Publication Information
ISSN: 2641-693X
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Small-Bore versus Large-Bore chest drain in the Management of Patients with Pleural Effusion at the Lagos University Teaching Hospital, Nigeria
Olugbemi Aj1*, Ogunleye Eo1, Olusoji Oo1, Ojo Oo1, Sanni Sb1, Olugbemi M2
1Cardiothoracic Surgery Unit, Department of Surgery, Lagos University Teaching Hospital, Nigeria
2General Surgery Unit, Department of Surgery, Lagos University Teaching Hospital, Nigeria
Received Date: February 17, 2020; Accepted Date: February 29, 2020; Published Date: March 06, 2020
*Corresponding author: Olugbemi Aj, Small-Bore versus Large-Bore chest drain in the Management of Patients with Pleural Effusion at the Lagos University Teaching Hospital, Nigeria. Tel: +2348082550192, +2348055463013. Email: austinjostin@yahoo.com
Citation: Olugbemi Aj, Ogunleye Eo, Olusoji Oo, Ojo Oo, Sanni Sb, Olugbemi M (2020) Small-Bore versus Large-Bore chest drain in the Management of Patients with Pleural Effusion at the Lagos University Teaching Hospital, Nigeria. Int Jr Cardiac Sci and Res: IJCSAR-116.
Abstract
Background: Many researchers have investigated the usefulness of small-bore chest drains in the management of pneumothoraces, pleural effusion and pleurodesis. The aim of this study is to compare the adequacy of drainage of small-bore catheters against large-bore catheters in the management of patients with pleural effusion.
Method: Eighty-six patients who presented with pleural effusions were recruited for this study. Two categories of patients were selected randomly and one group was intubated with large-bore (28-32Fr) chest tube, while the other was intubated with small-bore (12-14Fr) chest tube. Adequacy of drainage was compared in terms of post-extubation adequate lung re-expansion, relief of dyspnea (from NYHA III-IV to I-II), tube blockage, and total amount of drainage and duration of drainage.
Results: Comparison between small-bore chest tubes and large-bore chest tubes showed that small chest drains were more desirable to use for the management of pleural effusions. Adequacy of drainage was assessed. In patients with small-bore chest drain, there was adequate lung re-expansion in 31 patients (72.1%), relief of dyspnoea (from NYHA III-IV to I-II) in 30 patients (70%), tube blockage in 5 patients (11.6%) and mean duration of drainage was 1-2 weeks. In those with large-bore chest drain, there was adequate lung re-expansion in 32 patients (74.4%), relief of dyspnoea (from NYHA III-IV to I-II) in 31 patients (72.1%), tube blockage in 4 patients (9.0%) and mean duration of drainage was 2 weeks. There was no significant statistical difference noted with regards to adequacy of drainage between small-bore and large-bore chest drains. (P-values – 0.810, 0.815, 0.147, 0.739, for adequate lung re-expansion, relief of dyspnoea (from NHYA grade III-IV to I-II), tube blockage, and duration of drainage respectively).
Conclusion: Small-bore chest drains were found to be as effective as large-bore chest drains in the drainage of pleural effusions.
Keywords: Chest drainage; Large-bore chest drain; Pleural effusion; Small-bore chest drain
Introduction
Hippocrates first advocated pleural drainage 2400 years ago when he described the use of incision, cautery and metal tubes to treat empyema [1]. The technique became widely used to drain post-pneumonic empyema during the influenza epidemic of 1917 [2]. In recent years, large-bore drains have been the mainstay of treatment for percutaneous drainage of pleural collections and they remain in use for post-surgical thoracic drainage. Indications for tube placement within the chest include pneumothorax, benign and malignant pleural effusion, parapneumonic effusion and empyema, and haemothorax. Traditional surgical teaching dictates that viscous fluid collections require large tubes for successful drainage [3, 4]. Publications [5, 6] suggest that a variety of pleural collections can be adequately drained using small-bore catheters, often inserted under imaging guidance, thereby avoiding the complications associated with the ‘blind’ insertion of large-bore chest tubes. It is likely that the evidence supporting the use of small-bore drains within the abdomen and pelvis may be applicable to the chest. It is also possible, but perhaps less likely, that dissemination of this evidence may end the not infrequent assertion by clinicians that patients ‘would have been better treated with a larger drain’.
