Publication Information
ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Soybean Milk Production: An Enzymatic Approach
Jyoti Rani1*, Vinay Gautam2
1Department of Nutrition and Dietetics, UIAHS, Chandigarh University, Gharuan, Mohali- 140413 (Punjab) INDIA
2Chitkara University Institute of Engineering and Technology, Chitkara University, Punjab, 140401, INDIA
Received Date: August 26, 2022; Accepted Date: September 01, 2022; Published Date: September 06, 2022;
*Corresponding author: Jyoti Rani, Department of Nutrition and Dietetics, UIAHS, Chandigarh University, Gharuan, Mohali-140413 (Punjab) INDIA. Email: jyoti.e8920@cumail.in
Citation: Rani J, Gautam V (2022) Soybean milk production: An enzymatic approach. Adv in Nutri and Food Sci: ANAFS-239.
DOI: 10.37722/ANAFS.2022402
Abstract
Commercialized method for soymilk extraction vs Enzyme assisted soymilk (Hi-Media cellulase enzyme from Trichoderma viride and pectinase enzyme from Rhizopus spp. were used at varied concentrations to temperature and time combinations) methods were studied. Standardizing the time period for pre-soaking of soybean grains followed by boiling of those pre-soaked grains in the above mentioned two methods. Later, observing the effects of pre-soaking time on extracted soymilk quantity and quality. The results have shown exactly double milk yield with enzyme assisted soymilk extraction method (cellulase enzyme was found substantial effective in milk extraction to pectinase enzyme) to the commercial soymilk extraction method. In terms of the soymilk quantity and quality, the enzyme assisted method of soymilk extraction can be considered as an alternative over commercial method of soymilk production for the soymilk production with rich nutrients. Thus, enzyme assisted extraction method can be considered as a novel processing method for soymilk extraction with enriched nutritional quality, double milk yield and less of byproduct (OKARA). As a whole de-hulled soybean can be completely converted to milk.
Keywords: (cellulase & pectinase) and commercialized soymilk extraction method; enzyme assisted soymilk extraction; soybean; soymilk
Introduction
With the rise in infection rate after COVID 19 globally the need and attention towards functional components or functional foods had gain wide publicity online and offline. Milk has been known as a complete food since ages. But due to instability of gut microbes the lactose intolerance is being reported after consuming lacteal milk that leads to development and evolution of certain other milk types obtained from rice, almond, wheat, coconut, soybean and so on. But among these soybean milk has got wide attention and acceptance because of its cheaper, low in fat and nutrient rich composition that has proven health benefits on brain, heart, hormonal etc. India ranked fifth in production with 11.22 million tons in 2019-20 as per the Soybean Outlook report, 2021. The Soybean (Glycine max) is composed of 45 percent protein and 20 percent fat that’s higher in comparison to 13 percent and 20 percent of protein in egg and meat, respectively. Soya products are more economic and cheaper than other products containing high-quality protein such as meat, fish, milk and other protein-rich legumes. In fact, the veganism trend is escalating after pandemic that highlighted the growth scale of market over 2021-2027. So, now more than 14% of global population could be going to be vegan. Soy milk is consumed in various forms like raw flavored, non-flavored soymilk and also used to produce soy cheese/tofu. The basic methods for producing soymilk have been commercialized and used widely that includes grinding hot soybean grains followed by straining and siphoning off the soybean flavor as compared to the enzyme assisted soymilk production.
There are some limitations which are associated with the soymilk like it has pronounced beany flavour most commonly not accepted by the consumers because of lipoxygenase enzyme that gets active when the cotyledons broke down and enzymes come in contact with water, oxygen and lipids; these all-in combination gives the beany flavour especially in manual method of soymilk extraction. But methods like commercial method where excessive heating enhanced sensory properties with reduction in nutritional qualities and also left with good amount of by-product called as Okara (Iwuoha et al., 1997). Okara is a soy pulp consisting of insoluble parts of soybean containing 25-28% of protein, carbohydrates (3.8-5.3%) as well as oil and fat (9.3-10.9%) that remains after filtration of soymilk and that maybe used sometimes for Tofu. Other anti-nutrients also limit its consumption such as lectins, phytic acid, indigestible oligosaccharides and the trypsin inhibitors (TI) (Carthy et al., 1987). So, by modifying the processing methods it could be an effective way to produce soymilk or to improve the health promoting bioactive compounds with restricted undesired compounds from raw soybeans to support the soymilk production (Yuan et al., 2008).
Enzyme assisted method can be used for soybean softening and increasing the porosity that may increase the milk recovery, minimize the residue and increase the overall efficiency of soymilk extraction (Rosenthal et al., 2009). It is an environment friendly method that lowered the protein damage during extraction. In this method enzymes hydrolyze the structural polysaccharides which form the cell walls of seed. Other conditions also favor the milk extraction like temperature, pH, particle size, agitation rate that only aimed to increase the extraction yield. So, different enzymes were used to enhance the product yield. Mainly the reasons to choose the enzyme assisted soymilk extraction method were to reduce the input energy in production of milk. Comparing the yield from manual soymilk extraction method to enzyme assisted soymilk extraction method was one of the major objectives with improvement in nutritional quality without loss of Iso-flavones and anti- oxidants that may get destroyed due to exposure to long period of heat. So, here the standardization of manual soymilk extraction method to the enzyme assisted soymilk extraction method along with the comparison of yield was done.
Materials and Methods
Soybean
The Soybean dried seeds were obtained from the market of Madhya Pradesh known as Soya State of India. Different packets of soybean were ordered from the local vendors of Madhya Pradesh at regular intervals and brought for research and experiment purpose.
