Publication Information
ISSN: 2641-6859
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Quantitative Characterization of Lateral Force Transmission in Passive Skeletal Muscle
Carina Abrams1, David Hawkins2*
1Human Performance Laboratory, Davis, CA, USA
2Department of Neurobiology, Physiology and Behavior, University of California, Davis, CA, USA
Received Date: June 12, 2020; Accepted Date: June 19, 2020; Published Date: June 26, 2020
*Corresponding author: David Hawkins, One Shields Avenue, Department of Neurobiology, Physiology and Behavior, Room 196 Briggs Hall, University of California, Davis, Davis, CA 95616, USA. Tel: +15307522748; Email: dahawkins@ucdavis.edu
Citation: Abrams C, Hawkins D (2020) Quantitative Characterization of Lateral Force Transmission in Passive Skeletal Muscle. Adv Ortho and Sprts Med: AOASM-123.
DOI: 10.37722/AOASM.20202
Abstract
Force generated within a muscle-tendon unit (either through muscle activation or stretch) is commonly thought to be transmitted serially from one structure to the next, referred to as myotendinous force transmission. However, there exist alternate pathways that employ intramuscular and intermuscular connective tissues to transmit force laterally, referred to as lateral force transmission (LFT). The extensiveness of LFT and the mechanisms responsible for it are poorly understood. Lateral force transmission was investigated in normal and partially compromised passive skeletal muscle systems to determine the fraction of total system force that can be transferred laterally and to investigate possible mechanisms contributing to LFT. Chicken peroneus longus (PL)/middle gastrocnemius (MG)/fascia complexes were isolated taking care to maintain their normal connection to each other. They were attached to a testing fixture that allowed removal and reattachment of the distal end of the muscles. Tensile tests were conducted under three levels of tenotomy (i.e. both muscles attached, only PL attached, only MG attached) and three levels of fasciotomy (100%, 66% and 33% intact). LFT between muscles was sizeable and showed directionality (32% of the force applied distally to the MG muscle was transferred laterally to the PL muscle while only 16% of the force applied distally to the PL was transferred laterally to the MG). There was poor correlation between LFT and the ratio of muscle elastic moduli. The contact area between muscles and the ratio of fascia to muscle elastic moduli were the greatest contributors to LFT.
Keywords: Fascia; Fasciotomy; Intermuscular; Tenotomy
Introduction
Muscle-tendon research is critical for advancing preventative, surgical and rehabilitative techniques in orthopedic and sports medicine. As muscle-tendon injuries are prevalent among recreational and competitive athletes and treatments thereof are largely qualitative in nature, basic research contributing to the collective understanding of these complex structures and their interactions is needed.
Force generated within a muscle-tendon unit (either through muscle activation or stretch) is commonly thought to be transmitted serially from one structure to the next [1-3]. However, alternate pathways exist that employ intra- and intermuscular connective tissues to transmit force laterally (lateral force transmission, LFT) [4-11]. The extensiveness of LFT and the mechanisms responsible for it are poorly understood, but anatomical and clinical evidence indicates the importance of LFT.
Although structural arrangement of tissues at the musculotendinous junction is highly specialized for force transmission [12], muscular force transmission does not occur exclusively at this site. Many muscles contain “non-spanning” fibers which terminate within the muscle, and this architecture indicates substantial mechanical coupling to allow transmission of force from these fiber ends through the intramuscular connective tissue and/or the common connective tissue sheath that wraps the distal tendons.
The mechanical redundancy and architectural complexity [6, 10, 13, 14] of muscle systems leads to difficulty in predicting their behavior. Although the anatomical path between origin and insertion of a muscle-tendon system tends to dictate the primary action of the muscle, research has shown that despite changes in insertion site, muscle function is not completely altered [15, 16]. The rectus femoris (RF) muscle can continue to generate an extensor moment at the knee despite repositioning its distal tendon to a site posterior to the joint axis. These results indicate that lateral force transmission between the proximal portion of the RF muscle and the other knee extensors must exist. Clinically, RF tendon transfer tends to be a successful means of reducing knee extensor spasticity, but such a discrepancy in the actual and expected RF moments are cause for further investigation. Selection criteria to appropriately characterize donor muscles for tendon transfer surgeries are based on individual architectural characteristics [7], but the functional outcome of muscle transfers depend on more than simply the architecture of the individual muscles themselves, and further understanding of the interface between them is required.
