Publication Information
ISSN: 2641-6859
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Bone fractures and cruciate ligament ruptures in competitive sports: are vitamin d receptor and collagen Ia1 polymorphisms causative and what impact do they show on the micronutrient status of elite athletes?
Klaus Erpenbach1*, Ann Sophie Erpenbach1, Wolfgang Mayer2, Robert Rein3, Stefan Mücke1
1Institut für medizinische Leistungsoptimierung und Trainingssteuerung, Marienstraße 1, 50374 Erftstadt, Germany
2Lab4more GmbH Bavariahaus, Augustenstraße 10, 80333 München, Germany
3Deutsche Sporthochschule Köln, Institut für Trainingswissenschaften und Sportinformatik, Am Sportpark Müngersdorf 6, 50933 Köln, Germany
Received Date: July 14, 2023; Accepted Date: July 19, 2023; Published Date: July 30, 2023;
*Corresponding author: Klaus Erpenbach, Institut für medizinische Leistungsoptimierung und Trainingssteuerung, Marienstraße 1, 50374 Erftstadt, Germany. Email: info@im-lot.org
Citation: Erpenbach K, Erpenbach A S, Mayer W, Rein R, Mücke S (2023) Bone fractures and cruciate ligament ruptures in competitive sports: are vitamin D receptor and collagen IA1 polymorphisms causative and what impact do they show on the micronutrient status of elite athletes? Adv Ortho and Sports Med: AOASM-192.
DOI: 10.37722/AOASM.2023301
Abstract
Bone fractures as well as anterior cruciate ligament ruptures are serious events in elite sports that dramatically interrupt the continuation of an athlete´s career. Are vitamin D receptor (VDR) and collagenIA1 (Col-IA1) polymorphisms responsible for these severe events in elite athletes and what impact do they show on the micronutrient status in elite athletes?
Methods
In 22 elite athletes [male: 15 - female: 7 / soccer: 12 - field hockey: 6 - Olympic: 4], the VDR (BsmI) or Col-IA1 polymorphisms (rs1800012), 25-OH vitaminD3, parathyroid hormone, vitamin-K2-MK7, calcium and magnesium in serum and calcium and magnesium in whole blood were determined and correlated with respect to spontaneous bone fracture and anterior cruciate ligament rupture events.
Results
A VDR polymorphism was detected in 15 athletes, a COL-IA1 polymorphism in 10 athletes and in 3 athletes both polymorphisms were detected. Competitive athletes with VDR or COL-IA1 polymorphisms frequently suffered bone fractures or severe muscle-tendon ruptures, respectively (fracture: 2.86 ± 1.61 / muscle-tendon ruptures: 3.14 ± 2.05), whereas athletes with VDR polymorphism significantly more often suffered from fractures (U=0.73 [0.5-1.0], p=0.046) and competitive athletes with COL-IA1 polymorphism experienced cruciate ligament (VDK) ruptures significantly more often (OD=0.056 [0.0051-0.6059], p=0.024). Vitamin D deficiency (25(OH)vitaminD3 = 15.14 ± 5.85 ng/ml) was detected in all competitive athletes suffering from these polymorphisms, severe deficiency in 82% [vitamin D deficiency < 20ng/ml - N = 18/22 (82%), insufficient vitamin D supply (20-30 ng/ml) - N = 4/22 (18%)]. 74% (N=14/19) of the athletes showed hyperphosphatemia, 9.5% (N=2/21) secondary hyperpara-thyroidism, 55% (N=12/22) hypocalcemia, and 36% (N=8/22) and 64% (N=14/22) intracellular calcium and magnesium deficiency, respectively. The blood vitamin K2-MK7 level was at the lower limit in all affected individuals (VitK2-MK7 norm: 2-6µg/l: 2.65 ± 0.42 µg/l) and significantly correlated negatively with the serum magnesium level (r = -0.537, p=0.048). Parathyroid hormone level correlated significantly negatively with intracellular calcium level (r= -0.608, p=0.0034). Strikingly, an atlas blockade was detectable on the same side of the VDK rupture in all affected individuals with VDK ruptures.