Processing of Soymilk by Commercial Method
For the processing of soymilk initially soybean seeds (dry, sound, mold and extraneous matter free seeds) were cleaned and washed followed by soaking in boiled water. Once the soybean absorbs water resulting in gaining weight and size then soybeans were boiled for 1 hour in water added sodium carbonate @ 0.5% to remove the beany flavor and for quick cooking. Then, hot soybean grains were grinded using a mixer grinder until the soft slurry obtained through the use of boiled water and simultaneously filtering through muslin cloth till okara left and soymilk extracted. Then, the pasteurization of soymilk was done at 80oC for few seconds, followed by immediate cooling through chilled water to make it free from microbes and to store it for the long time.
Standardization of Commercial Soymilk extraction method
At first, the soaking time (different temperatures were 45oC for 6hrs, 50oC for 5hrs, 55oC for 4hrs, 60oC for 3hrs and 65oC for 2hrs) with boiling conditions like autoclaving, pressure cooking and boiling in open was standardized that subjected to de-hulling of soybeans and further extraction of soybean milk.
Standardization of enzyme assisted soymilk extraction method using cellulase enzyme
Cellulase enzyme was used for the extraction of soymilk. Initially, pre-weighed soybeans were cleaned, soaked for 5 hours and then de-hulled beans were cooked in the pressure cooker for 10 min and poured in to the beaker. To standardize the units of enzyme (0.05%, 0.08%, 0.1%, 0.2% and 0.3% of cellulase enzyme on the basis of wet weight of soybean) required for consistent production of milk through enzyme using different enzymes concentrations. The cellulase enzyme was used in triplicate samples where soybean grains were boiled and kept in water bath at different temperatures (37oC, 40oC, 45oC, 50 oC, 55 oC, 60oC, and 65oC) at different incubation time like 2hrs, 3hrs, 4hrs, 5hrs and 24hrs. After this treatment the samples were removed from water bath and soymilk was extracted and yield was calculated. The extracted milk was pasteurized and stored at 0-6 oC. All the steps were performed under sterile conditions.
Standardization of enzyme assisted soymilk extraction method using pectinase enzyme
Similar to cellulase enzyme the pectinase enzyme units per gram was standardized. So, pre-weighed soybean grains were soaked overnight, boiled for 1 hr in water, de-hulled and pressure cooked followed by mixing with pectinase enzyme 0.05%, 0.08%, 0.1%, 0.2% and 0.3% concentrations on the basis of the wet basis of soybean grains. The grains with enzyme were kept in water bath at different temperatures (37 oC, 40 oC, 45 oC, 50 oC, 55 oC, 60 oC, and 65 oC) for different incubation durations like 2hrs, 3hrs, 4hrs, 5hrs and 24hr then samples were removed from water bath and filtered through muslin cloth to obtain soymilk which was pasteurized and stored at 0-6 oC. All these processes were performed under aseptic conditions.
Results and Discussions
Standardization of Manual Soy Milk Extraction Method
Initially the soybeans were soaked at different temperatures with varied soaking time before actual boiling to produce soymilk as shown in the table (1). The best result was 200 ml of soymilk obtained from 40g of soybean grains at 55 oC after 4hrs of soaking time while at 60 oC little browning observed in soymilk leads to color change.
Different boiling conditions before grinding of soybeans for soymilk
Before soybean grinding they were pressure cooked for 10 min. and found this method more suitable as compared to the boiling of soybean grains in open pan. The pressure cooking of soybeans was time efficient and also soymilk production was more as compared in autoclaving the soymilk obtained had brown color and bitter flavor may be due to due to high temperature & longtime processing.
Sr.no
Temperature ( oC)
Time (Hrs)
Soymilk obtained (ml per 4g of wet soya)
1
45
6
16
2
50
5
17
3
55
4
20
4
60
3
20
Standardization of Enzyme Assisted Soymilk Extraction Method using Cellulase Enzyme
Time and temperature considered as the dependent factors for enzyme activity. The two factors were implied at different enzymes concentration followed by the analysis on the basis of production of milk and okara. The trials were repeated thrice.
Production of soymilk and okara at different concentrations of cellulase enzyme
Cellulase enzyme (percentage weight of the soaked soybeans), a macerating enzyme helps in the hydrolysis of cell wall by converting the cellulose in to glucose. The average values for soymilk after thrice attempt of trials were 17.26 ml (okara: 2.52g), 18.16 ml (okara: 2.73g), 19.16 ml (okara: 2.53g), 20.2 ml (okara: 1.32g) and 18.03 ml (okara: 2.23g) at 0.05 %, 0.08 %, 0.10 %, 0.20 % and 0.30 %. So, maximum production of soymilk and minimum okara was observed at the 0.20 % concentration of cellulase enzyme. Similar results were obtained at 0.30 % and 0.20 % of cellulase enzyme. As a result, 0.20 % concentration of enzyme was chosen for producing the milk because the production rate observed was same for all the concentrations. Comparing the yield from manual soymilk extraction method the production of soymilk was more at 0.20 % cellulase enzyme.
Production of soymilk and okara at 0.2 % concentration of cellulase enzyme with varying incubation time
It was observed that varying the incubation time for the production of soymilk and okara for 2 hrs, 3 hrs, 4 hrs, 5 hrs and 24 hrs resulted in 14.43 ml (okara of 3.16 g), 18.12 ml (2.53 g), 20.11 ml (1.70 g), 19.43 ml (1.93 g) and 14.43 ml (2.30 g). The maximum soymilk obtained was at 4 hrs of incubation while after 5 hrs of incubation time there was a slight change in texture and color of the milk. After 24 hrs of incubation the curdling observed in soymilk. From the results, it can be concluded that the quantity and quality of soymilk produced from cellulase activity was obtained maximum after 4 hrs of incubation time.