In the current study, lateral force transmission was investigated in normal and partially compromised muscle systems to identify factors contributing to LFT and to quantify the fraction of total system force that can be transferred laterally. We hypothesized that the extent of LFT would depend on the relative elastic moduli of the muscles and fascia and the extent of the fascia connecting the muscles. Specifically, LFT was quantified in a passive two-synergist chicken muscle system for various tenotomy and fasciotomy conditions.
Research Objectives
- Quantify the magnitude of LFT that can occur between passive muscles using a chicken model, specifically the chicken peroneus longus/middle gastrocnemius/fascia complex.
- Test the hypothesis that LFT depends on the relative elastic moduli of the muscles and fascia and the extent of fascia connecting the muscles.
Materials and Methods
The peroneus longus (PL) and middle head of the gastrocnemius (MG) muscles of the chicken (N=6) were selected for their similarity in size and architecture (e.g. resting lengths, cross-sectional areas, and fascicle orientation), and for the fascia that connects them along 60-80% of their muscle belly lengths. This fascia is delicate in appearance and therefore should provide a conservative estimate of the magnitude of LFT that may occur between muscle complexes having more extensive fascia. The chicken PL and MG have similar overall size, but they have roughly a 3-fold difference in their elastic moduli so this muscle complex was deemed appropriate for testing the stated hypothesis. The PL was left intact at its origin on the patella and proximal aspect of the tibia, and the MG was severed from its origin on the distolateral aspect of the femur to facilitate attachment to a force transducer. The distal tendons were severed from their insertions (PL inserts on the tendon of the digital flexors, and MG inserts on the tarsometatarsus of the foot) as distally as possible. The muscles and associated fascia were dissected from surrounding tissues, taking care to maintain their normal connection to each other, and fixed to the testing apparatus (Figure 1). The testing apparatus did not maintain the exact three-dimensional in-vivo orientation of the two muscles, however, the general spacing and alignment of the muscles was similar to in-vivo conditions (Figure 1) and adequate for testing the stated objectives. The two muscles wrap around a bone in-vivo, but for testing purposes the muscles were supported on a plexiglass plate creating a planar orientation of the muscles.


Figure 1: Illustration of the peroneus longus (PL) and middle gastrocnemius (MG) orientation within the leg (A) and as attached to the testing frame (B). Markers shown on the muscles were used for reference purposes. The proximal MG marker in (A) was removed during clamping because it was located within the clamping area shown in (B).
The testing apparatus consisted of modified Cambridge Industries, Inc. series 300B lever system (modified to give linear actuation), a plate with clamps to secure the specimen, and an Omega Engineering load cell (model # LCCA-50, 225 N capacity). A custom pulley replaced the manufacturer’s lever arm attached to the rotating shaft of the Cambridge system. With this pulley, the maximum deformation allowed by the system was 1.8 centimeters. The maximum sustainable force was 96 Newtons. The system pulley was attached, via flexible, non-stretch cables and a brass rod, to the clamps holding the distal PL and MG tendons. Through this connection, force and displacement of the distal end of the muscle complex could be measured. A plexiglass shelf, placed under the muscles after their fixation to the testing apparatus, supported the weight of the muscles. The Cambridge System was controlled and data collected using custom LabVIEW data acquisition software (National Instruments LabVIEW version 6.1) and a PCI-6036E analog data acquisition card. Data were collected at 10 Hz.