Small-caliber tube thoracostomy is a valuable treatment for various pathologic conditions of the pleural space. Smaller caliber tubes placed under image guidance are becoming increasingly useful in numerous situations, are less painful than larger surgical tubes, and provide more accurate positioning when compared with tubes placed without image guidance. The use of small-caliber chest tubes in developed countries may be enhanced by active drainage, use of VATS or image guidance and perhaps, early presentation of patients with pleural effusion (to be researched).
Recent literature suggests that treatment of pleural effusion with small caliber tube thoracostomy is equally effective and less painful than treatment with large caliber tube thoracostomy. [7, 8] Additionally, it has been shown that wire-guided chest tube placement allows for more accurate positioning when compared with the classic surgical technique. [9] Consequently, the role of small caliber tube thoracostomy is increasing and often performed under image guidance by Interventional Radiologists.
Drainage catheter needs to be of sufficient bore to allow adequate flow for fluid or air collection to be drained successfully, to remain patent and for all or the majority of the collection being drained to be in communication with the catheter. It is also desirable to use the catheter that is the safest and most comfortable for the patient. A large-bore catheter would appear to fulfil the first two criteria best. However, review of the published evidence suggests that small-bore catheters are at least as effective as large-bore tubes, implying that other factors need to be considered.
Reports on drainage techniques suggest that, before drainage of a collection, diagnostic needle aspiration should be performed, initially via a 22G needle and, if this is unsuccessful, via a 20 or 18G cannula [10]. This has the dual purpose of determining the contents of the collection and guiding subsequent drain insertion. It has been postulated that if pus can be aspirated by such a needle it should be drainable through a catheter twice the size i.e. 6 F or above [11]. As such, if drain patency can be maintained (for instance by flushing or the use of suction) the maximum flow rate of the catheter is unlikely to be the limiting factor for most pleural collections. Although no comparative studies for pleural collections have been performed to date, two series have compared the effectiveness of different sized catheters for draining intra-abdominal abscesses [12, 13]. Gobien et al. [12] drained 51 abdominal abscesses with a variety of catheters ranging from 5 to 18 F. Five patients initially had 5 or 6 F catheters inserted but these repeatedly blocked and had to be exchanged for larger catheters. Above 8F, increasing catheter size did not improve outcome as defined by length of post-procedure fever, duration of drainage or overall technical success. Rothlin et al. [13] retrospectively reviewed 64 patients with abdominal abscesses drained with either a 7 F pigtail catheter or a 14 F sump drain. No significant difference was found in drainage success rates or drainage time. In a review of the literature, Park et al [14] assessed the effect of catheter diameter on abscess drainage from 25 studies and found that duration of drainage was shorter for smaller diameter catheters. Although this appears counterintuitive, it may reflect bias introduced by the operator’s choice of smaller catheters for thinner pus, identified on initial diagnostic aspiration.
Further support for the use of small-bore chest drains is found in the extensive body of work using small-bore catheters to drain abdomino-pelvic abscesses, both as a primary treatment and as a temporizing measure prior to definitive surgery [15-17].
Success rates compare well with surgical series, even in difficult areas such as the pancreas. These studies suggest that pus can be adequately drained via small bore catheters, although early advocates of abdominal abscess catheter drainage encountered considerable skepticism from surgical colleagues about the efficacy of these small catheters [10, 15-17].
It is worth noting that there is paucity of published work on this topic in this environment. Hence, this study was being undertaken to bridge this identified knowledge gap. The aim of this study was to determine the adequacy of drainage, comparing small-bore catheter to large-bore catheter in terms of; rate of blockage, total amount of drainage, duration of drainage of effusion, adequate lung re-expansion and relief of dyspnea.
Chest tubes have been classified into small-bore, medium – bore and large – bore based on their sizes [18].
- Small bore tube (8–14 F). Small bore chest tubes are usually inserted with the aid of a guidewire by a Seldinger technique.
