Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Nutritional, Physiological, and Morphological Effects on Almond Trees Grown by Fertigation with the Nitrification Inhibitor 3, 4-Dimethylpyrazole-Succinic Acid
Mario Ferrández-Cámara1, Juan J Martínez-Nicolás1, José M Cámara-Zapata1, Juan Carlos Fernández-Zapata1, Marina Alfosea-Simón2, Francisco García-Sánchez2*
1Centro de Investigación e Innovación Agroalimentaria and Agroambiental (CIAGRO-UMH), Miguel Hernández University, 03312 Orihuela, Spain
2Centro de Edafología and Biología Aplicada del Segura, CEBAS-CSIC, 30100 Murcia, Spain
Received Date: April 12, 2022; Accepted Date: April 23, 2022; Published Date: May 11, 2022;
*Corresponding author: Francisco García-Sánchez, Centro de Edafología and Biología Aplicada del Segura, CEBAS-CSIC, 30100 Murcia, Spain, Tel: +34-968-396200. Email: fgs@cebas.csic.es
DOI: 10.37722/AAHAE.2022201
Abstract
Nitrogen fertilization is key to improve crop yield. However, due to the harmful environmental effects of fertilizers, farmers and governments are searching agronomic practices that provide nutrients to the crops with minimal environmental impact, as for example the use of nitrification inhibitors. These compounds act on ammonium reducing its oxidation to nitrate, and soil´s nitrogen remains longer in the form of ammonium helping prevent nitrate lixiviation. The objective of the experiment was to evaluate the effect of a new nitrification inhibitor (DMPSA) in almond trees. We studied the effect of three solutions on vegetative growth, nutrition, and physiology on Alvijor almond plants irrigated with one of three possible study solutions i) 3:1 nitrate:ammonium rate solution ii) 1:1 nitrate:ammonium rate solution, or iii) 1:1 nitrate:ammonium rate solution plus a nitrification inhibitor (3,4-dimethylpyrazole-succinic acid). Plants were grown in a greenhouse in calcareous and alkaline soil from the Spanish Levante area. Macro and micronutrients were determined from drainage samples collected throughout the experiment. At the end of the experiment, gaseous exchange and chlorophyll fluorescence parameters were measured and plants harvested to analyse morphological characteristics (leaf, stem and root fresh and dry weight, aerial part rate, and trunk diameter) and N, P, K, Ca, and Mg content in the leaves.
We found higher levels of ammonium and lower nitrates in the roots and higher vegetative growth with the irrigation solution containing the nitrification inhibitor in comparison with treatment solutions 1:1 and 3:1. The drainage showed that NI reduces the level of nitrates in the leachate, limiting its discharge to the subsoil.
In conclusion, the nutrient solution with the nitrification inhibitor has a positive impact on Alvijor almond plants and the environment.
Keywords: Ammonium; Avijor almond variety; DMPSA; Irrigation nutrient solutions; Leaf analysis; Nitrate
Introduction
Today's agriculture aims to produce quality foods while safeguarding the environment and natural resources, such as soil fertility. To accomplish this, products added to nourish the plants or improve soil characteristics must meet two key requirements: agronomic effectiveness and be harmless to the environment and health. Nitrogen (N) is a key nutrient in agriculture required in large quantities by croplands. However, its excessive use pollutes soils and marine environments, causing serious environmental problems [1]. Spain stands out in the use of national and international fertilizers. In the 2017-2018 business year, dealers sold around 1,075, 424, and 420 thousand tons of N, P2O5, and K2O fertilizer units, respectively, with the highest consumptions reported for the autonomous communities of Andalusia and Castile-Leon [2]. Ammonium nitrate and urea were among the leading products, with a sales volume of 729 and 694 thousand tons, respectively, in 2020 [3].
Plants take in N through the roots in the form of nitrate and/or ammonium. Depending on the crop and edaphic/climate conditions, plants absorb N more effectively from one or the other source. Inside the plant, ammonium reacts with glutamate to form glutamine, allowing the assimilation of N. When the plant absorbs nitrate as the N source, a reduction of the nitrate occurs to ammonium in a previous step [4].
In agriculture, indiscriminate use of N fertilizers is increasing the release of nitrous oxide (N2O) to the atmosphere, which directly associates to climate change and creates eutrophication problems in many scenarios [5]. Furthermore, there is a relationship between nitrate and ammonium nitrification and volatilization processes with the addition of nitrogen inhibitors to the crops, influenced by environmental conditions, crop management, and type of soil.