The specimen was positioned to simulate a knee position of 90 degrees of flexion as closely as possible. That is, the natural relative positions of the patella, tibia, and muscles at this joint angle before dissection were reproduced as closely as allowed by the testing apparatus. The proximal end of MG was clamped to a load cell that measured the force in the proximal aspect of the MG. The distal tendon clamps were adjusted to produce an initial system preload of 1N. Markers were placed along the muscles and fascia for reference purposes and to measure the initial length of each muscle.
Specimen information recorded included the length of the muscles (distance between the most proximal and most distal markers on each structure), thickness (distance from superficial surface to deep surface), and width (distance from medial to lateral edges) of each muscle (taken at the midpoint between the proximal and distal ends of the muscle belly), and the length and thickness of the fascial interface between them (thickness measured at the distal end, where calipers could be used to measure fascial thickness without damaging the structure). Cross-sectional areas of the muscles were calculated as the product of the width and thickness, and that of the fascia was calculated as the product of its thickness and its length. Cross-sectional area of the fascia was calculated to represent the contact area between the muscles.
Testing consisted of preconditioning the muscle system followed by a series of loading protocols applied to the complete and partially compromised muscle system. The initial 1N preload force was not regained after preconditioning as the tissue within clamps adjusted slightly, however, within a specimen, the force present after three minutes of recovery from the preconditioning was the same as that observed during the start of all subsequent tests. The preconditioning protocol involved 50 ramp loading cycles performed at 0.5 Hz from 0-20% strain of the PL (20%/second strain rate). The 20% strain range was based on estimated in-vivo passive muscle length changes incurred during a normal range of motion. Following preconditioning, experimental trials consisted of a ramp to the specified deformation (20% strain in PL) at a strain rate of 1% PL strain per second, followed by return to the initial position at the same rate (Figure 2). Each test was performed twice with at least three minutes of recovery between tests. Results from the two trials were averaged.
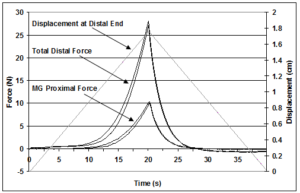 Figure 2: Illustration of the force-displacement data collected during two repeat trials of the whole muscle complex. Force was recorded at the distal end of the muscles where the tendons were clamped and pulled. Force was also recorded at the proximal attachment of the middle gastrocnemius (MG). Between each trial the muscles were held in a slack position to allow the muscles to recovery before the next test.
Figure 2: Illustration of the force-displacement data collected during two repeat trials of the whole muscle complex. Force was recorded at the distal end of the muscles where the tendons were clamped and pulled. Force was also recorded at the proximal attachment of the middle gastrocnemius (MG). Between each trial the muscles were held in a slack position to allow the muscles to recovery before the next test.
Testing was performed for intact (meaning the fascia was totally intact between the two muscles and proximal and distal ends of the muscles were attached to clamping devices), tenotomy, and fasciotomy conditions. The intact test was performed first. This was followed by two tenotomy cases in which the distal end of one muscle-tendon unit remained attached to the clamp and was pulled while the distal end of the other muscle-tendon unit was released from the clamp. The two tenotomy conditions were repeated with two fasciotomy conditions, 1/3 of the fascia transected distally and 2/3 of the fascia transected distally. Individual muscles were tested after complete fasciotomy.
Friction, though small, was present in the system and was accounted for in the force analysis. Friction existed due to the brass rod sliding through the track pulleys and from the muscles contacting the plexiglass shelf. Friction in the pulleys was determined by applying known weights to the brass rod and moving the rod at a rate similar to that used in the muscle testing. The difference between the weight and the recorded Cambridge force was used to adjust muscle forces calculated from the Cambridge data. During the isolated muscle tests, the difference between the recorded Cambridge force and the proximal muscle force was used to identify the force acting between the muscle and the plexiglass shelf.
