Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Pathogenicity and Antibiotics Sensitivity Profile of Aeromonas Bestiarum Used In Experimental Infection of Different Developmental Stages of Clarias Gariepinus
Olakunle S Tiamiyu1*, Oladosu G.A1, Anifowose O.R1, Ajayi O.L2
1University of Ibadan, Nigeria
2Federal University of Agriculture Abeokuta, Nigeria
Received Date: February 27, 2020; Accepted Date: March 4, 2020; Published Date: March 13, 2020
*Corresponding author: Olakunle S Tiamiyu, University of Ibadan, Nigeria. Tel: +2348060715385, +2348098715385; Email: tiamiyukunle69@gmail.com
Citation: Tiamiyu OS, Oladosu GA, Anifowose OR, Ajayi OL (2020) Pathogenicity and Antibiotics Sensitivity Profile of Aeromonas Bestiarum Used In Experimental Infection of Different Developmental Stages of Clarias Gariepinus. Jr Aqua Mar Bio Eco: JAMBE-103.
Summary
Motile Aeromonas Septicaemia (MAS) known to be the commonest bacterial infection of cultured fish is mostly ascribed to Aeromonas hydrophila. This study was therefore conducted to determine the pathogenicity of Aeromonas bestiarum in fry, juvenile and post-juvenile of Clarias gariepinus, and evaluate the antibiotic sensitivity profile of the organism for effective control. Aeromonas bestiarum was isolated from dead fry in Ijebu Ode. The organism was characterized and used for this study. Two-hundred apparently healthy fry collected from a commercial hatchery were randomly divided into four experimental groups of 50 fry. One hundred and twenty apparently healthy juvenile and post juvenile collected from a commercial hatchery were randomly divided into four experimental groups of 15 juvenile and post juvenile respectively. Three groups were infected with 1x108 (cfu)/ml of Aeromonas bestiarum by immersion in 2L of water, while the fourth group were not infected. Fish in the infected and control groups were monitored daily for 21 days for signs of infection and mortality. The cumulative mortality in fry, juvenile and post-juvenile were 85%, 82% and 50% respectively. Gross lesions observed in post-juvenile fish were bulgy eyes, swollen dorsal muscle caudal to the cranium, congested kidney and skin depigmentation. Histological lesions were equally recorded in the hepatic tissue, diffuse degeneration and necrosis of the tubular epithelium in the interstitium of kidney. It was re-isolated from infected fish in the different developmental stages, while the organism was observed to be sensitive to two antibiotics. Groups of survivors in the different stages were treated for five days. On the second day of treatment, there was 15%, 14% and 0% mortality in the treated fry, juveniles and post-juveniles respectively, while the mortality rate of untreated but infected group (control) were 40%, 42% and 42% for fry, juveniles and post-juveniles fish respectively. This shows Aeromonas bestiarum causes high mortality in fry, juvenile and post-juvenile of Clarias gariepinus. It's however sensitive to Enrofloxacin and Gentamicin which can be used for treatment of infection by Aeromonas bestiarum for now.
Introduction
African catfish also called African sharp tooth catfish, Clarias gariepinus (Burchell), is an economically important fish species in West African countries including Nigeria [1]. This dominance of African catfish production is related to their aquaculture attributes which include ability to withstand handling stress, disease resistance, fast growth rate, high fecundity and palatability [2]. Bacterial diseases in fish cause disease outbreaks that could lead to high economic losses. A few examples are Vibriosis, Pseudomonadiasis, Staphylococcosis, Streptococcosis, hemorrhagic septicaemia, and Columnaris disease, Mycobacteriosis, Yersiniosis (enteric red-mouth disease), Motile Aeromonas Septicaemia and Edwardsiellosis [3]. Economic losses due to disease are likely to increase as aquaculture expands and intensifies [4].
This then shows why thorough investigation into the nature of these disease conditions is important, especially through experimental infections. Experimental infection of animals has been a very important tool in establishing aetiology of specific disease conditions and of studying their pathogenesis and pathophysiology.