In the process of nitrification, soil´s nitrifying bacteria convert ammonium ( to nitrate ( , with the consequent destructive effects to the soil due to nitrate lixiviation ( and the emission of nitric oxide (NO) and N2O to the atmosphere. These phenomenas lower the effectiveness in the agricultural use of nitrogen fertilizers and has an impact on the atmosphere (greenhouse gases), aquifers, and seas, were huge amounts of nitrites end up. Because of this, it is often desirable to reduce nitrification in agricultural soils [6]. Nitrification inhibitors decrease the nitrification rate by slowing down the conversion of NH4+ to , allowing plants to absorb N easier in the form of ammonium and avoid the energy expenditure needed by the plant to convert nitrate to ammonium, which can be destined to other physiological processes. Nitrification inhibitors (NIs) slowdown microbial conversion from ammonium-N to nitrate-N (nitrification), decreasing the loss due to nitrate lixiviation, which results in efficient use of fertilizers. Many synthetic NIs inhibit ammonia monooxygenase, an enzyme that uses ammonia as substrate in the conversion of ammonium to nitrate [7].
There is a range of nitrification inhibitors widely used in agriculture, including (1) 2-chloro-6-trichloromethyl pyridine (nitrapyrin), (2) dicyandiamide (DCD), and (3) 3,4-dimethylpyrazole phosphate (DMPP). A new NI was recently developed, 3,4-dimethylpyrazole-succinic acid (DMPSA). The difference between DMPP and DMPSA is the succinic bond in the latter, which provides many advantages such as stability under basic conditions and the possibility of combining it with other fertilizers like calcium ammonium nitrate or diammonium hydrogen phosphate [8]. These compounds reduce nitrate lixiviation, emission of N2O, and emission of NO in 48%, 44%, and 24%, respectively [9-11]. The effectiveness of fertilizers formulated with NIs rely on factors as the soil (pH and texture), irrigation management, and fertilization (irrigation and fertilization rate with N), temperature, as well as the formulation and chemical characteristics of the fertilizers [12-14].
Almonds are the most important nut crops among dry farming in areas with Mediterranean climate. This fruit tree requires little water and is adapted to adverse soil and climate conditions, occupying areas usually unsuitable for other fruit tree species. Current global production of shelled fruits is 3.5 Tg. World´s top almond producing countries are the USA (over 50%), Spain (9%), Iran (5%), Turkey, and Australia (4%) [15]. To improve the economic performance of the farms, almond growers must establish technical advances: develop varieties that are more productive, introduce irrigation and fertilization systems, improve mechanical harvesting, and increase plant density. Among these advances, fertigation using fertilizers with improved effectiveness regarding N use is providing good results. The aim of this study was to assess the effect of irrigating almond plants with a nutrient solution that includes a new nitrification inhibitor (DMPSA). We grew the plants in pots with soil and irrigated them by fertigation. We expect that the inhibition of ammonium to nitrate will ease plant N absorption and assimilation and reduce nitrate lixiviation.
Materials and Methods
Growing conditions and plants
This study was performed in a multi-tunnel greenhouse in the CEBAS experimental farm La Matanza located in the municipal area of Santomera (18 km from Murcia, Spain). A cooling system and aluminium shade cloth (30%) maintained the temperature under control. Mean daytime temperature and moisture were kept at 29 ºC and 55%, respectively. Avijon almond trees (Prunus dulcis (Mill) D.A. Webb) were used, purchased from a commercial nursery (EF Viveros del Sureste S.L., Carretera de Granada, Km 10,5, 30412 Barranda, Caravaca de la Cruz, Murcia, Spain).
The Avijon variety almond plants were transplanted into 20-liter pots containing soil from Vega Baja del Segura (Orihuela, Comunidad Valenciana, Spain). This clay loam soil has a pH of 8.1 and electrical conductivity of 0.82 (mS cm-1). Concentrations of Cl-, NO3-, PO43-, SO42- anions, extracted in water, were 265.6, 1,029.7, <1.0, 368.2 (mg/Kg of soil), respectively. Concentrations of macro- and micronutrients, following digestion with HNO3:H2O2 was as follows (expressed as g/100 g of soil): Ca = 17.50, K = 0.78, Mg = 1.34, Na = 0.06, and P = 0.08. Once transplanted, the plants were irrigated according to their needs using water from the Tagus-Segura water transfer. For the irrigation, 4 L h-1 self-compensating dripper system was used, with sufficient irrigation volume to produce drainage every time.
