that cultivars have a multiple origin. Several related genotypes, including polyploid variants, may have concurred in the development of a single cultivar. Events of sexual reproduction may be expected to occur also within already established cultivars since some of the fig cultivars are totally or partially dependent on pollination for fruit production. After pollination these plants develop their fruits which contains numerous fertile seeds. Plants derived from seed, exhibiting desirable characteristics may have been selected by fig breeders and growers and propagated as new clones. Both situations could have occurred during the long history of fig cultivation and must be taken into account for explaining the existence of the polyploid cytotypes in the cultivated fig tree, such as the triploid plants discovered within the cultivars Verdino and Verdolino [10]. In conclusion it can be asserted that the discovery of polyploid cytotypes demonstrates that individuals belonging to the same cultivar may derive from genetically different lineages.
Acknowledgements
The author wishes to thank Mr Agostino Mario Di Mino for having provided images and information on the practice of caprification. This work was funded by the Fondazione Cassa di Risparmio di Perugia (Italy), Project code: 2019.0317.029.
References
- Chaudhary LB, JV Sudhakar JV, Kumar A, Bajpai O, Tiwari R et al. (2012) Synopsis of the Genus Ficus (Moraceae) in India. Taiwania 57:193-216.
- Condit IJ (1964) Cytological studies in the genus Ficus. III. Chromosome numbers in sixty-two species. Madroño 17:153-155.
- Weiblen GD (2000) Phylogenetic relationships of functionally dioecious Ficus (Moraceae) based on ribosomal DNA sequences and morphology. Am J Bot 87:1342-1357.
- Bronstein J (1992) Seed predators as mutualists: ecology and evolution of the fig pollinator interaction. In E A Bernais ed. Insect-plant interaction. CRC Press, Boca Rato, Florida USA 1-47.
- Valdeyron G, Lloid DG (1979) Sex differences and flowering phenology in the common fig, Ficus carica. Evolution 33:673-685.
- Beck NG, Lord EM (1988) Breeding System in Ficus carica, the common fig. II. Pollination events. Am J Bot 75:1913-1922.
- Fuller DQ, Stevens CJ (2019) Between domestication and civilization: the role of agriculture and arboriculture in the emergence of the first urbal societies. Vegetation History and Archaeobotany 28:263-282.
- Storey, WB (1975) Figs. In J Janick and JN Moore ed. Advances in Fruit Breeding. Purdue University Press, West Lafayette, Indiana 568-589.
- Condit IJ (1933) Chromosome number and morphology in thirty-one species. Cytological and morphological studies in the genus Ficu University of California publications in botany. University of California Press.
- Falistocco E (2009) Presence of triploid cytotypes in the common fig (Ficus carica ). Genome 52:919-25.
- Falistocco E (2016) Recurrent events of polyploidy in Ficus carica (Moraceae). Int J Plant Sci 177:319-325.
- Khadari B, Grout C, Santoni S, Kjellberg F (2005) Contrasted genetic diversity and differentiation among Mediterranean populations of Ficus carica : A study using mtDNA RFLP. Genet Resour Crop Ev 52:97-109.