Materials and Method
The Aeromonas bestiarum used for this study was isolated from the case of fingerlings mortalities that were presented for bacteria isolation, identification and Antibiotics sensitivity Test at Animal Care Veterinary Diagnostic Laboratory, Ogere, Ogun State, Nigeria. Two hundred pieces of 14 days old Clarias gariepinus fry, 100 pieces of 11 weeks old Juvenile and 100 pieces of 13 weeks old Post Juvenile African Catfish were collected and the fry were randomly divided into four groups each of 50 fish, while the Juvenile and post juvenile were divided into four groups of 15 fish each. Twelve Experimental tanks (Figure 1) were used and properly labeled as Fry Control, Fry A, Fry B, Fry C, Juvenile Control, Juvenile A, Juvenile B, Juvenile C, and Post Juvenile Control, post Juvenile A, Post Juvenile B and Post Juvenile C. Each tank contained 4 liters of water. The microbial and aflatoxin analysis of the Artemia and Skretting fry and Juvenile wean diet (0.7mm and 1.8mm) fed to the fry Juvenile and Post Juvenile were carried out. The infection trial was carried out by immersion of fish in water containing 1.8 X 108cfu/ml of the isolates of Aeromonas bestiarum at the rate of 5ml/L while the control group was not infected [5]. After 5 days post infection, half of water in each experimental tank was replaced 24 hours to ensure good water quality [6]. Samples of dead fry, Juvenile and post Juvenile were cultured for bacteria using standard methods and re-isolation of the bacteria isolates used for the experimental infection. The samples of dead Juvenile and post Juvenile under hygienic condition were transported to the pathology laboratory of Federal University of Agriculture Abeokuta for histopathology reports.

Figure 1: Experimental Tanks.
Results and Discussion PAREMETERS CF = Control for Fry CJ = Control for Juvenile F = Fry Tank J = Juvenile CPJ = Control for Post Juvenile PJ = Post Juvenile
The microbial analysis result of the feed sample was 0 x 105cfu/g as shown in (Table 1) included total aerobic count, coliform count, fungal count and vibrio count while Total Aflatoxin test for Artemia was 36ppb and Fish feed was 34ppb respectively. The 2 results above indicated there was no feed contamination during this study. There was high total aerobic counts in all the infected tanks (448 ± 206 cfu/ml) (Table 2) compared to control tanks (120 ± 56 cfu/ml) within 21 days meanwhile there was low dissolved oxygen in all the infected tanks (4.5 ±0.75 ppm) (Table 3) compared to control tanks (6.4 ± 0.48 ppm). There was high percentage mortality in all the infected tanks, fry 85%, Juvenile 82% and Post Juvenile 50% (Table 4) compared to control tanks, fry 10%, Juvenile 6.7% and Post Juvenile 6.7% within 21 days. The Histopathological lesions caused by the infection of Aeromonasbestiarum at cellular levels were obvious in the liver and the kidney. In the liver, there was loss of cohesion in the hepatic tissue, as well as the presence of emboli in hepatic blood vessels (Figure 4), diffuse degeneration and necrosis of the tubular epithelium with odema fluid in the interstitium of kidney (Figure 5). The infected and re-isolated bacteria Aeromonasbestiarum was only sensitive to Enrofloxacin and Gentamicin (Figure 6). It was intermediate to Furaltadone, Streptomycin and resistant to Colistin, Penicillin-Streptomycin and Oxytetracycline.
PARAMETERS
Artemia cfu/g x 105
0.7mm Fish Feed cfu/g x105
1.8mm Fish Feed cfu/g x105
Total Aerobic Counts
0
0
0
Coliform Counts
0
0
0
Fungal counts
0
0
0
Vibrio counts
0
0
0
Escherichia coli
0
0
0
CF Tank water cfu/ml
F Tank water cfu/ml
CJ Tank water cfu/ml
J Tank water cfu/ml
CPJ Tank water cfu/ml
PJ Tank water cfu/ml
DAY 12
Total Aerobic Counts
75
216
91
502
117
710
Coliform Counts
32
23
41
355
49
396
Fungal counts
0
0
0
0
0
0
Vibrio counts
0
18
0
38
0
46
Escherichia coli
0
0
0
0
0
0
DAY 21
Total Aerobic Counts
82
300
112
710
241
250
Coliform Counts
40
95
49
480
121
115
Fungal counts
0
0
0
0
0
0
Vibrio counts
0
25
0
58
0
72
Escherichia coli
0
0
0
0
0
0
Infected tanks total aerobic count (448 ± 206 cfu/ml), Control tanks total aerobic count (120 ± 56 cfu/ml) CF = Control for Fry CJ = Control for Juvenile F = Fry Tank J = Juvenile CPJ = Control for Post Juvenile PJ = Post Juvenile