Standardization of enzyme assisted soymilk extraction method using cellulase enzyme
Considering the time and temperature as the dependent factors for enzyme activity. The two factors have been matched with different concentration of enzymes at different time temperatures combinations followed by the analysis on the basis of production of milk and okara. The trials were repeated thrice and then means, standard variation has been shown in tables below.

Figure 1: Cellulase enzyme assisted Milk extraction

Figure 2: Cellulase enzyme assisted Okara extraction.
Production of milk at different concentration of cellulase enzyme
Using different concentration of cellulase enzyme (percentage weight of the soaked soybeans), a macerating enzyme helps in the hydrolysis of cell wall by converting the cellulose in to glucose. It was observed that average values for the production of milk after thrice attempts of trials 17.26ml, 18.16ml, 19.16ml, 20.2ml and 18.03ml at 0.05percent, 0.08percent, 0.10percent, 0.20 percent and 0.30 percent, respectively. Table 2 showed that maximum production of the milk was observed at the 0.20 percent concentration of cellulase enzyme with a mean value of 20.2 with a standard variation of 0.06. Similar quantity of milk was obtained either 0.30 percent or 0.20percent of cellulase enzyme. As a result, the 0.20 percent concentration of enzyme was chosen for producing the milk as the production rate observed were same. Comparing to the commercial soymilk extraction method the production of milk 0.20 percent concentration of cellulase enzyme was more as shown in figure 3.
Sr no.
Cellulase enzyme conc. (% of wet weight of soya)
Production of milk (ml)
1
0.05
17.26±0.17
2
0.08
18.16±0.08
3
0.1
19.16±0.08
4
0.2
20.2±0.06
5
0.3
19.67±0.08
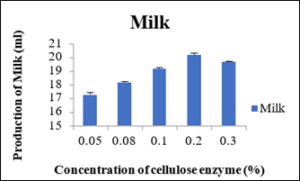
Figure 3: Production of Milk.
Production of Okara at different concentration of cellulase enzyme
After extraction of soymilk the left-over solid matter i.e., okara was observed with the mean values 2.52g, 2.73g, 2.53g, 1.32g and 2.23g at concentration of 0.05percent, 0.08percent, 0.10percent, 0.20percent and 0.30percent, respectively as shown in table 3. As the figure 2 explained that the least production of okara was noted concentration of 0.20percent of cellulase enzyme with the mean value of 1.32 with the standard variation of 0.05. Comparing to the production of okara in enzyme assisted soymilk extraction method it was very less as compare to the okara produced by the commercial method. So that may be inferred that the full conversion of cellulose has taken place with the help of cellulase enzyme.
Sr no.
Cellulase enzyme conc. (% of wet weight of soya)
Production of Okara (g)
1
0.05
2.52±0.15
2
0.08
2.73±0.03
3
0.1
2.53±0.03
4
0.2
1.32±0.05
5
0.3
2.23±0.08

Figure 4: Okara Produced.
Production of milk at 0.2 percent concentration of cellulase enzyme with variation in incubation time
It was observed that varying the incubation time for the production of milk such as 2hrs, 3hrs, 4hrs, 5hrs and 24hrs the production of milk observed was 14.43ml, 18.12ml, 20.11ml, 19.43ml and 14.43ml, respectively as shown in the table 4. From the table the maximum production of milk observed was at 4 hrs of incubation time in water bath at optimum temperature while after 5hrs of incubation time there was a slight change in texture and color of the milk. After 24hrs of incubation the curdling observed in milk. From the results the quantity and quality of milk was produced from cellulase activity on soya maximum for 4 hrs as shown in the figure 5.
Sr.no
Time (Hrs)
Production of milk (ml)
1
2
14.43±0.33
2
3
18.12±0.05
3
4
20.11±0.05
4
5
19.43±0.30
5
24
14.43±0.28
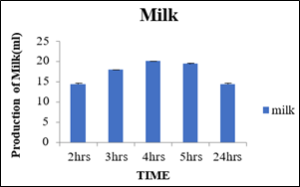
Figure 5: Production of Milk.
Production of Okara at 0.2percent concentration of cellulase enzyme with variation in incubation time
Results shown in the table 5 explained that variation in incubation time amount of okara produced was also varied like at 2hrs, 3hrs, 4hrs, 5hrs and 24hrs the okara was 3.16g, 2.53g, 1.70g, 1.93g and 2.30g, respectively. The maximum okara produced was after 2hrs and the minimum was after 4hrs. So, it may be inferred that the optimum time for the production of minimum okara with maximum milk percentage was at 0.2percent cellulase enzyme concentration after incubation time for 4hrs.
Sr.no
Time (Hrs)
Production of okara (g)
1
2
3.16±0.08
2
3
2.53±0.03
3
4
1.70±0.25
4
5
1.93±0.26
5
24
2.30±0.05
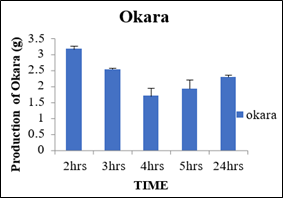
Figure 6: Production of Okara.