Data Analysis
Force-deformation data were analyzed to determine the magnitude of LFT and stress-strain data were used to determine the elastic moduli of the muscles and fascia. The magnitude of the force transmitted laterally (FLFT) was measured for the tendon transection cases, in which only one tendon was attached distally to the moveable bar- either the PL tendon (TPL) or MG tendon (TMG). Both muscles were attached proximally at all times. The distally attached muscle was referred to as the transmitter or transmitting muscle (designated with subscript T), as a portion of the force generated within it could be transmitted to the adjacent muscle that was attached only proximally, referred to as the receiver or receiving muscle (designated with subscript R). The fascia force contribution to total distal force during the intact muscle testing was determined by subtracting force profiles, obtained for each muscle tested by itself after complete fasciotomy, from the total force obtained from the total force profile obtained during the first tests (Figure 3). Stress was calculated by dividing the force in each structure by the structure’s cross-sectional area. Strain was defined as the displacement of the distal clamp divided by the initial length of the structure. Stress-strain properties were plotted (Figure 4). The Elastic moduli of the muscles and fascia were defined as the slope of the last 2% of the stress-strain relationship (Figure 4).
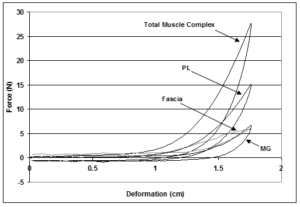
Figure 3: Illustration of the force-deformation behavior of the whole muscle complex and individual peroneus longus (PL) and middle gastrocnemius (MG) muscles tested alone, and the fascia. Force-deformation data for the muscle complex and individual muscles were obtained from the experimental testing while data for the fascia was calculated by subtracting the individual muscle forces from the force of the entire muscle complex.

Figure 4: The average stress-strain curves for the muscles and fascia of a representative sample. The slope of these curves over the last 2% strain was used to determine the elastic modulus for the structure.
Statistical Analysis
An ANOVA (a = 0.05, specimen as a random factor in the generalized linear model) was used to analyze differences between treatment conditions. The tendon attached, and the fraction of fascia remaining for each case was treated as covariates in this analysis. Tukey’s post hoc test (a = 0.05) was used to determine between which treatment combinations significant differences in FLFT existed.
An ANCOVA was performed to determine the relative roles of the various factors hypothesized to contribute to LFT. Specimen was treated as a random factor and fasciotomy level (fraction of fascia intact), the ratio of muscle moduli (ER/ET), and the ratio of fascia and transmitting muscle moduli (EF/ET) were treated as covariates. A statistical regression model of the form
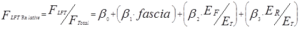
was derived, expressing relative LFT (FLFT/FTotal) as a function of relative contact area and material properties of the muscles and fascia. β0, β1, β2, etc. are coefficients and fascia (percent of intermuscular fascia remaining), ER/ET, and EF/ET (ratios of elastic moduli of receiving and transmitting muscles, and fascia and transmitting muscle, respectively) are the factors determined to be statistically significant contributors to FLFT,
Results
Muscle and fascia lengths, cross sectional areas and elastic moduli are reported in (Table 1). The PL had a longer initial length, a smaller CSA, and a greater elastic modulus than the MG in all cases. The fascia was shorter than both muscles in all cases, had very small CSA, and showed considerable variance in elastic modulus.
During TPL tests, FLFT was equal to the force measured by the load cell attached to the proximal end of the MG, which was unattached at the distal end. Since only TPL was attached distally, any force reaching the proximal load cell must have been transferred laterally from PL to MG. Conversely, during TMG tests, FLFT was measured as the force difference between the distal force measurement (Ftotal) and the measured proximal force (FProximal). That is, any force not reaching the proximal load cell when only the MG was attached distally must have been transferred laterally to the PL. FLFT for the various fasciotomy and tenotomy tests were determined and normalized to total system force measured at the distal end (FTotal) to give relative FLFT, or the fraction of total force that was transmitted laterally (Table 2, Figure 5).