- Medium bore tube (16–24 F). Medium sized chest drains may be inserted by a Seldinger technique or by blunt dissection.
- Large bore tube (>24 F). Blunt dissection into the pleural space is performed.
Materials and Methodology
Study Design
The study was a prospective hospital based involving all patients including children, presenting at the Lagos University Teaching Hospital (LUTH) (in the period of the study) with pleural effusion. The study spanned 12 months (April 2013 – Mar 2014). The study included all patients with pleural effusion requiring drainage, presenting at the Lagos University Teaching Hospital.
The following categories of patients were excluded:
- Patients with minimal or non-free flowing pleural effusion not requiring drainage
- Patients with complicated pleural effusions such as loculated pleural effusion
- Patients with pleural effusion secondary to surgical intervention
- Patients who are less than five years old, in whom pain assessment was difficult.
Ethical Approval
Approval for the study was obtained from the Research and Ethic Committee of the Lagos University Teaching Hospital before the commencement of the study. Informed consent was also obtained from the patients or parents (for children) before being administered with the questionnaires. Approval was also obtained from the managing consultant before enrolling the patients
Study Population
The study was conducted on patients including children with pleural effusion, presenting at the Accident and Emergency centre (A/E), Children Emergency Room (CHER), consults from various wards in the hospital and surgical out-patient clinics, at the Lagos University Teaching Hospital (LUTH). LUTH is a major referral centre in Lagos State. It is an 850-bed hospital with various out-patients clinics and two emergency rooms - Accidents and Emergency room and Children’s Emergency Room (CHER).
Lagos state is the commercial nerve centre of Nigeria with a population of 9,113,605 (Based on 2006 Census). LUTH is located within Surulere Local Government Area (LGA) and also bordered by Mushin LGA. Both Local Government Areas have a combined population of 1,134,722 (Based on 2006 Census).
Data Collection
All patients who met the inclusion criteria presenting in the period of the study (1 year) in Accident and Emergency centre, Children Emergency Room, consults from various wards in the hospital and surgical out-patient clinics, were administered with the Proforma after obtaining consent. Data was collected in such areas as biodata, history, physical examination, investigations, diagnosis, treatment and outcome.
Informed consent was obtained from all patients. Consented patients had proper history, physical examination and confirmation of pleural effusion by thoracocenthesis, pre-drainage chest radiography and chest CT scan (which was done to assess the underlying lung condition). Two groups of patients were recruited for this study. One group was intubated with small-bore chest tubes while the other, with large-bore chest tubes. Patient selection into those who were intubated with small-bore chest tubes and those who were intubated with large-bore chest tubes was by randomized systematic approach. All the patients were assigned numbers consecutively as they presented. Patients with odd numbers (serial number at presentation) were intubated with small-bore chest tubes while those with even numbers were intubated with large-bore chest tubes, i.e, patients 1,3,5,7...were intubated with small-bore chest tube while patients 2,4,6,8…were intubated with large-bore chest tubes.
For those in the small bore chest tube group, chest tubes were inserted by Seldinger’s technique (Figures 1 and 2). Prophylactic antibiotic was administered (IV Ceftriaxone, 1g). After local anaesthetic (1% Lidocaine in Adrenalin – 5mg/kg) was administered, an 18-gauge trocar needle was inserted into the pleural space at the safety triangle. A guide wire was placed through the needle, and dilation was done using the dilator. A size 12Fr -14Fr chest drain catheter was then inserted, clamped and secured to the chest wall of the patient using non-absorbable Prolene 1 suture. The catheter was then placed in continuous drainage with an underwater seal bottle and dressing done. Patient was then administered analgesics - Tab Diclofenac 75mg bd, tabs Paracetamol 1g tds. This procedure was done at the bedside of the patient.

Figure 1: Small-bore chest drain kit.
 Figure 2: A patient with small-bore chest drain (size 12F).
Figure 2: A patient with small-bore chest drain (size 12F).