Production of milk with 0.2percent cellulase enzyme concentration after 4hrs of incubation with variation in temperature
The trials were performed at different temperatures for 4 hrs and results postulated that at 37⁰C, 40⁰C, 45⁰C, 50⁰C, 55⁰C and 60⁰C the milk production was 14.76ml, 15.13ml, 20.1ml, 18.9ml, 17.86ml respectively. The minimum milk obtained was at 37⁰C that means the cellulase has not shown any activity at this temperature. The maximum activity by cellulase enzyme causing maximum production of milk was observed at temperature of 45⁰C. At 60⁰C the texture and the colour of the milk changed like the browning of milk has taken place due to high temperature. So, the optimum temperature for high percentage of quantity and quality in soymilk was observed at 45⁰C as shown in the table 6 and figure 5.
Sr.no
Temperature (⁰C)
Production of milk(ml)
1
37
14.76±0.14
2
40
15.13±0.06
3
45
20.1±0.05
4
50
18.96±0.03
5
55
17.86±0.08
6
60
16.96±0.03

Figure 7: Production of Milk.
Production of Okara at 0.2percent cellulase enzyme after 4hrs of incubation with varied temperature
The okara produced at different temperatures 37⁰C, 40⁰C, 45⁰C, 50⁰C, 55⁰C and 60⁰C were 3.63g, 2.70g, 1.90g, 2.72g and 2.63g respectively as depicted in the table 7. The minimum okara was produced at 45⁰C and maximum at 37⁰C as shown in the figure 8. So, this shows at cellulase works at optimum temperature of 45⁰C.
Sr no
Temperature (⁰C)
Production of Okara (g)
1
37
3.63±0.08
2
40
2.70±0.05
3
45
1.90±0.05
4
50
2.72±0.15
5
55
2.63±0.03
6
60
2.3±0.05
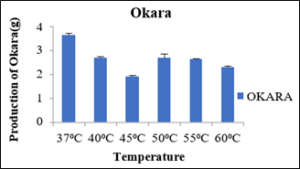
Figure 8: Production of Okara.
Standardization of enzyme assisted soymilk extraction method using pectinase enzyme
Here, the pectinase enzyme was used to dissolve the pectin content in the soybean in the form insoluble carbohydrates. The different concentrations of pectinase enzyme used at different temperatures with different time combinations for the standardization of the method and showed below in the form of tables and graphs.

Figure 9: Pectinase enzyme assisted Milk.

Figure 10: Pectinase enzyme assisted okara.
Production of milk at different concentration of Pectinase enzyme
From the table 8 it was observed that at different concentration of 0.05percent, 0.08 percent, 0.10percent, 0.20percent and 0.30percent percent the produced milk was 18.4ml, 18.03ml, 17.06ml, 17.0ml and 17.10ml, respectively. So, the maximum production of milk was observed at 0.05percent and at 0.08percent of pectinase enzyme concentration The production of milk was also nearly equal to 0.05percent but the concentration of 0.05percent was preferred more as the small amount of enzyme was producing equal amount of milk as compare to other concentrations of the enzymes. The milk produced with the pectinase enzyme was also more as compare to the milk produced by commercial method but the only lacking point of this milk was the texture compared to the milk obtained with the cellulase and by commercial method.
Sr.no.
Pectinase enzyme conc. (% of wet weight of soya)
Production of milk(ml)
1
0.05
18.4±0.20
2
0.08
16.03±0.08
3
0.1
17.06±0.12
4
0.2
17.01±0.11
5
0.3
17.10±0.05
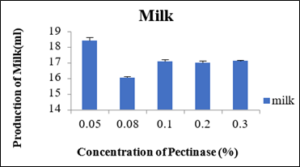
Figure 11: Production of Milk.
Production of Okara at different concentration of Pectinase enzyme
As shown in the table 9 that at different concentrations i.e., 0.05percent, 0.08percent, 0.10percent, 0.20percent and 0.30percent of pectinase enzyme the produced okara was 2.40g, 2.76g, 3.01g, 3.03g and 2.96g. as shown in the figure 12 the minimum okara was produced at 0.05percent.
Sr.no.
Pectinase enzyme conc. (% of wet weight of soya)
Okara produced (g)
1
0.05
2.40±0.03
2
0.08
2.76±0.03
3
0.1
3.01±0.05
4
0.2
3.03±0.08
5
0.3
2.96±0.12
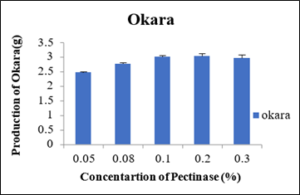
Figure 12: Production of Okara.
Production of milk at 0.05percent concentration of pectinase enzyme with variation in incubation time
From the below table 10 the produced milk after 2hrs, 3hrs, 4hrs and 5 hrs was 15.46ml, 16.23ml, 18.03ml, 18.60ml and 15.06ml respectively. So, this from this figure 13 it was analyzed that the optimum time for the production of milk at 0.05percent concentration for 5hrs in water bath was found best. After 24hrs, the curdling took place and in 2hrs the pectinase has not shown any activity in the soybean slur.
Sr.no
Time (Hrs)
Production of Milk (ml)
1
2
15.46±0.12
2
3
16.23±0.08
3
4
18.03±0.08
4
5
18.60±0.05
5
24
15.06±0.08
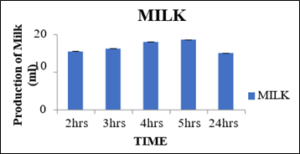
Figure 13: Production of okara.
Production of Okara at 0.05percent concentration of pectinase enzyme with variation in incubation time
As shown below in the table 16 the amount of okara resulted was different at different incubation time like at 2hrs, 3hrs, 4hrs and 5hrs the okara was 3.16g, 2.76g, 2.73g, 2.46g and 2.83g produced, respectively. It may be postulated that after 2hrs of pectinase enzyme activity the maximum okara was produced against 5hrs when it was minimum. So, the optimum time for the production of milk was found to be 5hrs using pectinase enzyme for the production of soymilk as shown in the figure 13.