| Sample | Peroneus Longus | Middle Gastrocnemius | Fascia | ||||||
| Length (cm) | CSA (cm2) | Elastic Modulus (N/cm2) | Length (cm) | CSA (cm2) | Elastic Modulus (N/cm2) | Length (cm) | CSA (cm2) | Elastic Modulus (N/cm2) | |
| 1 | 8.55 | 1.47 | 165.87 | 7.19 | 2.34 | 44.33 | 5.51 | 0.28 | 193.52 |
| 2 | 9.50 | 1.73 | 135.44 | 7.10 | 2.92 | 39.64 | 6.93 | 0.35 | 214.14 |
| 3 | 8.54 | 1.62 | 215.13 | 7.17 | 2.38 | 55.93 | 6.81 | 0.34 | 13.34 |
| 4 | 9.20 | 1.77 | 165.06 | 7.34 | 2.64 | 80.65 | 5.39 | 0.27 | 53.74 |
| 5 | 8.40 | 2.14 | 166.92 | 7.01 | 3.35 | 58.16 | 6.86 | 0.34 | 156.68 |
| 6 | 8.84 | 2.01 | 213.55 | 6.72 | 2.45 | 48.26 | 5.57 | 0.28 | 83.09 |
| Average | 8.84 | 1.79 | 177.00 | 7.09 | 2.68 | 54.49 | 6.18 | 0.31 | 119.08 |
| Std. Error | 0.18 | 0.10 | 12.76 | 0.09 | 0.16 | 5.95 | 0.31 | 0.02 | 33.03 |
Individual muscle information from isolated testing (adjusted for force degradation), fascia force calculated by subtraction. Cross-sectional area (CSA) equal to product of width and thickness of each muscle. CSA for fascia equal to product of length and thickness, representing fascia-muscle contact area. Elastic Modulus equal to slope of stress-strain curve over last 2% strain of specific structure.
| Sample | TPLIntact | TPLFas1 | TPLFas2 | TMGIntact | TMGFas1 | TMGFas2 |
| 1 | 0.19 | 0.14 | 0.10 | 0.30 | 0.22 | 0.09 |
| 2 | 0.26 | 0.22 | 0.13 | 0.35 | 0.29 | 0.25 |
| 3 | 0.16 | 0.08 | 0.08 | 0.14 | 0.14 | 0.04 |
| 4 | 0.25 | 0.11 | 0.07 | 0.10 | 0.07 | 0.07 |
| 5 | 0.17 | 0.19 | 0.09 | 0.23 | 0.12 | 0.09 |
| 6 | 0.16 | 0.17 | 0.12 | 0.16 | 0.09 | 0.08 |
| Average | 0.16 | 0.12 | 0.08 | 0.32 | 0.27 | 0.22 |
| Std. Error. | 0.05 | 0.05 | 0.02 | 0.10 | 0.09 | 0.07 |
‘Intact’, ‘Fas1’, and ‘Fas2’ represent complete fascia intact, 66% of the fascia intact and 33% of the fascia intact respectively. ‘TPL’ and ‘TMG’ indicate the tendon attached during the test, either the peroneus longus (PL) or the middle gastrocnemius (MG).
Tenotomy and fasciotomy had significant effects (P<0.05) on the extent of relative LFT in this muscle system. Tukey’s post hoc test revealed that each level of tenotomy and fasciotomy was significantly different from every other condition (p < 0.05, Figure 5). That is, the differences in relative LFT for each of the fasciotomy levels was greater than could be expected by random error after accounting for differences attributed to which muscle was attached/unattached distally. There was not a significant interaction between tenotomy and fasciotomy (p = 0.728). That is, the effect of one factor was not dependent on the level of the other.

Figure 5: Illustration of the force transmitted laterally (FLFT), normalized with respect to the total force measured at the distal end (FTotal), as a function of progressive fascia removal for when the Peroneus Longus (PL) tendon was attached to the distal clamp (triangle symbols) and for when the Middle Gastrocnemius (MG) tendon was attached to the distal clamp. Error bars represent the standard error of the mean for each set of tests.