The large bore chest tubes were inserted by blunt dissection (Figures 3 and 4). Intravenous infusion was set up using appropriate IV fluid, after gaining peripheral venous access. Prophylactic antibiotic was then administered (IV Ceftriaxone, 1g). Local anaesthetic (1% Lidocaine in Adrenalin – 5mg/kg) was administered using a size 18-guage needle in the safety triangle. An incision, 1-1.5cm, was then made just above and parallel to the rib (where the tube was placed). Blunt dissection of the subcutaneous tissue and muscle into the pleural cavity was then done. This track was explored with a finger through into the thoracic cavity to ensure there was no underlying organs that might then be damaged as the tube was inserted. A size 28F or 32F chest tube was then inserted, clamped, secured to the chest wall using Prolene 2 suture and a purse-string stitch applied around the tube. The tube was then connected to an underwater seal bottle and dressing done. Patient was then administered analgesics - Tab Diclofenac 75mg bd, tabs Paracetamol 1g tds. This procedure was done in the theatre except in patients with severe respiratory distress, in which case, the procedure was done at patient’s bedside. However, patients still had to pay for theatre use as the sterile tray for the procedure was obtained from the theatre.
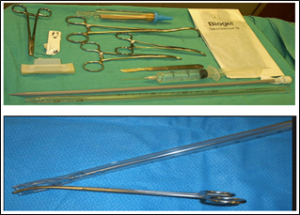
Figure 3: Large-bore chest drain and materials for insertion.

Figure 4: Patient with large-bore chest drain (size 28F).
Post intubation chest radiograph was done to confirm placement of the chest tube for both groups of patients. The chest tubes were monitored and managed. Daily drainage, characteristics of effluent and resolution of symptoms.
Extubation was done in the two groups of patients when the drainage output over three consecutive days was less than 100ml each (in patient with purulent effluent, the chest drains were removed when there was no drainage in three consecutive days) and chest radiograph showed little or no residual fluid. Immediately after the Catheters were removed, post-extubation chest radiographs were obtained. Pleurodesis was done when clinically indicated before the removal of the chest tube Immediate post-extubation chest radiographs were assessed and analysis was done based on lung expansion, (adequate lung expansion: complete expansion, more than 2/3 expansion, and inadequate lung expansion: 1/2 expansion and less than 1/2 expansion) (Figure 5, 6).
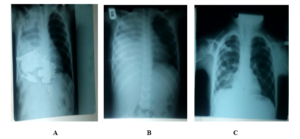
Figure 5: A: Pre-intubation CXR, B: Intubation CXR, C: Post-extubation CXR with complete lung re-expansion (adequate lung expansion).

Figure 6: D: Pre-intubation CXR, E: Post- extubation CXR with more than 2/3 lung re-expansion (adequate lung expansion).
Assessment was also done including rate of blockage of the chest tubes, total amount of drainage, duration of drainage of effusion and relief of dyspnea (comparing the degree of dyspnea (NYHA) pre-intubation and post-extubation).
Data Analysis
Data collected was collated and analysed using statistical package for social science (SPSS) version 21. Results were presented using tables, charts and diagrams. Test of significance was used where necessary.
Results
A total of 86 patients who met the inclusion criteria were recruited for this study. The results and findings are depicted in the following tables and pictograms. There were 29 males (33.7%) and 57 females (66.3%) as depicted in Table 1. Male: Female ratio was 1:1.97.
Frequency
Percent
Male
29
33.7
Female
57
66.3
Total
86
100
In this study, 81.4% of patients were within the age group of 21-65 with 6 patients (7.0%) older than 65 years and 4 patients (4.7%) between the ages of 5-10 years, as illustrated in (Table 2) below. The mean age group was 36-65, while the median age group was 21-35years.
Frequency
Percent
10-May
4
4.7
20-Nov
6
7
21-35
36
41.8
36-65
34
39.5
>65
6
7
Total
86
100
(Table 3) shows the mode to be difficulty in breathing with 84 patients (97.7%) presenting with dyspnoea (III-IV). Other common presenting symptoms included Cough – 63 patients (73.3%) and anorexia – 68 patients (79.1%).