Sr.no.
Time (Hrs)
Production of Okara (g)
1
2
3.16±0.08
2
3
2.76±0.06
3
4
2.73±0.03
4
5
2.46±0.03
5
24
2.83±0.03
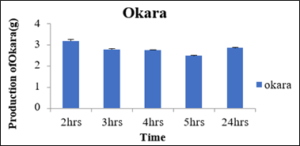
Figure 14: Production of Okara.
Production of milk at 0.05percent Pectinase enzyme concentration of after 5 hrs at different temperatures
From the table 12 the milk produced at different temperatures 37⁰C, 40⁰C, 45⁰C, 50⁰C, 55⁰C and 60⁰C was 18.01ml, 16.90ml, 15.06ml, 15.42ml, 15.23ml and 16.03ml, respectively. From the figure 15 it was observed that at temperature of 37⁰C the maximum production of milk was there and minimum at the temperature of 45⁰C. So, the optimum temperature for the production of milk with the help of pectinase enzyme was 37⁰C in the water bath for 5 hrs.
Sr.no
Temperature (⁰C)
Production of Milk (ml)
1
37
18.01±0.14
2
40
16.90±0.05
3
45
15.06± 0.06
4
50
15.42±0.05
5
55
15.23± 0.08
6
60
16.03±0.08

Figure 15: Production of Milk at different Temperatures.
Production of milk at 0.05percent Pectinase enzyme concentration of after 5 hrs at different temperatures
From the table 13 it was observed that the minimum production of okara was at the temperature of 37⁰C which was 2.92g and the maximum was at the temperature of 40⁰C of 3.03g. Hence, the optimum temperature for the activity of pectinase and for the minimum production of okara was 37⁰C.
Sr.no
Temperature (⁰C)
Production of Okara(g)
1
37⁰C
2.92±0.05
2
40⁰C
3.03±0.08
3
45⁰C
2.43±0.14
4
50⁰C
2.46±0.03
5
55⁰C
2.93±0.03
6
60⁰C
2.72±0.15
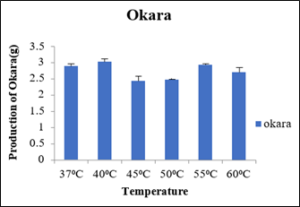
Figure 16: Production of Okara.
Standardization of commercial soymilk extraction method
Initially the soybeans were soaked at different temperatures at different soaking time before boiling for the production of milk as shown in the table (14). So, the best result was 20ml of milk produced at 55⁰C after 4hrs of soaking time and at 60⁰C the browning of soymilk was observed due to which the color changed.
Sr.no
Temperature (⁰C)
Time (Hrs)
Production of milk (ml per 4g of wet soya)
1
45
6
16
2
50
5
17
3
55
4
20
4
60
3
20

Figure 17: Milk produced after the autoclaving, boiling and blanching of soybeans.
Different boiling conditions before the grinding of soybeans for soymilk
Before grinding of soybean the cooking of soyabeans in pressure cooker for about 10mins was found the most suitable method as compare to the boiling. As cooking of soyabeans in pressure cooker was time efficient and also the production of milk was more and in autoclave due to high temperature and more timing it changed the color, flavor and produced the brown color of milk.
Conclusion
Soybean is an allrounder bean possessing good oil percentage with protein that subjected it to others uses and that is soymilk that is most commonly found useful to replace the lacteal milk because of lactose intolerance very common now a days. If novel method of enzyme assisted soymilk production method found successful at industry level, it can be a boon with energy less production, least wastage and more soymilk production.
References
- Soyabean Outlook (2021) Agricultural Market Intelligence Centre, PJTSAU
- Abraham, A. G., & de Antoni, G. L. (1999). Characterization of kefir grains grown in cows' milk and in soy milk. Journal of Dairy Research, 66(02), 327-333.
- Adlercreutz, H., & Mazur, W. (1997). Phyto-oestrogens and Western diseases. Annals of medicine, 29(2), 95-120.
- Aubert, C., & Xigang, Z. (2002). The changing role of soybean in China's food system: A study in its production, processing, consumption and trade. EU-China Joint Research Project (INCO-DC, n° ERBIC18CT970178). China agriculture press.
- Afrose, S., Hossain, M. S., & Tsujii, H. (2010). Effect of dietary karaya saponin on serumand egg yolk cholesterol in laying hens. British poultry science, 51(6), 797-804.
- Achi O K (2005). Traditional fermented protein condiments in Nigeria. African Journal of Biotechnology, 4(13).
- Arya M, Bhatia V, Ansari M, Husain S Soybean (Glycine Max L.) Genotypes for water logging tolerance. Soybean Research, 15.
- Bhatia, V. S., Singh, P., Wani, S. P., Chauhan, G. S., Rao, A. K., Mishra, A. K., & Srinivas, K. (2008). Analysis of potential yields and yield gaps of rainfed soybean in India using CROPGRO-Soybean model. agricultural and forest meteorology, 148(8), 1252-1265.
- Bansal, S., Mangal, M., Sharma, S. K., Yadav, D. N., & Gupta, R. K. (2015). Optimization of Fermentation Conditions for Probiotic Soy Yoghurt Using Response Surface Methodology. Journal of food processing and preservation, 39(6), 1809-1816.