Conclusion
Competitive athletes with a VDR polymorphism are more likely to suffer a bone fracture while athletes with a COL-IA1 polymorphism are significantly more likely to experience a severe tendon rupture (VDK, Achilles tendon). Severe vitamin D3 deficiency with concomitant borderline vitamin K2-MK7 supply and the resulting intracellular calcium and magnesium deficiency seem to be considered as a genetically dysregulated cause.
Randomized therapy studies with high-dose vitamin D3-/K2-MK7-substitution and the goal to permanently reach a parathyroid hormone value at the lower limit of 16 pg/ml have to show whether fatigue fractures or severe tendon ruptures (VDK, Achilles tendon) can be effectively prevented in athletes with VDR- / COL-IA1-polymoprhisms.
Introduction
Nontraumatic spontaneous hip and lumbar vertebral fractures or bone bruises at other skeletal sites, as well as severe nontraumatic tendon-muscle tears (Achilles tendon or anterior cruciate ligament ruptures), make a vitamin D-dependent gene defect (VDR or Col-IA1 polymorphisms) likely in elite athletes despite optimal serum vitamin D levels (1). Skeletal muscle is closely related to bone tissue (2-4), and recent evidence suggests that vitamin D plays a direct role in both muscle and bone homeostasis (1,5,6). Numerous clinical studies demonstrated the relationship between serum vitamin D levels [25(OH)-vitaminD3] and muscle mass or strength (8,9). In vitro experiments showed that vitamin D is able to induce calcium influx, intracellular signal transduction, and gene expression in muscle stem cells (9,10). Thus, vitamin D exhibits a wide range of biological functions: Regulation of calcium and phosphate homeostasis in muscle and skeletal metabolism and vascular function (11).
One of the most studied genes for bone-muscle metabolism is the vitamin D receptor (VDR) because of its important regulatory role in calcium homeostasis and skeletal muscle function (12). Identification of the VDR on muscle cells provided further support for a direct effect of vitamin D on muscle tissue (13). The VDR is a nuclear receptor of 50 kDa that belongs to class 2 of the steroid receptor family. The VDR gene is located on chromosome 12q.13.1 and contains nine exons (14-18). The VDR gene has several polymorphic sites that have been studied in relation to bone and skeletal muscle traits such as FokI, BsmI, TaqI, and Cdx2. The Bsml polymorphism of the VDR gene is most commonly associated with bone density (and osteoporosis). In the VDR gene cluster, this polymorphism is detected with two alleles: BsmI (b and B) [19].
The collagen IA1 gene is another very interesting candidate gene with respect to muscle tendon/osteoporosis risk. Type I collagen is a triple-stranded fibrillar protein and the major collagen of tendon and bone and is also found in the epimysium and perimysium of skeletal muscle (17). It consists of two a1 polypeptide chains (encoded by the COL-IA1 gene) and one a2 chain (encoded by the COL-IA2 gene). While fast-twitch muscle (fiber type 2) has more type III collagen, slow-twitch muscle fibers (fiber type 1) contain more type I collagen. Both types serve as a supporting structure in muscle tissue, where they attach myocytes and muscle bundles to each other (17). The collagen fiber network of skeletal muscle has been shown to contribute significantly to the integrity and tensile strength of muscle tendons and bone (18,20). A polymorphic binding site of the Sp1 transcription factor exists in the gene encoding the a1 chain of type I collagen, and the s (rather than S) allele of this polymorphism has been associated with lower grip and biceps strength on the dominant side (20).
The aim of the present cohort study is to investigate an association between the VDR (Bsml)/Col-IA1 gene polymorphisms, muscle and bone metabolism, micronutrient parameters, and injury severity in competitive athletes.
Methodology
Participants
22 competitive athletes from different sports were included in the cohort study. All athletes gave written informed consent for data collection. Using a questionnaire, the frequency of medically confirmed muscle-tendon ruptures (muscle bundle tears, syndesmosis tears, VDK ruptures, Achilles tendon ruptures) and medically confirmed fractures during the entire sports career were collected. Inclusion criterion: more than 2 fractures and/or more than 2 muscle-tendon ruptures in the previous sports career. In all 22 athletes, VDR or Col-IA1 polymorphism, 25-OH vitaminD3, parathyroid hormone, vitamin-K2-MK7, phosphate, calcium and magnesium in serum, and calcium and magnesium in whole blood were determined and correlated in relation to the events of spontaneous / bone fracture and anterior cruciate ligament rupture.