Frequency
Percent
Dyspnoea (III-IV)
84
97.70%
Cough
63
73.30%
Chest pain
37
43.00%
Anorexia
68
79.10%
(Figure 7) below shows the distribution of the nature of pleural effusion among patients. Thirty four patients (39.5%) had serous effusion and 26 patients (30.2%) had serosanguinous effusion. purulent effusion made up only 7%.of the cases (6 patients).
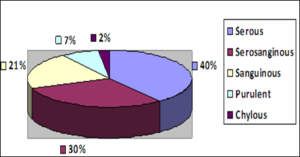
Figure 7: Distribution of nature of Pleural effusion.
The result of this study revealed that the two main causes of pleural effusion are malignancy and chest infection. Of the infective causes, Tuberculosis made up 23.3% (20 patients) - (Table 4). Breast cancer, being the commonest malignant cause of pleural effusion was seen in 32 patients - 37.2%. Gynaecological malignancies were responsible for pleural effusion in 13 patients (15.1%) – Cervical cancer, 5 patients; Ovarian cancer, 3 patients and Endometrial cancer, 5 patients. Three patients (3.5%) had Catamenial effusion. Other cancers (Laryngeal cancer - 1 patient and Chest wall tumour – 1 patient) was the cause in 2.3%.
Frequency
Percent
Malignancy
Breast cancer
32
37.2
Gynaecological malignancy
13
15.1
Lung cancer
4
4.7
Mesothelioma
2
2.3
Mediastinal - lymphoma
1
1.2
Oesophageal cancer
1
1.2
Other cancers
2
2.3
Infection
Tuberculosis
20
23.3
Pneumonia
6
7
Others
Catamenial effusion
3
3.5
Chylothorax
2
2.3
Total
86
100
This study also showed that in terms of adequacy of drainage, small-bore was as effective as large-bore tubes in the drainage of Pleural effusion as illustrated in (Table 5) below. Post drainage with small-bore, 31 patients (72.1%) had adequate lung re-expansion, 30 (70%) of the patients had relief of dyspnoea (from NHYA grade III-IV to I-II), 5 patients (11.6%) had blocked tubes. The mean duration of drainage for small-bore was 1-2 weeks and large-bore drain was 2 weeks. The mean volume of total drainage for small-bore was 1500-3000mls, while large-bore drains was 3000mls. There was no significant statistical difference noted with regards to adequacy in drainage between small-bore and large-bore chest drains. (P-values – 0.810, 0.815, 0.147, 0.06, 0.739, for adequate lung re-expansion, relief of dyspnoea (from NHYA grade III-IV to I-II), tube blockage, total amount of drainage and duration of drainage respectively).
Adequacy of Drainage
Small-Bore Frequency
Large-Bore Frequency
Adequate lung expansion
31 (72.1%)
32 (74.4%)
Relief of dyspnoea (from NHYA grade III-IV to I-II)
30 (69.7%)
31 (72.1%)
Blockage
5 (11.6%)
4 (9.0%)
Kinked
3 (6.9%)
-
Duration of drainage
3-5 days
10 (23.3%)
16 (37.2%)
6-7 days
10 (23.3%)
6 (14.0%)
1-2 weeks
23 (53.3%)
16 (37.2%)
3-5 weeks
0 (0%)
5 (11.6%)
Total amount of drainage
<500
1 (2.3%)
0 (0%)
500-1500
2 (4.7%)
15 (34.9%)
1500-3000
16 (37.2%)
11 (25.6%)
3000-5000
16 (37.2%)
9 (20.9%)
>5000
8 (18.6%)
8 (18.6%)
Discussion
The use of small-bore chest drains in the management of pleural effusion has gained a lot of attraction worldwide. However, its use in the West African sub region is yet to be popularized. In this study, there were 29 males (33.7%) and 57 females (66.3%). This gender disparity was due to the fact that majority of the patients were diagnosed with Breast and Gynaecological malignancies (45 patients - 52.5%). Compared to a study done by Chetty et all, there were 53% male and 47% female patients [19]. Thirty-six (42%) of the patients were young, within the age of 21-35 years. The middle age group (36-65yrs) accounted for another 40% (34 patients). 6 patients (7.0%) were elderly and 4 patients (4.7%) between the ages of 5-10 years. Chetty et al found the mean age to be 63.5 years (range, 31-83 years) [19]. A total of 84 patients (97.7%) presented with difficulty in breathing. 53 (61.6%) presented with dyspnoea on exertion (NHYA III), while 31 (36%) presented with dyspnoea at rest (NYHA IV) with oxygen saturation ranging between 90-95% and requiring administration of intranasal oxygen. The cause of dyspnoea in these patients is multifactorial, including space occupation of the pleural space by the effusion with reduction in the elastic recoil of the lungs; increase intrathoracic pressure, leading to reduction in venous return to the heart and reduction in cardiac output; involvement of the lung parenchyma in the disease process and collapse of the lung. Other common presenting symptoms included cough (73.3%) and anorexia (79.1%).