We hypothesized that the relative elastic moduli of the fascia and muscles affects the magnitude of LFT. To test this idea the elastic modulus of the individual muscles (Table 1) was determined and the association of FLFT to the ratio of the elastic modulus of the receiving muscle to that of the transmitting muscle (ER/ET) was examined. For the TPL fasciotomy conditions, relative FLFT tended to decrease as elastic modulus ratio (modulus of the MG receiving muscle divided by modulus of PL transmitting muscle, ER/ET) increased (Figure 6), but the elastic modulus ratio explained only a fraction of relative FLFT (R2 < 0.33). For the TMG intact and fasciotomy cases, in which the receiving muscle was stiffer than the transmitting muscle, relative FLFT increased slightly with increasing ER/ET ratio, but ER/ET ratio explained only a fraction of relative FLFT (R2 < 0.06). As the ratio of receiving modulus to transmitting modulus approached 1.0 (properties more similar), percent of total force transmitted laterally tended to decrease. None of these regressions (relative FLFT with respect to ER/ET) were significant (all p > 0.05).
The effects of the material properties of the fascia were also considered. As the elastic modulus of the fascia increased relative to that of the transmitting muscle, the fraction of the total force that could be transmitted through the interface increased (Figure 7). The effects the EF/ET ratio had on relative FLFT were similar (indicated by similar slopes of trend lines, Figure 7), regardless of the muscle-tendon unit that was attached distally. The relationship between relative FLFT and EF/ET was statistically significant (p < 0.001), with 21-95% of the variability in FLFT/FTotal accounted for by variability in EF/ET.
The influences of three covariates (fasciotomy level (“fascia”), ratio of elastic moduli of the muscles (ER/ET), and ratio of elastic moduli of the fascia and transmitting muscle (EF/ ET)) were evaluated by ANCOVA to determine the relative importance to the magnitude of relative FLFT. Variation in the six specimens was accounted for by designating specimen as a random factor in this analysis. Over the range of values observed in this study, the quantity of the fascia interface and the relative moduli of the fascia and transmitting muscle had the greatest effect on FLFT/FTotal. Although the effect of ER/ET was significant, the effect of this variable on FLFT/FTotal was lower than the other two factors. The statistical model (Eq. 1) accounts for all but 17% of the variation in the empirical data (R2 = 0.83).
Eq. 1
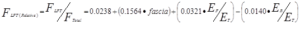
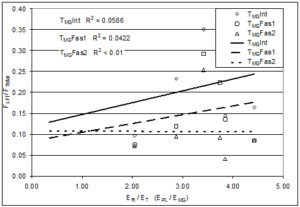
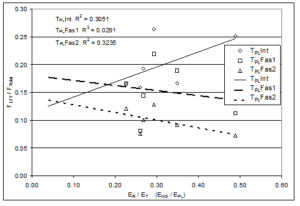 Figure 6: Illustration of the laterally transferred force (FLFT) normalized with respect to the total force measured at the distal end (FTotal) expressed as a function of the ratio of elastic moduli of the receiving (ER) and transmitting (ET) muscles (either the Peroneus Longus modulus (EPL) or Middle Gastrocnemius modulus (EMG)). Results show very poor correlation between the elastic moduli ratio and the extent of lateral force transmission (R2 values less than 0.33 for all conditions). ‘Int’, ‘Fas1’, and ‘Fas2’ represent intact fascia, 1/3 fasciotomy, and 2/3 fasciotomy, respectively.
Figure 6: Illustration of the laterally transferred force (FLFT) normalized with respect to the total force measured at the distal end (FTotal) expressed as a function of the ratio of elastic moduli of the receiving (ER) and transmitting (ET) muscles (either the Peroneus Longus modulus (EPL) or Middle Gastrocnemius modulus (EMG)). Results show very poor correlation between the elastic moduli ratio and the extent of lateral force transmission (R2 values less than 0.33 for all conditions). ‘Int’, ‘Fas1’, and ‘Fas2’ represent intact fascia, 1/3 fasciotomy, and 2/3 fasciotomy, respectively.