VDR polymorphism - rs1544410 (EDTA)
Extraction of genomic DNA from peripheral blood leukocytes, method: nucleic acid amplification (NNA), genotype detection by RFLP.
Col-IA1 polymorphism - rs1800012 (EDTA)
Extraction of genomic DNA from peripheral blood leukocytes, method: nucleic acid amplification (NNA), genotype detection by specific fluorescent probes.
Vitamin D 25-OH (serum)
The quantitative determination of total 25-OH vitamin D in serum was performed according to the manufacturer's instructions using a direct competitive chemiluminescence immunoassay (CLIA) on the Liasion-XL automated laboratory instrument from Diasorin, Dietzenbach, Germany (order no.310600).
Intact parathormone (plasma)
Intact parathormone in EDTA plasma was determined with an Alinity I automatic laboratory instrument from Abbott, Germany, according to the manufacturer's instructions (Order No. 08P3124).
Vitamin K2 - MK7 (serum)
Measurements were performed using a Shimadzu LCMS-8060 triple quadrupole mass spectrometer coupled to a Nexera X2 UHPLC system and equipped with a Shim-pack GIST C18 column (Shimadzu, Kyoto, Japan). 1.25 ml of serum was mixed with 625 μl of ZnSO4 solution and shaken for 10 minutes. After addition of 5 ml acetonitrile/methanol (50/50 v/v), the mixture was shaken for 4 min. Finally, the mixture was centrifuged at 4500 rpm and 20 °C for 10 min. The supernatant was injected directly.
Whole blood magnesium and whole blood calcium (Na-Heparin blood) = vMg - vCa
Magnesium and calcium in whole blood were determined by atomic absorption spectrometry (AAS) after acid digestion.
Magnesium, phosphate and calcium (serum) = sMg - sPhosphate - sCa
Calcium, phosphate and magnesium in serum were determined using an Abbott Alinity C laboratory analyzer according to the manufacturer's instructions (Ca/Mg ref. no. 8P1920, phosphate ref. no. 8P4020).
Statistical analysis
Statistical analysis of the data was performed using Pearson's correlation analysis for correlation comparisons. Effect sizes were divided into small ρ = 0.1, medium ρ = 0.3, and large ρ = 0.5 according to Ellis (40). Frequency comparisons were examined via cross-tabulations using a Pearson χ2 test, whereas mean differences were hedged using a Wilcox-Mann Whitney U test. The significance level for all tests was set at α = 0.05 (p≤0.05 significant, p≤0.01 highly significant). All analyses were performed using R software (version 4.1.2).
Results
22 competitive athletes (age: 23.68 ± 5.86 - male: 15 - female: 7) from different sports (soccer: 12 - field hockey: 6 - track and field: 4) fulfilled inclusion criteria: Bone fractures 2.86 ± 1.61 / Muscle-tendon ruptures: 3.14 ± 2.05. A VDR polymorphism was detected in 15 athletes, a COL-IA1 polymorphism in 10 athletes and in 3 athletes both polymorphisms were detected (Table 1). Competitive athletes with VDR polymorphism suffered from fractures statistically significantly more frequently (U=0.73 [0.5-1.0], p=0.046) [Figure 1] and with COL-IA1 polymorphism significantly from cruciate ligament ruptures (VDK) (OddsRatio=0.056 (0.0051-0.6059), p=0.024) [Table 4] were found. All three athletes with both polymorphisms suffered from severe atraumatic fracture (LWK-4: N=2, femoral neck fracture: N=1) [Table 1] and demonstrated confirmed vitaminD3 deficiency (norm 30-80ng/ml: 15.43 ± 2.97 ng/ml) and cellular magnesium deficiency (norm 1.29-1.69 mmol/l: 1.26 ± 0.14 mmol/l) in whole blood. Vitamin D deficiency (25(OH)vitaminD3 = 15.14 ± 5.85 ng/ml) was detected in all athletes with VDR/ Col-IA1 polymorphism, severe deficiency in 82% [vitamin D deficiency < 20ng/ml - N = 18/22 (82%)] and insufficient vitamin D level [(20-30 ng/ml) in 18% - N = 4/22 (18%)]. 86% (N=19/22) of the athletes showed hyperphosphatemia, 23% (N=5/22) secondary hyperpara-thyroidism, 41% (N=9/22) hypocalcemia and 27% (N=6/22) and 64% (N=14/22) intracellular calcium and magnesium deficiency, respectively (Table 2). The blood vitamin K2-MK7 level was at the lower limit [VitK2-MK7 (norm: 2-6µg/l): 2.65 ± 0.42 µg/l] in all athletes and correlated significantly negatively with the serum magnesium level (r = -0.537, p=0.048) [Table 3a]. The intact parathyroid hormone level negatively correlated significantly with the intracellular calcium level (r= -0.608, p=0.0034) [Table 3b]. Interestingly, in all patients suffering from a VDK rupture an atlas blockage was detected to the same side of the VDK rupture (Table 4).