Chernow and Sahn [20] and Weick et al. [21] reported that up to 25% of patients with carcinoma or lymphoma of the pleura, respectively, may be relatively asymptomatic when the pleural effusion is initially discovered on a routine chest radiograph. Chernow and Sahn [20] noted that signs of a pleural effusion are typically found on physical examination and cachexia and lymphadenopathy may be seen in cancer. The nature of effluent varied. Serous effluent accounted for 39.5%, Serosanguinous, 30.2%, Sanguinous 20.9%, Purulent 7% and chylous 2.3%. Results from this study revealed that the most common cause of pleural effusion was breast cancer (37.2%), followed by pulmonary tuberculosis infection. This agrees with results obtained by Parulekar et al. [15]. Their study showed that majority of patients had breast cancer (55%). Results obtained by Reinhold C and colleagues [22] when they analyzed 42 consecutive patients who had pleural effusions. 16% had benign exudates, 29% malignant exudates, 35% empyemas. This is also in keeping with a study by Ogunleye et al. [23] who retrospectively analysed 372 patients with pleural effusion over a 55-month period and noted that malignant effusions constituted majority of the sample size and concluded that advanced malignancies are the commonest causes of symptomatic pleural effusions and that within the group breast carcinoma exerts much weight over and above other malignancies.
Adequacy of drainage was also assessed in this study by comparing palliation of dyspnoea (from NYHA III-IV to I-II), adequate lung re-expansion, tube blockage, total amount of drainage and duration of drainage – after removal of both small-bore and large-bore chest drains. For both small-bore and large-bore chest drains 70% of the patients had relief of dyspnea from NYHA III-IV to I-II. Dyspnoea was not relieved in some patients possibly due to the fact that some of the patients had lung parenchymal disease from malignancy e.g in lung cancer; or from extensive metastasis to the lungs as was the case for some patients with breast and gynaecological cancers; or from lung parenchymal destruction as was the case in some patients with PTB.
There was adequate lung re-expansion, 72.1%, tube blockage, 11.6% and average duration of drainage of 1-2 weeks, in patients with small-bore chest drain. In Patients with large-bore drains, there was adequate lung re-expansion in 74.4%, tube blockage in 9.0% and average duration of drainage of 2 weeks. There was no significant statistical difference noted with regards to adequacy in drainage between small-bore and large-bore chest drains. (P-values – 0.810, 0.815, 0.147, 0.060, 0.739, for adequate lung re-expansion, relief of dyspnoea (from NHYA grade III-IV to I-II), tube blockage, total amount of drainage and duration of drainage respectively). These results were similar to those obtained by Filosso et al. [24] who studied 7 patients with malignant pleural effusion using small-bore chest drain, there were no clinical complications following the placement of the drainage: its placement was easy, safe and well-tolerated by all patients. A satisfactory radiological lung expansion was achieved in all cases; Dyspnea reliefs were complete in 4 cases. A study by Sahin et al. [25] showed that of the 24 patients analyzed, 2 patients (8%) did not show lung expansion after pleural drainage, which is also in keeping with results of this study.