- Bellaloui, N., & Mengistu, A. (2008). Seed composition is influenced by irrigation regimes and cultivar differences in soybean. Irrigation Science, 26(3), 261-268.
- Baumann, U., & Bisping, B. (1995). Proteolysis during tempe fermentation. Food microbiology, 12, 39-47.
- Banaszkiewicz, T. (2011). Nutritional value of soybean meal. INTECH Open Access Publisher.
- Bolla K N (2015). Soybean Consumption and Health Benefits. International Journal of Scientific and Technology Research, 4, 50.
- Bau H M, Villaume C, Nicolas J P, Méjean L (1997). Effect of germination on chemical composition, biochemical constituents and anti nutritional factors of soybean (Glycine max) seeds. Journal of the Science of Food and Agriculture, 73:1-9.
- Behrens, J. H., Villanueva, N. D., & Da Silva, M. A. (2007). Effect of nutrition and health claims on the acceptability of soy milk beverages. International journal of food science & technology, 42(1), 50-56.
- Byun, M. W., Kang, I. J., & Mori, T. (1995). Properties of soy milk and tofu prepared with γ‐irradiated soybeans. Journal of the Science of Food and Agriculture, 67(4), 477-483.
- Badenhop, A. F., & Hackler, L. R. (1971). Protein quality of dry roasted soybeans: Amino acid composition and protein efficiency ratio. Journal of Food Science, 36(1), 1-4.
- Cant A J, Bailes J A, Marsden R A (1985). Cow's milk, soy milk and goat's milk in a mother's diet causing eczema and diarrhoea in her breast fed infant. Acta Paediatrica, 74:467-468.
- Cohn, J. S., Kamili, A., Wat, E., Chung, R. W., & Tandy, S. (2010). Reduction in intestinal cholesterol absorption by various food components: mechanisms and implications. Atherosclerosis Supplements, 11(1), 45-48.
- Cai, T. D., & Chang, K. C. (1997). Dry tofu characteristics affected by soy milk solid content and coagulation time. Journal of food quality, 20(5), 391-402.
- Carrão-Panizzi, M. C., Beléia, A. D. P., Prudêncio-Ferreira, S. H., Oliveira, M. C. N., & Kitamura, K. (1999). Effects of isoflavones on beany flavor and astringency of soy milk and cooked whole soybean grains. Pesquisa Agropecuária Brasileira, 34(6), 1044-1052.
- Cassidy, A., Bingham, S., & Setchell, K. (1995). Biological effects of isoflavones in young women: importance of the chemical composition of soybean products. British Journal of Nutrition, 74(04), 587-601.
- Chumchuere, S., & Robinson, R. K. (1999). Selection of starter cultures forthe fermentation of soy milk . Food Microbiology, 16(2), 129-137.
- Cassidy, A., Albertazzi, P., Nielsen, I. L., Hall, W., Williamson, G., Tetens, I., ... & Steiner, C. (2006). Critical review of health effects of soybean phyto-oestrogens in post-menopausal women. Proceedings of the Nutrition Society, 65(01), 76-92.
- Dhar, T., & Foltz, J. D. (2004). Is soy milk? The economics of the soy milk market. In American Association of Agricultural Economics Meeting, Denver, USA.
- Dong, J. Y., & Qin, L. Q. (2011). Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast cancer research and treatment, 125(2), 315-323.
- Ensminger, M. P., & Abney, T. S. (1978). Effect of foliar fungicides on fungal colonization and seed quality of soybeans in the Midwest. Phytopathology News.
- Evans, D. E., Tsukamoto, C., & Nielsen, N. C. (1997). A small scale method for the production of soymilk and silken tofu. Crop Science, 37(5), 1463-1471.
- Fasina, Y. O., Garlich, J. D., Classen, H. L., Ferket, P. R., Havenstein, G. B., Grimes, J. L & Christensent, V. L. (2004). Response of turkey poults to soybean lectin levels typically encountered in commercial diets. 1. Effect on growth and nutrient digestibility. Poultry science, 83(9), 1559-1571.
- Guo, Y., Wang, S., Hoot, D. R., & Clinton, S. K. (2007). Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. The Journal of nutritional biochemistry, 18(6), 408-417.
- Galante, Y. M., & Formantici, C. (2003). Enzyme applications in detergency and in manufacturing industries. Current organic chemistry, 7(13), 1399-1422.
- Ghosh, P., Pamment, N. B., & Martin, W. R. B. (1982). Simultaneous saccharification and fermentation of cellulose: Effect of β-D-glucosidase activity and ethanol inhibition of cellulases. Enzyme and Microbial Technology, 4(6), 425-430.
- Garcia, M. C., Torre, M., Marina, M. L., Laborda, F., & Rodriquez, A. R. (1997). Composition and characterization of soybean and related products. Critical Reviews in Food Science & Nutrition, 37(4), 361-391.
- Hooper, L., Ryder, J. J., Kurzer, M. S., Lampe, J. W., Messina, M. J., Phipps, W. R., & Cassidy, (2009). Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Human reproduction update, 15(4), 423-440.
- Hartman, J. W., Tang, J. E., Wilkinson, S. B., Tarnopolsky, M. A., Lawrence, R. L., Fullerton, V., & Phillips, S. M. (2007). Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. The American journal of clinical nutrition, 86(2), 373-381.
- Hajirostamloo, B. (2009). Comparison of nutritional and chemical parameters of soymilk and cow milk. World Acad Sci Eng Technol, 57, 436-438.
- Halpern, S. R., Sellers, W. A., Johnson, R. B., Anderson, D. W., Saperstein, S., & Reisch, J. S. (1973). Development of childhood allergy in infants fed breast, soy, or cow milk. Journal of Allergy and Clinical Immunology, 51(3), 139-151.