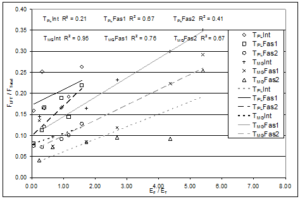
Figure 7: Illustration of the laterally transferred force (FLFT) normalized with respect to the total force measured at the distal end (FTotal) expressed as a function of the ratio of elastic moduli of the fascia (EF) and transmitting (ET) muscle (either the Peroneus Longus modulus (EPL) when the PL tendon (TPL) was attached or the Middle Gastrocnemius modulus (EMG) when the MG tendon (TMG) was attached). The relationship between relative FLFT and EF/ET was statistically significant (p < 0.001), with 21-95% of the variability in FLFT/FTotal accounted for by variability in EF/ET. ‘Int’, ‘Fas1’, and ‘Fas2’ represent intact fascia, 1/3 fasciotomy, and 2/3 fasciotomy, respectively.
Discussion
The use of chicken muscles in this study provided a cost effective and convenient model for studying LFT between passive muscles. Data from chicken muscles were useful for comparing to related studies performed on rat muscles [5, 7-9]. Chicken specimens were obtained through a commercial distributor and though animal histories (living conditions, age of animals, diet, activity level, etc.) were unknown, it was assumed that the population was homogeneous in these aspects and that the time from termination of the animals to testing was similar for all specimens.
Efforts were made to mimic the in-vivo anatomical organization and orientation of the chicken muscles during testing. However, to accommodate the testing apparatus, there were some differences in the relative positions of the structures. The muscles typically reside on a curved bony surface in-vivo, however, the muscles were placed on a flat surface during testing. Separation of natural attachments and fixing tissues to the testing apparatus may have generated transverse forces within the system that were not present in-vivo and not detected by the sensors. Reproducing the exact in-vivo conditions was not deemed necessary to achieve the stated objectives.
The intermuscular interface was hypothesized to be a significant force transmission pathway, and data indicate that as the interface is removed, the capacity for lateral force transmission indeed decreases significantly. Both the muscle-tendon unit attached at the distal end and the amount of remaining fascia intact were significant contributors to the magnitude of LFT in this muscle preparation. Although there is a high degree of variability in the data, generally, as the intermuscular connections facilitating LFT were removed (from Intact to Fasciotomy 1 to Fasciotomy 2 tests), LFT was diminished. This is consistent with findings that show increases in passive force measured in the rat extensor digitorum longus (EDL) with increasing relative muscle position [18]. It seems that, despite its apparently delicate nature, intramuscular [19] intermuscular connective tissue, as studied here, can transmit considerable force.
Similar to findings from active rat EDL muscles [5], lateral force transmission was less affected in this study by tenotomy than fasciotomy. Huijing et. al. observed a 16% decrement in active force with tenotomy of approximately 55 % of the active muscle mass. Though the actual force developed by the 45% of EDL muscle mass directly associated with the one attached digit tendon was not measured in the Huijing et al., study, it is reasonable to assume that nearly 40% of the force developed in the attached tendon resulted from forces transferred laterally. This compares to the 16 % to 32% force transmitted laterally in this study (depending on which muscle was detached). Huijing et al. observed an additional 36 % decrement in tendon force following removal of 2/3 of the fascia between the active muscles. We found that lateral force transmission decreased by 8-10 % as 2/3 of the fascia was transected between passive muscles. It would be interesting to perform tests similar to those described here on both passive and active muscle systems to determine how LFT is affected by the active state of the muscles. Muscle activation changes the stiffness of the muscle, but it is not clear to what extent these changes affect LFT.
The ANCOVA used to evaluate the contributions of contact area (fraction of fascia remaining), relative material properties of the receiving and transmitting muscles (ER/ET) and the relative material properties of the fascia and transmitting muscle (EF/ET) to FLFT indicated that all these factors were significant. Relative FLFT in all experimental treatment conditions (tenotomy and fasciotomy conditions) were statistically different from each other, with the relative properties of the fascia to transmitting muscle, as well as the quantity of intermuscular interface (fascia) remaining playing a greater role than the relative properties of the receiving to transmitting muscles.