After a two-week acclimation period, the almond plants were separated into three groups, each irrigated with different nutrient solutions prepared with KNO3, Ca(NO3)2, MgSO4, NH4NO3, (NH4)H2PO4, (NH4)2SO4, K2SO4, micronutrients, and/or the nitrification inhibitor (DMPSA). N, P, K, and Ca at concentrations in the nutrient solutions were 5.18 mM, 0.6 mM, 2.6 mM, and 4 mM, respectively and differed in the NO3-/NH4+ ratio and presence of the NI. Treatment solution 1) 1:1 nitrate/ammonium ratio, without the NI; Treatment solution 2) 3:1 ratio, without the NI; Treatment solution 3) 1:1 ratio plus the NI.
At the beginning, irrigation of the plants with the above-described nutrient solutions was done once a day for five minutes. Once adapted to greenhouse conditions, irrigation was increased to 10 minutes every day. The amount of added water aimed to obtain a minimum drainage of 15% of total irrigation volume every time. Irrigation frequency and volume were adjusted weekly based on the drainage data collected from the recipients in the pots. By using computer-controlled irrigation network sectoring (tanks, pipes, electrovalves, pumps, and so on), irrigation of each treatment group was independent from each other. Plants were placed in four blocks, each of which had four almond plants per treatment randomly distributed, namely 16 almond plants per treatment.
Parameters analysed
Measurements of plant drainage
Every four weeks, follow-up of the experiment was carried out. After irrigating, drainage samples were collected for each of the treatments using four containers per treatment. Next, the volume was measured and drainage samples stored to analyse cation, anion, and ammonium content.
Measurement of chlorophyll content and trunk height and diameter
On the day drainage samples were collected, chlorophyll content and trunk height and diameter were measured in all experimental plants. Fully developed leaves in all orientations were used to measure chlorophyll with a portable Cl-01 chlorophyll content meter (Hansatech) (SPAD units). That device determines the relative content of chlorophyll using dual-wavelength optical absorbance (620 and 940 nm) from sample leaves. Heights were determined with a tape measure from the base of the pot to the higher cross of the plants. For trunk diameter measurements, a digital caliper (Digimatic, Mitutoyo CD-15D) with 0.1mm accuracy was used.
Soil moisture and crop transpiration measurements
Soil moisture measurements were obtained throughout the whole study using a TDR 100 (Time-Domain Reflectometer, Fieldscout) portable soil moisture (relative) meter. Loss of moisture from soil was measured in a control pot with no plant. Values are presented as percentage (%) of transpired water/hour.
Gaseous exchange parameters
Gaseous exchange parameters were measured on fully expanded and developed leaves between 8:30 and 11:00 am in all plants and for each of the treatments few days before harvesting them. A portable photosynthesis system was used (PP System Ciras-2, UK), adjusted to 1.200 mmol m-2 s-1 for photosynthetically active radiation light and 400 ppm CO2 concentration in the measuring chamber. That device measured the net rate of photosynthetic CO2 (ACO2) assimilation and stomatal conductance (gs), and calculated water use efficiency (WUE = ACO2/Eleaf, were Eleaf is the leaf transpiration value obtained in each measurement) and Ci/Ca relationship (Ci and Ca represent substomatal and external CO2 concentrations, respectively).
Chlorophyll fluorescence parameters
Chlorophyll fluorescence was measured on the same plants used to determine gaseous exchange parameters with the aid of a portable FMS-2 pulse-modulated chlorophyll fluorometer (Hansatech Instruments Ltd., UK). The following chlorophyll fluorescence parameters were measured: quantum efficiency PSII (ΦPSII = (Fm'-Fs) / Fm'), efficiency of PSII antennas (Fv′/Fm′= (Fm′-F0′)/Fm′), and photochemical quenching coefficient (Pq=(Fm′-Fs)/(Fm′-F0′)). Fs represents the steady state fluorescence yield. Fm' represents the maximum value when all reaction centres are closed after a saturating pulse of light (12,000 μmol m-2 s-1 for 0.8 s) was applied, and F0′ the minimal fluorescence in a light-adapted state obtained when the actinic light source is shut out temporarily and a pulse of far-red light (735 nm) applied to drain the PSII electrodes.