The average duration of chest drain with large-bore was 2 weeks with an average total drainage of 3000mls of effluent; while average duration with small-bore drain was 1-2 weeks with average total drainage of 3000-5000mls of effluent. This result is similar to that obtained by Gammie, et al. [26]. They retrospectively reviewed 109 consecutive pigtail catheter placements. Mean effusion volume decreased from 43 to 9 percent, and drainage averaged 2899 ml over 97 hours. This result is also in keeping with that obtained by Singh et al. [27] who studied 10 patients with pleural effusion in intensive care whom they managed with small-bore chest drain. The mean and standard deviation of the volumes drained at 1, 6 and 24 hours post catheter insertion were 454 +/- 241 ml, 756 +/- 403 ml and 1010 +/- 469 ml, respectively. The largest volume drained in a single patient was 6030 ml over 11 days.
Conclusion
There was predominance of females in patients who presented with pleural effusion who subsequently had chest tube insertion done, with co-dominant young and middle age groups. Difficulty in breathing was the most common presenting symptom - ninety-eight percent of patients (84 patients). This study revealed that the majority of the effusion was serous in nature – 39.5%, and that the two main causes of pleural effusion in this environment are malignancy (Breast cancer dominates) and Infection (Pulmonary tuberculosis dominates). Small-bore chest drains were found to be as effective as large-bore as they give similar results with large-bore chest drains in terms of adequacy of drainage with respect to post-extubation adequate lung re-expansion, relief of dyspnoea, tube blockage, total amount of drainage and duration of drainage, in the drainage of pleural effusion, irrespective of the nature of effusion. Routine use of small-bore chest tube is therefore advocated in the drainage of pleural effusion.
NYHA Class
Symptoms
I
Cardiac disease, but no symptoms and no limitation in ordinary physical activity, e.g. no shortness of breath when walking, climbing stairs etc.
II
Mild symptoms (mild shortness of breath and/or angina) and slight limitation during ordinary activity.
III
Marked limitation in activity due to symptoms, even during less-than-ordinary activity, e.g. walking short distances (20–100 m).
Comfortable only at rest.
IV
Severe limitations. Experiences symptoms even while at rest. Mostly bedbound patients.
References
- Hippocrates, Genuine Works Of Hippocrates, London: Sydenham Society, 1847
- Graham Ea, And Bell Rd (1918) “Open Pneumothorax: Its Relation To The Treatment Of Empyema,” Am J Med Sci 156: 839-871.
- Light Rw, Rodriguez RM (1981) “Management Of Parapneumonic Effusions,” Arch Intern Med 141: 1339-1341.
- Miller Ks, And Sahn Sa (1987) “Chest Tubes. Indications, Technique, Management And Complications,” Chest 91: 258-264.
- Parulekar W, Di Primio G, Matzinger F, Dennie C, Bociek G (2001) “Use Of Small-Bore Vs Large-Bore Chest Tubes For Treatment Of Malignant Pleural Effusions,” Chest 120: 19-25.
- Crouch Jd, Keagy Ba, Delany Dj (1987) “"Pigtail" Catheter Drainage In Thoracic Surgery,” Am Rev Respir Dis 136: 174-175.
- Rahman NM, Maskell NA, Davies CW, Hedley EL, Nunn AJ (2010) “The Relationship Between Chest Tube Size And Clinical Outcome In Pleural Infection,” Chest 137: 536-543.
- Rivera L, O'Reilly EB, Sise MJ, Norton VC, Sise CB, et al. (2009) “Small Catheter Tube Thoracostomy: Effective In Managing Chest Trauma In Stable Patients,” J Trauma 66: 393-399.
- Protic A, Barkovic I, Bralic M, Cicvaric T, Stifter S, et al. (2010) “Targeted Wire-Guided Chest Tube Placement: A Cadaver Study,” Eur J Emerg Med 17: 146-149.
- Van Sonnenberg E, Ferruci Jt, Mueller Pr, Wittenberg J, Simeone Jf (1982) “Percutaneous Drainage Of Abscesses And Fluid Collections: Technique, Results And Applications,” Radiology 142: 1-10.