| Total (N = 22) | VDR | Col-IA1 | beide |
| WT | 7
(b/b) |
12
(G/G = SS) |
-- |
| Heterozygous | 9
(B/b) |
10
(G/T = Ss) |
2 *
(B/b + G/T) |
| Homozygous | 6
(B/B) |
--
(T/T = ss) |
1 #
(B/B + G/T) |
| VDR = vitamin D receptor - COL-IA1 = collagen-1A1 - WT = wild type
* = both athletes suffered an LWK-4 fracture without trauma # = athlete suffered a femoral neck fracture without trauma |
|||
| Gesamt
(N=22) |
VitaminD3 > 30 ng/ml
(N=0) |
VitaminD3
20 -30 ng/ml
(N=4) |
VitaminD3
< 20 ng/ml
(N=18) |
|
| VitaminD3
(30 - 80 ng/ml) |
15,14
± 5,85 |
- | 24,83
± 2,86 |
13,31
± 4,03 |
| vCalcium
(1,14 - 1,68 mmol/l) |
1,27
± 0,15 |
- | 1,32
± 0,13 |
1,27
± 0,15 |
| sCalcium
(2,29 - 2,68 mmol/l) |
2,34
± 0,13 |
- | 2,34
± 0,09 |
2,34
± 0,15 |
| vMagnesium
(1,29 - 1,69 mmol/l) |
1,29
± 0,09 |
- | 1,25
± 0,01 |
1,30
± 0,10 |
| sMagnesium
(0,72 - 0,91 mmol/l) |
0,84
± 0,07 |
- | 0,92
± 0,02 |
0,83
± 0,07 |
| sPhosphate
(2,3 - 4,7 mg/dl) |
5,20
± 0,84 |
-
|
5,63
± 0,95 |
4,99
± 0,77 |
| PTH intact
(15 - 68,3 pg/ml) |
59,01
± 6,01 |
- | 57,68
± 7,68 |
59,28
± 5,93 |
| VitaminK2-MK7
(2 - 6 µg/l) |
2,65
± 0,42 |
- | 2,61
± 0,27 |
2,70
± 0,42 |
| M-S-R | 3,14
± 2,05 |
- | 2,95
± 1,80 |
3,44
± 1,84 |
| Fractur | 2,86
± 1,61 |
- | 2,63
± 0,47 |
3,06
± 1,43 |
| Vitamin D3
(30 - 80 ng/ml) |
vMagnesium
(1,29 - 1,69 mmol/l) |
|
| Gen-Study
N = 22 |
15,14 *
± 5,85 |
1,29 #
± 0,09 |
| Athletes with
Deficiency |
82%
(18/22) |
64%
(14/22) |
| OM-Study (1)
N = 111/70 |
31,10 *
± 10,61 |
1,40 #
± 0,15 |
| Athletes with
Deficiency |
13,5%
(15/111)
* p<0,00001 |
27,1%
(19/70)
# p<0,001 |
| VitaminD3 | VitaminK2-MK7 | PTH | sCalcium | sMagnesium | Phosphate | |
| VitaminD3 | 1 | -0,314 | -0,050 | -0,108 | 0,422 | 0,413 |
| Vitamin K2-MK7 | -0,314 | 1 | -0,320 | -0,114 | -0,537 * | -0,279 |
| PTH | -0,050 | -0,320 | 1 | -0,117 | 0,095 | 0,145 |
| sCalcium | -0,108 | -0,114 | -0,117 | 1 | 0,489 # | -0,296 |
| sMagnesium | 0,422 | -0,537 * | 0,095 | 0,489 # | 1 | 0,262 |
| Phopshate | 0,413 | -0,279 | 0,145 | -0,296 | 0,262 | 1 |
| PTH = parathyroid hormone, s = serum, * p=0.048, # p=0.025 - all other correlations not significant | ||||||
| VitaminD3 | VitaminK2-MK7 | PTH | vCalcium | vMagnesium | Phosphate | |
| VitaminD3 | 1 | -0,314 | -0,050 | 0,202 | -0,111 | 0,413 |
| VitaminK2-MK7 | -0,314 | 1 | -0,320 | 0,033 | 0,219 | -0,279 |
| PTH | -0,050 | -0,320 | 1 | -0,608 ** | -0,095 | 0,145 |
| vCalcium | 0,202 | 0,033 | -0,608 ** | 1 | -0,154 | -0,109 |
| vMagnesium | -0,111 | 0,219 | -0,095 | -0,154 | 1 | -0,446 |
| Phopshate | 0,413 | -0,279 | 0,145 | -0,109 | -0,446 | 1 |
| ** p=0,0034 wouldn´t it be better to put it directly to the same side of table5 | ||||||
| Muscle-tendon tears | Fractures |
| 2,86 ± 1,61 | 3,14 ± 2,05 |
| prominent:
anterior cruciate ligament tear: 11 (45,6% - 10/22) |
prominent:
Stress fractures (100% - 22/22) |
| all 10 athletes have
ATLAS pathology on the same side |
all athletes suffer the
fractures already in youth |
| Relationship with
Col-IA1 polymorphism OD = 0,056 [0,0051-0,6059] p=0,024 |
Difference due to
VDR polymorphism U = 0,73 [0,5-1,0] p=0,046 |
| OD = Odds Ratio (Lo-95% - Hi-95%) - U = Difference | |