- Iancu, C., Barbu, V., Nicolau, A., & Iordachescu, G. (2010). Attempts to obtain a new symbiotic product based on soy milk. Innovative Romanian Food Biotechnology, 7, 21.
- Iwuoha, C. I., & Umunnakwe, K. E. (1997). Chemical, physical and sensory characteristics of soy milk as affected by processing method, temperature and duration of storage. Food Chemistry, 59(3), 373-379.
- Joshi, O. P. (2003). Future perspectives of soybean in India. Soybean Research, 2, 29-42.
- Kakade, M. L., Simons, N. R., Liener, I. E., & Lambert, J. W. (1972). Biochemical and nutritional assessment of different varieties of soybeans. Journal of Agricultural and Food Chemistry, 20(1), 87-90.
- Kiuchi, K. (2001). Miso & Natto. Food Culture, 3, 7-10.
- Khare, S. K., Jha, K., & Gandhi, A. P. (1995). Citric acid production from okara (soy-residue) by solid-state fermentation. Bioresource Technology, 54(3), 323-325.
- Kalita, P., Tapan, B. K., Pal, T. K., & Kalita, R. (2013). Estimation of total flavonoids content (TFC) and anti-oxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. Journal of Drug delivery and Therapeutics, 3(4), 33-37.
- Khajeh-Hosseini, M., Powell, A. A., & Bingham, I. J. (2003). The interaction between salinity stress and seed vigour during germination of soybean seeds. Seed Science and technology, 31(3), 715-725.
- Kwok, K. C., Liang, H. H., & Niranjan, K. (2002). Optimizing conditions for thermal processes of soy milk. Journal of agricultural and food chemistry, 50(17), 4834-4838.
- Kalia, V. C., Lal, S., & Gupta, M. N. (2001). Using enzymes for oil recovery from edible seeds.
- Kim, J. S., & Kwon, C. S. (2001). Estimated dietary isoflavone intake of Korean population based on National Nutrition Survey. Nutrition Research, 21(7), 947-953.
- Lucas, A., Gibbs, J. A., Lyster, R. J. L., & Baum, J. D. (1978). Creamatocrit: simple clinical technique for estimating fat concentration and energy value of human milk. Br Med J, 1(6119), 1018-1020.
- Iancu, C., Barbu, V., Nicolau, A., & Iordachescu, G. (2010). Attempts to obtain a new symbiotic product based on soy milk. Innovative romanian food biotechnology, 7, 21.
- Lander, E. S., Linton, L. M., Birren, B., Nusbaum, C., Zody, M. C., Baldwin & Funke, R. (2001). Initial sequencing and analysis of the human genome. Nature, 409(6822), 860-921.
- Liener, I. E., & PAELANSCH, M. (1952). Purification of a toxic substance from defatted soy bean flonr. Journal of Biological Chemistry, 197, 29-36.
- Liu, J. R., Wang, S. Y., Chen, M. J., Chen, H. L., Yueh, P. Y., & Lin, C. W. (2006). Hypocholesterolaemic effects of milk-kefir and soy milk -kefir in cholesterol-fed hamsters. British journal of nutrition, 95(05), 939-946.
- Larmond, E. (1977). Laboratory methods for sensory evaluation of food. Research Branch, Canada Dept. of Agriculture.
- Li, Y., Griffing, E., Higgins, M., & Overcash, M. (2006). Life cycle assessment of soybean oil production. Journal of food process engineering, 29(4), 429-445.
- Martínez-Montemayor, M. M., Otero-Franqui, E., Martinez, J., De La Mota-Peynado, A., Cubano, L. A., & Dharmawardhane, S. (2010). Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clinical & experimental metastasis, 27(7), 465-480.
- Messina, M. J., Persky, V., Setchell, K. D., & Barnes, S. (1994). Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutrition and cancer, 21(2), 113-131.
- Marshall, W. E., Wartelle, L. H., Boler, D. E., Johns, M. M., & Toles, C. A. (1999). Enhanced metal adsorption by soybean hulls modified with citric acid. Bioresource Technology, 69(3), 263-268.
- McCarthy, R. D., Porter, G. A., & Griel, L. C. (1968). Bovine Ketosis and Depressed Fat Test in Milk: A Problem of Methionine Metabolism and Serum Lipoprotein Aberration1. Journal of dairy science, 51(3), 459-462.
- Nelson, A. I., Steinberg, M. I., & Wei, L. S. (1976). Illinois process for preparation of soy milk. Journal of Food Science, 41(1), 57-61.
- Noguchi, A. (1987). S. Patent No. 4,642,241. Washington, DC: U.S. Patent and Trademark Office.
- Oboh, G. (2006). Coagulants modulate the hypo-cholesterolemia effect of tofu (coagulated soy milk). African Journal of biotechnology, 5(3), 290-294.
- Oliveira, G. D. L., & Schneider, M. (2016). The politics of flexing soybeans: China, Brazil and global agro-industrial restructuring. The Journal of Peasant Studies, 43(1), 167-194.
- Onuorah, C. E., Adejare, A. O., & Uhiara, N. S. (2007). Comparative physico-chemical evaluation of soymilk and soya cake produced by three different methods.
- Önning, G., Åkesson, B., Öste, R., & Lundquist, I. (1998). Effects of consumption of oat milk, soy milk , or cow’s milk on plasma lipids and antioxidative capacity in healthy subjects. Annals of nutrition and metabolism, 42(4), 211-220.