- Mueller Pr, Van Sonnenberg E, Ferruci Jt (1984) “Percutaneous Drainage Of 250 Abdominal Abscesses And Fluid Collections. Part Ii: Current Procedural Concepts,” Radiology 151: 343-347.
- Gobien Rp, Stanley Jh, Schabel Si, Curry NS, Gobien BS, et al. (1985) “The Effect Of Drainage Tube Size On Adequacy Of Percutaneous Abscess Drainage,” Cardiovasc Intervent Radiol 8: 100-102.
- Rothlin Ma, Scho¨B O, Klotz Hp, Candinas D, Largiade`R (1998) “Percutaneous Drainage Of Abdominal Abscesses. Are Large-Bore Catheters Necessary?,” Eur J Surg 164: 419-424.
- Park Jk, Kraus Fc, Haaga Jr (1993) “Fluid Flow During Percutaneous Drainage Procedures: An In Vitro Study Of The Effects Of Fluid Viscosity, Catheter Size, And Adjunctive Urokinase,” Am J Roentgenol 160: 165-169.
- vanSonnenberg E, Mueller PR, Ferrucci JT Jr (1984) “Percutaneous Drainage Of 250 Abdominal Abscesses And Fluid Collections. Part I: Results, Failures And Complications,” Radiology 151: 337-341.
- Lambiasere, Deyoe L, Cronan Jj, Dorfmangs (1992) “Percutaneous Drainage Of 335 Consecutive Abscesses: Results Of Primary Drainagewith 1-Year Followup,” Radiology 184: 167-179.
- vanSonnenberg E, Wittich GR, Chon KS, D'Agostino HB, Casola G, et al. (1997) “Percutaneous Radiologic Drainage Of Pancreatic Abscesses,” Am J Roentgenol 168: 979-984.
- Dev SP, Nascimiento B Jr, Simone C, Chien V (2007) “Videos In Clinical Medicine: Chest-Tube Insertion,” N Engl J Med 357:15.
- Chetty Gk, Battula Nr, Govindaswamy R, Elahi Mm ()2006 “Comparative Analysis Of The Bonanno Catheter And Tube Thorocostomy In Effective Aspiration Of Pleural Effusion,” Heart Surg Forum 9: 731-734.
- Chernow B, Sahn Sa (1977) “Carcinomatous Involvement Of The Pleura: An Analysis Of 96 Patients,” Am J Med 63: 695-702.
- Weick Jk, Kiely Jm, Harrison Eg (1973) “Pleural Effusion In Lymphoma. Cancer,” 31: 848-853.
- Reinhold C, Illescas Ff, Atri M, Bret Pm (1989) “Treatment Of Pleural Effusions And Pneumothorax With Catheters Placed Percutaneously Under Imaging Guidance,” Am J Roentgenol 152: 1189-1191.
- Ogunleye E, Thomas E, And Olusoji O (2013) “Aetiology And Demographic Attributes Of Common Pleural Collections In An African Population,” Surgical Science 4: 332-338.
- Filosso PL1, Sandri A, Felletti G, Ruffini E, Lausi P, et al. (2011) “Preliminary Results Of A New Small-Bore Percutaneous Pleural Catheter Used For Treatment Of Malignant Pleural Effusions In Ecog Ps 3-4 Patients.,” Eur J Surg Oncol 37: 1093-1098.
- Sahin U, Unlü M, Akkaya A, And OZ (2001) “The Value Of Small-Bore Catheter Thoracostomy In The Treatment Of Malignant Pleural Effusions.,” Respiration 68: 501-505.
- Gammie JS1, Banks MC, Fuhrman CR, Pham SM, Griffith BP (1999) “The Pigtail Catheter For Pleural Drainage: A Less Invasive Alternative To Tube Thoracostomy,” 3: 57-61.
- Singh K, Loo S, Bellomo R (2003) “Pleural Drainage Using Central Venous Catheters.,” Crit Care 7: 191-194.
- C. C. O. T. N. Y. H. Association., Nomenclature And Criteria For Diagnosis Of Diseases Of The Heart And Great Vessels., 9th Ed. Ed., P.^Pp. 253-256: Boston: Little, Brown & Co., 1994.