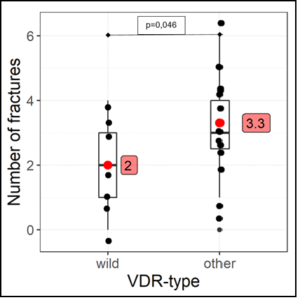
Figure 1: Correlation VDR polymorphism and bone fracture.
wild = wild type - other = heterozygous + homozygous
Discussion
The present study shows that detection of the B allele (VDR polymorphism BsmI, rs1544410) in competitive athletes correlates with a significantly increased risk of atraumatic stress fractures regardless to the type of sport and load confirming the results of Chatzipapas et al. (21), who found a 5.3-fold increased risk of stress fractures in competitive athletes with a VDR polymorphism BsmI [rs1544410, p=0.050] (21) and Varley et al., who demonstrated a 1.4-fold increased risk for the VDR polymorphism FokI [rs10735810, p=0.02] (22).
47% of our study participants with VDR polymorphism (Bsm-1) had serum hypocalcemia and 13% had cellular calcium deficiency and 73% had cellular magnesium deficiency. Parathyroid hormone was at the upper normal limit (PTH = 59.1 ± 5.3 pg/ml - norm to 68.5 pg/ml) and vitamin K2 (MK7) was at the lower normal limit (VitK2-MK7 = 2.7 ± 0.4 µg/l) in all athletes with VDR polymorphism. A possible relationship between VDR genotype (Bsml) and calcium homeostasis via calcium intake was investigated in a longitudinal study (23). Ferrari et al. (23) demonstrated genotype-related differences in the change of bone density over time: the "Bb" heterozygotes responded to calcium intake with an increase in bone density, the "bb" wild types maintained their bone density while the "BB" homozygotes actually lost bone density over time independent of calcium intake. These various calcium absorption and response studies suggest that a potential VDR genotype effect would be largely masked at high effective calcium intakes; suggesting that VDR genotype may be considered as an indicator to identify individuals in whom calcium supplementation might be most effective. Therefore, calcium supplementation would be most effective (and justifiable) in "BB" and possibly "Bb" genotype subjects with little or no value in "bb" genotype subjects. Despite some conflicting data that may relate to ethnic and environmental heterogeneity, it seems clear that polymorphisms of the VDR gene are associated with differences in bone density, bone size, intestinal calcium absorption, and bone turnover. These data provide a basis for understanding the studies of differential bone density responses of the different VDR genotype subjects not only to long-term calcium supplementation but also to vitamin D intake to prevent the risk of hyperparathyroidism and thus osseous calcium deprivation. In our study, parathyroid hormone was at the upper normal limit in all athletes with VDR polymorphism (PTH = 59.1 ± 5.3 pg/ml - norm up to 68.5 pg/ml), all athletes were vitamin D deficient (25(OH)vitaminD3 = 15.14 ± 5.85), and vitamin K2 (MK7) levels were at the lower normal limit (VitK2-MK7 = 2.7 ± 0.4 µg/l). Indeed, VDR polymorphisms have been reported to have effects on parathyroid regulation (24-26). This suggests differences in PTH regulation as a possible pathway for subtle differences in vitamin D regulation of bone and calcium homeostasis.