Conclusions
- Lateral force transmission occurs between lower leg chicken muscles (in addition to rat and human muscles as previously demonstrated) and is dependent not only on the contact area between the muscles via the intermuscular fascia (quantity of the interface), but also on the relative elastic moduli of the connecting interface and the muscles (quality of the interface). Between 8 and 35 % of the total system force under various test conditions was laterally transferred to the adjacent muscle via the intermuscular interface.
- In cases in which the transmitting muscle was more compliant than the receiving muscle, the relative magnitude of force transmitted was larger. The relative material properties of the muscles and fascia were significant contributors to lateral force transmission in this preparation, and those of the fascia appear to be more important than those of the muscles.
- Results from this study provide further understanding of the role of intermuscular fascia in lateral force The small fascia can transmit considerable force, and presumably increased fascial connections between muscles would increase the occurrence of LFT between them. These results provide explanation for clinical observations of LFT following trauma and transfer procedures.
References
- Davis JR, Mirka GA (2000) Transverse-contour modeling of trunk muscle-distributed forces and spinal loads during lifting and twisting. Spine 25: 180-189.
- Boriek AM, Zhu D, Zeller M, Rodarte JR (2001) Inferences on force transmission from muscle fiber architecture of the canine diaphragm. Am J Physiol 280: R156-165.
- Perumal R, Wexler AS, Ding J, Binder-Macleod SA (2002) Modeling the length dependence of isometric force in human quadriceps muscles. J Biomech 35: 919-930.
- Street SF, Ramsey RW (1965) Sarcolemma: Transmitter of Active Tension in Frog Skeletal Muscle. Science, New Series 149: 1379-1380.
- Huijing PA, Baan GC, Rebel GT (1998) Non-myotendinous force transmission in rat extensor digitorum longus muscle. J Exp Bio 201: 682-691.
- Monti RJ, Roy RR, Hodgson JA, Edgerton VR (1999) Transmission of forces within mammalian skeletal muscles. J Biomech 32: 371-380.
- Maas H, Baan GC, Huijing PA (2001) Intermuscular interactions via myofascial force transmission: effects of tibialis anterior and extensor hallucis longus length on force transmission from rat extensor digitorum longus muscle. J Biomech 34: 927-940.
- Huijing PA, Baan GC (2002) Myofascial force transmission: muscle relative position and length determine agonist and synergist muscle force. J Appl Phys 94: 1092-1107.
- Maas H. (2003) Myofascial force transmission: Intra-, inter- and extramuscular pathways. Doctoral Dissertation. Digital Printing Partners Utrecht, Houten, Amsterdam.
- Huijing PA (2009) Epimuscular myofascial force transmission: A historical review and implications for new research. International Society of Biomechanics Muybridge award lecture, Taipei, 2007. J Biomech, 42: 9-21.
- Maas H, Sandercock TG (2010) Force transmission between synergistic skeletal muscles through connective tissue linkages. J Biomedicine and Biotechnology 1-9.
- Tidball JG (1991) Force transmission across muscle cell membranes. J Biomech 24: Suppl 1: 43-52.
- Huijing PA (1999) Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech 32: 329-345.
- Bloch RJ, Gonzalez-Serratos H (2003) Lateral Force Transmission Across Costameres in Skeletal Muscle. Exer Sport Sci Rev 31: 73-78.
- Riewald SA, Delp SL (1997) The action of the rectus femoris muscle following distal tendon transfer: does it generate knee flexion moment? Dev Med Child Neuro 39: 99-105.
- Asakawa DS, Blekmer SS, Gold GE, Delp SL (2002) In vivo motion of the rectus femoris muscle after tendon transfer surgery. J Biomech 35: 1029-1037.
- Lieber RL, Friden J (2001) Clinical Significance of Skeletal Muscle Architecture. Clin Orthop Rel Res 383: 140-151.
- Maas H, Baan GC, Huijing PA (2004) Muscle force is determined also by muscle relative position: isolated effects. J Biomech 37: 99-110.