PAREMETERS
CF Tank water cfu/ml
F Tank water cfu/ml
CJ Tank water cfu/ml
J Tank water cfu/ml
CPJ Tank water cfu/ml
PJ Tank water cfu/ml
Standard Values for Fish Farming
Mean ± S.D
DAY 12
pH
7.6
7.8
7.9
8.8
8.4
9.1
6.5 – 8.5
8.27±0.60
Total Hardness (ppm)
95
98
96
98
97
99
50 – 150
97.2±1.47
Nitrite (ppm)
0.11
0.17
0.11
0.18
0.18
0.2
0.05 max
0.16±0.04
Ammonia (ppm)
1.1
1.5
1.3
1.8
1.82
2.2
2.0 max
1.62±0.4
Dissolved Oxygen (ppm)
7
6
6.0
4
6.5
4
5.0 min
4.83±1.33
DAY 21
pH
8
8.3
8.9
9.4
8.8
9.1
6.5 – 8.5
8.75±0.52
Total Hardness (ppm)
96
98
97
100
96
100
50 – 150
97.8±1.83
Nitrite (ppm)
0.12
0.19
0.11
0.19
0.18
0.21
0.05 max
0.17±0.04
Ammonia (ppm)
1.1
1.5
1.45
2
1.9
2.4
2.0 max
1.73±0.46
Dissolved Oxygen (ppm)
5.5
5
6.5
4
6.6
4.5
5.0 min
4.6±0.58
Dissolved oxygen in Control tanks (6.4 ± 0.48 ppm) Dissolved Oxygen in infected tanks 4.5 ±0.75 ppm)
PARAMETERS
Control Fry
Fry
Control Juvenile
Juvenile
Control Post Juvenile
Post Juvenile
% MORTALITY at Day 7
2
13
0
11
0
6.7
% MORTALITY at Day 14
4
79
0
51
0
35.5
% MORTALITY at Day 21
10
85
6.7
82
6.7
50
The treated Fry, Juvenile and Post Juvenile with Enrofloxacin 20% at 10mg/kg body weight reduced the mortality rate to 15%, 14% and 0% respectively while the mortality rate of untreated Fry, Juvenile and Post Juvenile was 40%, 42% and 42% respectively 5 days post treatment. Aeromonas bestiarum [8] was previously known as Aeromonas hydrophila genomospecies 2 hence the reason why not so much has been said of Aeromonas bestiarum in Tropical Africa. It is however a growing problem in Poland [8]. Other Aeromonas species such as Aeromonas hydrophila, Aeromonas salmonicidae, Aeromonas caviae have all been reported in Nigeria. The most important fish pathogen was Aeromonas hydrophila [9] and highest prevalence is in polluted waters [10]. The mortality rates of 85% and 82% Tab. 4were observed in Fry and Juvenile fish tanks, infected with Aeromonas bestiarum, respectively in this study. This result is similar to the report of Madubuike et al. [5] who observed cumulative mortality rate of (30 – 90%) in catfish infected with Aeromonas hydrophila at the rate of 1 X 108cf.u/ml of the pond water. The variation in the pattern and mortality rates may be related to the species of fish, strain of Aeromonas species, experimental conditions, dose of the infective pathogen given, route of administration of the pathogen and duration of the experiment [5]. The high mortality observed in 14 days post infection with Aeromonas bestiarum in this study might have resulted from the alteration of homeostasis of the fry due to necrosis of the skinand fins (Figure 3) that are likely to affect the osmoregulatory and respiratory function of the fish. In this study, some fish showed marked hemorrhages on the base of the fins and vent (Figure 2). These were the findings of Laith AR and Najiah [11] who reported similar fingings in Clarias gariepinus. At 4 days post infection with Aeromonas bestiarum, hyperemic spots was observed on the base and tip of the skin which is also similar to what Madubuike et al, [5] observed in fingerlings that were infected with Aeromonas hydrophila. Camus et al, [11] also reported exophthalmia, pale gills and stomach filled with cloudy fluid similar findings were observed in this study. Laith and Najiah [12] discovered degenerative changes in the glomerular epithelium of the kidney of diseases of catfish infected with Aeromonas hydrophila which was similar to the report of histopathology in this study (Figure 4, 5). Laith and Najiah [12] reported that the Aeromonas hydrophila strain was resistant to Ampicillin and Colistin Sulphate. In this study, resistance of Aeromonas bestiarum to some antibiotics were also observed but it is only sensitive to Enrofloxacin and Gentamycin (Figure 6). Kozińska [8] reported that Aeromonas hydrophila and A. sobria were both sensitive to Enrofloxacin which is also similar to observation of this study. Enrofloxacin was therefore, the antibiotics that could be used for the treatment of Aeromonas bestiarum infection in catfish by bath at 10mg/kg body weight [13].