Several Japanese studies have reported differences in bone density response to 1a-hydroxylated vitamin D metabolites or analogs (27-29). The "bb" genotype, which is the most common in Japanese cohorts (75% of subjects), responded better to vitamin D compounds than the "Bb" genotype, which either did not respond as well or actually worsened with treatment. Given that the "Bb" genotype is the most common (50%) in Caucasian populations, VDR genotype differences may contribute to the variable and generally less impressive responses to vitamin D metabolites and analogues in the Caucasus as opposed to Japanese studies. A Dutch study of simple vitamin D supplementation for hip fracture prevention found that the bone density response to supplementation varied according to VDR genotype (30). In this relatively small study, bone density increased significantly in "BB" and "Bb" genotype subjects (0.4%) but not in "bb" genotype subjects (20.3%). These two sets of studies, albeit in different ethnical groups, suggest that "BB" and "Bb" subjects may respond positively to simple 25-hydroxylated vitamin D but not to 1a-hydroxylated vitamin D. In contrast, "bb" subjects can respond positively to 1a-hydroxylated vitamin D but not to simple vitamin D. These data suggest that some of the differences observed in VDR alleles and bone density end points may be related to their environment. For example, differences between "BB" and "bb" genotypes might be expected to be least evident in a population with relatively high calcium or relatively high vitamin D intakes and to be amplified in those with low calcium and thus habitually relatively high 1,25-dihydroxyvitamin D levels. However, prospective randomized trials have yet to demonstrate whether VDR genotype-related differences determine bone density responses. Data on the influence of vitamin K2-MK7 on bone density or a possible influence on fracture rates in competitive athletes with VDR polymorphism are lacking at present and more research is warranted.
The collagen IA1 gene is another very important gene in the research for candidate genes associated with fracture and osteoporosis risk. In the initial studies, polymorphism in intron 1 of the collagen Ia1 gene was shown to be associated with differences in bone density (31,32). Collagen type I fibrils are a major component of the bone matrix and form strong parallel fiber bundles in tendons and ligaments. The two major genes regulating collagen production are the collagen-Iα1 (COLIA1) and collagen-Iα2 (COLIA2) genes. The COLIA1 and COLIA2 polypeptides encode collagen Iα1 and collagen Iα2, respectively, which associate in a 2:1 ratio to collagen type I (33). The COL1A1 gene (located on chromosome 17q21.33) contains a polymorphism in the region of intron 1 (rs1800012), a predicted binding site for the transcription factor Sp1 (34). Some studies have shown that functional polymorphism of the Sp1 binding site is associated with various complex diseases, including osteoporotic fractures (35), osteoarthritis (36), myocardial infarction (36), intervertebral disc disease (37), and muscle-tendon ruptures (38,39). In our gene study, only the sS genotype but not the ss genotype was detected in Col-IA1 polymorphism-affected competitive athletes confirming previous studies in muscle-tendon ruptures (frequency of ss genotype: 0.4-4.6% of study participants) [38,39]. Detection of the sS genotype (Col-IA1 polymorphism, rs1800012) in competitive athletes regardless to the type of sport or the load correlated with a significantly increased risk of anterior cruciate ligament ruptures in our study (OD = 0.056 [0.0051-0.6059], p=0.024). International comparative studies of this sS genotype are not currently available. Severe 25(OH)-vitamin D3 deficiency with concomitant borderline vitamin K2 MK7 supply and resulting intracellular calcium and magnesium deficiency seem to be possible genetic dysregulated causes. Randomized therapy studies with high-dose vitamin D3-/K2-MK7-substitution and the goal to permanently reach a parathyroid hormone value at the lower limit at 16 pg/ml have to show whether fatigue stress fractures or severe tendon ruptures (VDK, Achilles tendon) can be effectively prevented in athletes with VDR- / COL-IA1-polymorphisms.