- PARMAR, D., & RAMGIRY, S. Stability Analysis for Growth and Yield Attributes in Soybean (Glycine max (L.) Merrill). SOYBEAN RESEARCH, 18.
- Rosenthal, A., Pyle, D. L., Niranjan, K., Gilmour, S., & Trinca, L. (2001). Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzyme and Microbial Technology, 28(6), 499-509.
- Setchell, K. D., Brown, N. M., & Lydeking-Olsen, E. (2002). The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. The Journal of nutrition, 132(12), 3577-3584.
- Su, N. W., Wang, M. L., Kwok, K. F., & Lee, M. H. (2005). Effects of temperature and sodium chloride concentration on the activities of proteases and amylases in soy sauce koji. Journal of agricultural and food chemistry, 53(5), 1521-1525.
- Sautier, C., Doucet, C., Flament, C., & Lemonnier, D. (1979). Effects of soy protein and saponins on serum, tissue and feces steroids in rat. Atherosclerosis, 34(3), 233-241.
- Steinkraus, K. H. (1983). Traditional food fermentations as industrial resources. Engineering in Life Sciences, 3(1), 3-12.
- Sairam, R. V., Franklin, G., Hassel, R., Smith, B., Meeker, K., Kashikar, N., & Goldman, S. L. (2003). A study on the effect of genotypes, plant growth regulators and sugars in promoting plant regeneration via organogenesis from soybean cotyledonary nodal callus. Plant Cell, Tissue and Organ Culture, 75(1), 79-85.
- Singh, R., Kumar, M., Mittal, A., & Mehta, P. K. (2016). Microbial enzymes: industrial progress in 21st century. 3 Biotech, 6(2).
- Serrato, A. G. (1981). Extraction of oil from soybeans. Journal of the American Oil Chemists' Society, 58(3), 157-159.
- Somersall, A. C. (2005). The Healing Power of 8 Sugars: An Amazing Breakthrough in Nutrition. Sciences and Medicine. Natural Wellness.
- Smith, K. J., & Huyser, W. (1987). World distribution and significance of soybean. Agronomy
- Take, H., Mtui, G., Kobayashi, F., & Nakamura, Y. (2005). Additive effect of soybean curd residue, Okara, for enhancement of methane production from pretreated woody waste. Food. Technol, 3, 535-537.
- Tindall, B. J., Sikorski, J., Smibert, R. A., & Krieg, N. R. (2007). Phenotypic characterization and the principles of comparative systematics. In Methods for General and Molecular Microbiology, Third Edition (pp. 330-393). American Society of Microbiology.
- Ugwu, B. O., Ukpabi, U. J. (2002). Potential of soy-cassava flour processing to sustain increasing cassava production in Nigeria. Outlook on Agriculture, 31:129-133.
- Van Eys, J. E., Offner, A., & Bach, A. (2004). Manual of quality analyses for soybean products in the feed industry. American Soybean Association, Brussels, Belgium.
- Vedavanam, K., Srijayanta, S, O’Reilly, J., Raman, A., & Wiseman, H. (1999). Antioxidant action and potential antidiabetic properties of an isoflavonoid‐containing soybean phytochemical extract (SPE). Phytotherapy Research, 13(7), 601-608.
- Velasquez, M. T., & Bhathena, S. J. (2007). Role of dietary soy protein in obesity. International journal of medical sciences, 4(2), 72.
- Verhoeck, K. C., Vissers, Y. M., Baumert, J. L., Faludi, R., Feys, M., Flanagan, S., & Wichers, H. (2015). Food processing and allergenicity. Food and Chemical Toxicology, 80, 223-240.
- Woo, H. D., Park, S., Oh, K., Kim, H. J., Shin, H. R., et al. (2014). Diet and cancer risk in the Korean population: a meta-analysis. Asian Pac J Cancer Prev, 15, 8509-8519.
- Wu, A. H., Yu, M. C., Tseng, C. C., & Pike, M. C. (2008). Epidemiology of soy exposures and breast cancer risk. British journal of cancer, 98(1), 9-14.
- Washburn, S., Burke, G. L., Morgan, T., & Anthony, M. (1999). Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause, 6(1), 7-13.
- Wiles, P. G., Gray, I. K., & Kissling, R. C. (1997). Routine analysis of proteins by Kjeldahl and Dumas methods: review and interlaboratory study using dairy products. Journal of AOAC International, 81:620-632.
- Xu, Z., Wang, Q., Jiang, Z., Yang, X. X., & Ji, Y. (2007). Enzymatic hydrolysis of pretreated soybean straw. Biomass and Bioenergy, 31:162-167.
- Yang, T., Qian, T., Wang, M., Shen, X., Xu, N., Sun, Z., & Yan, C. (2016). A sustainable route from biomass byproduct okara to high content nitrogen‐doped carbon sheets for efficient sodium ion batteries. Advanced Materials, 28(3), 539-545.
- Yuan, S., Chang, S. K., Liu, Z., & Xu, B. (2008). Elimination of trypsin inhibitor activity and beany flavor in soy milk by consecutive blanching and ultrahigh-temperature (UHT) processing. Journal of agricultural and food chemistry, 56:7957-7963.
- Zhao Y L, Cai G M, Hong X, Shan, L. M., Xiao, X. H. (2008). Anti-hepatitis B virus activities of triterpenoid saponin compound from Potentilla anserine L. Phytomedicine, 15:253-258.
- Soy Milk Market Size, Regional Outlook, Application Development Potential, Price Trends, COVID-19 Impact Analysis, Competitive Market Share & Forecast, (Report ID: GMI1388), Global Market Insights, Insights to Innovation, 2022-2028.