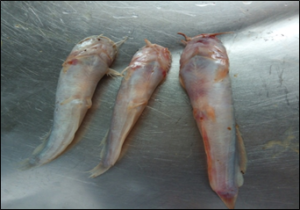
Figure 2: Hyperemia of the caudal and ventral fins and skin depigmentation of infected post juvenile fish.

Figure 3: Bulgy eyes and depigmentation of the skin of infected juvenile fish.
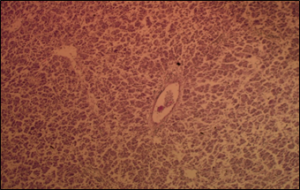
Figure 4: Photomicrograph of section of the liver showing loss of hepatic cohesion with presence of emboli within the blood vessel in the infected Post Juvenile Fish, H& E Stain x40.
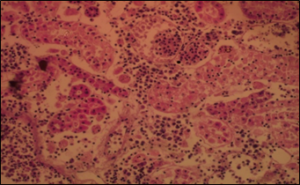
Figure 5: Photomicrograph of section of the kidney showing oedema of the interstitium with presence of protein cast in the glomerulus and the tubules of the Kidney of the infected Juvenile Fish, H&E x40.

Figure 6: Antibiotics sensitivity pattern.
Conclusion
Aeromonas bestiarum causes high mortality in fry, Juvenile and Post Juvenile of African Cat fish. The organism is however sensitive to Enrofloxacin and Gentamicin during treatment.
Reference
- Olatoye IO, Basiru A (2013) Antibiotic usage and Oxytetracycline residue in African catfish (Clariasgariepinus) in Ibadan, Nigeria. World Journal of Fish and Marine Sciences 5: 302-309.
- Ikpi G, Offem B (2011) Bacterial infection of mudfish Clarias gariepinus (Siluriformes: Clariidae) fingerlings in tropical nursery ponds. Reviews in Biology of Tropics 59: 751-759.
- Becky AL (1995) Introduction to fish health management (2nd Edition), U.S. Fish and wildlife service unit.
- Mohan CV, Bhatta R (2002) Social and economic impacts of aquatic animal health problems on aquaculture in India. p. 63-75. In: Arthur, J.R. Phillips, M.J., Subasinghe, R.P. Reantaso, M.B. and MacRae, I.H. (eds.) Primary Aquatic Animal Health Care in Rural, Small- scale, Aquaculture Development. FAO Fish. Tech. Pap. No. 406.
- Madubuike UA, Kennedy FC, Vincent SS (2015) Evaluation of pathogenicity of motile Aeromonas species in African catfish International Journal of Fisheries and Aquatic Studies 2: 93-98.
- Thomas J, Madan N, Nambi KSS, Majeed SA, Basha AN, et al. (2013) Studies on ulcerative disease caused by Aeromonas caviae-like bacterium in Indian catfish, Clarias batrachus (Linn). Aquaculture; 376- 379: 146-150.
- Ali A, Carnahan AM, Altwegg M, Luthy-Hottenstein J, Joseph SW (1996) Aeromonas bestiarum sp. Nov. (formerly genomospecies DNA group 2 A. hydrophila), a new species isolated from non-human sources. Microbiol. Lett 5: 156-165.
- Kosinzka A, Figueras MJ, Chacon MR, Soler L (2002) phenotypic characteristics and pathogenicity of Aeromonas genomospecies isolated from common carp (Cyprinus carpio). J Appl Microbiol 93: 1034-1041.
- Carnahan AM, Altwegg MM (1996) Taxonomy: The Genus Aeromonas, John Wiley, Chichester, UK, pp. 1-38.
- Hazen TC, Raker ML, Esch GW, Fliermans CB (1978) Ultrastructure of red-sore lesions on largemouth bass (Micropterus salmoides): Association of the ciHate Epistylis sp. and the bacterium Aeromonas hydrophila. Journal of Protozoology 25: 351-355.
- Camus AS, Durborrow RM, Hemstreet WG, Thune RL, Hawke JP (1998) Aeromonas Bacterial Infections Ñ Motile Aeromonad Septicemia. SRAC Publication No. 478.
- Laith AR, Najiah M (2014) Aeromonas hydrophila: Antimicrobial susceptibility and histopathology of isolates from diseased catfish, Clarias gariepinus (Burchell). Journal of Aquaculture Research and Development 2014: 5.
- Reimschuessel RL, Stewart E, Squibb K, Hirokawa T, Brady D, et al. (2005) Fish drug analysis--Phish-Pharm: a searchable database of pharmacokinetics data in fish. AAPS J 7: E288-E327.