References
- Erpenbach K, Erpenbach MC, Mayer W, Rein R, Mücke S (2021) 25-OH-VitaminD3 Level in Elite Sports: Is high-Dose Vitamin D Supplementation Effective In Preventing Muscle Damage and Infections? Adv Orthopedics Sports Med 137:1-7.
- Tarantino U, Piccirilli E, Fantini M (2015) Sarcopenia and fragility fractures: molecular and clinical evidence of the bone-muscle interaction. J Bone Joint Surg Am, 97:429-37.
- Brotto M, Johnson ML (2014) Endocrine crosstalk between muscle and bone Curr Osteoporos Rep, 12:135-41.
- Sartori R, Sandri M (2015) BMPs and the muscle-bone connection. Bone, 80:37-42.
- Gunton JE, Girgis CM, Baldock PA (2015) Bone muscle interactions and vitamin D. Bone, 80: 89-94.
- Sanders KM, Scott D, Ebeling PR (2014) Vitamin D deficiency and its role in muscle-bone interactions in the elderly. Curr Osteoporos Rep, 12:74-81.
- Tanner SB, Harwell SA (2015) More than healthy bones: a review of vitamin D in muscle health. Ther Adv Musculoskelet Dis, 7:152-9.
- Resmini G, Tarantino U, Iolascon G (2013) Vitamin D: role and opportunity to prescribe. Aging Clin Exp Res, 25 Suppl 1: S125-7.
- Boland RL (2011) VDR activation of intracellular signaling pathways in skeletal muscle. Mol Cell Endocrinol, 347:11-16.
- Abboud M, Puglisi DA, Davies BN (2013). Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology, 154:3022-3030.
- Zhao B, Zhang W, Du S, Zhou Z (2016) Vitamin D receptor BsmI polymorphism and osteoporosis risk in post-menopausal women. Archives of medical science. AMS 12:25-30.
- Tan LJ, Liu SL, Lei SF (2012). Molecular genetic studies of gene identification for sarcopenia. Hum Genet, 131:1-31.
- Ceglia L (2008). Vitamin D and skeletal muscle tissue and function. Mol Aspects Me., 29:407-414.
- Monticielo OA, Teixeira TDM, Chies JAB (2012). Vitamin D and polymorphisms of VDR gene in patients with systemic lupus erythematosus. Clin Rheumatol, 31:1411-1421.
- Smolders J, Peelen E, Thewissen M (2009). The relevance of vitamin D receptor gene polymorphisms for vitamin D research in multiple sclerosis. Autoimmun Rev Elsevier BV, 8:621-626.
- Whitfield GK, Remus LS, Jurutka PW (2001). Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol, 177:145-159.
- Jarvinen TA, Jozsa L, Kannus P (2002) Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles: an immunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil 245-54.
- Takala TE (2000) Biochemical composition of muscle extracellular matrix: the effect of loading. Scand J Med Sci Sports 10:321-5.
- Cooper GS, Umbach DM (1996) Are vitamin D receptor polymorphisms associated with bone mineral density? A metaanalysis. J Bone Min Research 11:1841-1849.
- Van Pottelbergh I GS, Nuytinck L, De Paepe A (2001) Association of the type I collagen alpha1 Sp1 polymorphism, bone density and upper limb muscle strength in community-dwelling elderly men. Osteoporos Int 12:895-901.
- Chatzipapas C, Boikos C, Drosos GI, Kazakos K, Tripsianis G, et al. (2009) Polymorphisms of the Vitamin D Receptor Gene and Stress Fractures. Horm Metab Res 41:635-640.
- Varley I, Hughes DC, Greeves JP, Stellingwerff T, Ranson C, et al. (2017) The association of novel polymorphisms with stress fracture injury in Elite Athletes: further insights from the SFEA cohort. J Sci Med Sport.
- Ferrari S, Rizzoli R, Chevalley T, Slosman D, Eisman JA, et al. (1995) Vitamin-D-receptor-gene polymorphisms and change in lumbar-spine bone mineral density. Lancet 345:423-424.
- Carling T, Kindmark A, Hellman P, Lundgren E, Ljunghall S, et al. (1995) Vitamin D receptor genotypes in primary hyperparathyroidism. Nat Med 1:1309-1311.
- Carling T, Kindmark A, Hellman P, Holmberg L, Akerstrom G, et al. (1997) Vitamin D receptor alleles b, a, and T: risk factors for sporadic primary hyperparathyroidism (HPT) but not HPT of uremia or MEN 1. Biochem Biophys Res Commun 231:329-332.
- Carling T, Ridefelt P, Hellman P, Rastad J, Akerstrom G (1997) Vitamin D receptor polymorphisms correlate to parathyroid cell function in primary hyperparathyroidism. J Clin Endocrinol Metab 82:1772-1775.
- Tokita A, Kelly PJ, Nguyen TV, Qi JC, Morrison NA, et al. (1994) Genetic influences on type I collagen synthesis and degradation: further evidence for genetic regulation of bone turnover. J Clin Endocrinol Metab 78:1461-1466.
- Yamagata Z, Miyamura T, Iijima A, Asaka A, Sasaki M, et al. (1994) Vitamin D receptor gene polymorphism and bone mineral density in healthy Japanese women [letter]. Lancet 344:1027.
- Shiraki M, Shiraki Y, Aoki C, THosoi T, Inoue S, et al. (1997) Association of bone mineral density with apolipoprotein E phenotype. J Bone Miner Res 12:1438.
- Graafmans WC, Lips P, Ooms ME, van Leeuwen JP, Pols HA, et al. (1997) The effect of vitamin D supplementation on the bone mineral density of the femoral neck is associated with vitamin D receptor genotype. J Bone Miner Res 12:1241-1245.
- Houston LA, Grant SF, Reid DM, Ralston SH (1996) Vitamin D receptor polymorphism, bone mineral density, and osteoporotic vertebral fracture: studies in a UK population. Bone 18:249-252.
- Grant SF, Reid DM, Blake G, Herd R, Fogelman I, et al. (1996) Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I a 1 gene. Nat Genet 14:203-205.
- Hoffmann A, Gross G (2007) Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop 31:791-797.
- Mann V, Hobson EE, Li B, Stewart TL, Grant SF (2001) A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest 107:899-907.
- Lian K, Zmuda JM, Nevitt MC, Lui L, Hochberg MC (2005) Type I collagen alpha1 Sp1 transcription factor binding site polymorphism is associated with reduced risk of hip osteoarthritis defined by severe joint space narrowing in elderly women. Arthritis Rheum 52:1431-1436.
- Speer G SP, Kósa JP, Tabák AG, Folhoffer A, Fuszek P, et al. (2006) Myocardial infarction is associated with Spl binding site polymorphism of collagen type 1A1 gene. Acta Cardiol 61: 321-325.
- Tilkeridis C BT, Garantziotis S, Stratakis CA (2005) Association of a COL1A1 polymorphism with lumbar disc disease in young military recruits. J Med Genet 42: e44.
- Khoschnau S, Melhus H, Jacobson A, Rahme H, Bengtsson H (2008) Type I collagen alpha1 Sp1 polymorphism and the risk of cruciate ligament ruptures or shoulder dislocations. Am J Sports Med 36:2432-2436.
- Posthumus M, September AV, Keegan M, O’Cuinneagain D, Van der Merwe W (2009) Genetic risk factors for anterior cruciate ligament ruptures: COL1A1 gene variant. Br J Sports Med 43:352-356.
- Ellis PD (2010) The essential guide to effect sizes: Statistical power, meta-analysis, and the interpretation of research results. Cambridge university press.