Establishment of growth parameters
After the experimental phase, the almond plants were harvested. Aerial parts (leaves and stems) and roots were weighted separately (expressed as grams of fresh weight per plant) with a Sartorius precision balance (Acculab, Gottingen, Alemania). Next, the tissues were washed with deionized water and placed in a drying oven at 60 ºC for at least 48 hours. Once dry, the weight of the tissues was obtained (dry weight). Fresh and dry tissue samples were stored until further analysis. Water content (%) was determined using the following formula:
Water content indicates the level of water in a tissue, namely, the hydric condition of the plant.
Analysis of minerals
Determination of nutrient mineral concentrations (K, Mg, Ca, P, Mn, Zn, Fe, and B) was done from dry grounded leaves by inductively coupled plasma spectroscopy (ICP, Iris Intrepid II, Thermo Electron Corporation, Franklin, USA), prior digestion with HNO3:H2O2 (5:3 volume) in a microwave oven (CERM Mars Xpress, North Carolina, USA) with temperature rise slope reaching 200 ºC. For total C and N, a C/N Thermo Finnigan elemental analyser was used (Milan, Italy). Additionally, anion concentration (F-, Cl-, NO2-, Br-, NO3- PO4-3 and SO4-2) and ammonium (NH4+) were determined in the drainage collected throughout the experimental period using the abovementioned spectrometer (ICP) and high-performance liquid chromatography.
Experimental design and statistical analysis
This is a unifactorial design experimental study based on three fertilization treatments. For the statistical analysis, analysis of variance (ANOVA) was first applied. For significant ANOVA, Duncan´s test was used to determine specific differences between pairs of means. For each treatment, four experimental replicates were done with four plants per replica. Statistical analysis was carried out using the IBM SPSS statistics v24 software (Armonk, New York, United States).
Results
Concentration of nutrients in plant drainage
We determined the mineral content of the drainage samples collected every month during the study. Figure 1 shows NO2-, NO3-, and NH4+ concentrations. No clear evolution of nitrate was seen throughout the experiment. However, when we performed the analysis of the data using the means of all samples for each treatment, concentrations increased as follows: NO3-/NH4+ ratio 1:1 (1,053 mg/L) > 3:1 (725 mg/L) > 1:1 + NI (646 mg/L). Differences in ammonium levels in the drainage were observed at the end of the experiment. Based on the treatment, significant increases were seen in the following order 1:1 + NI > 1:1 > 3:1 (Figure 1).
Figure 2 shows Ca2+, K+, and PO4-3 values per month and treatment. There was no clear trend throughout the experiment with regard to the sampling month, except for K and P, which increased as the experiment progressed, although decreases in P were detected from May on. Again, when the analysis was done with the means for all dates and treatment types, we observed higher Ca+2 (596 mg/L), K+ (258 mg/L), and Mg+2 (400 mg/L) concentrations in drainages from Treatment NO3-/NH4+ ratio 1:1, although Ca and K were similar to those determined for Treatment 1:1 + NI (Ca+2 = 606 mg/L; K+ = 227 mg/L).
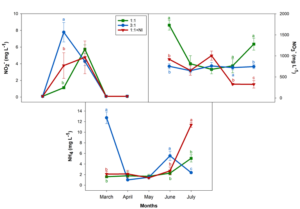
Figure 1: Plant drainage NO2-, NO3-, and NH4+ (mg L-1) levels in greenhouse-grown almond plants between March and July 2021. For significant ANOVA results, Duncan´s test was performed; lowercase letters denote significant differences among the treatments for each sampling date. Vertical bars indicate the standard error of the mean (n=4).
Figure 2: Plant drainage Ca, K, and PO43- (mg L-1) levels in greenhouse-grown almond plants between March and July 2021. For significant ANOVA results, Duncan´s test was performed; lowercase letters denote significant differences among the treatments for each sampling date. Vertical bars indicate the standard error of the mean (n=4).
Growth parameters
The tested treatments significantly affected vegetative growth regarding total biomass, and leaf and branch biomass; no effects were seen in almond plant roots (Figure 3). Highest total biomass for aerial parts was seen for plants treated with NO3-/NH4+ ratio 1:1 + NI (DMPSA); this is because both leaf and branch biomasses were greater than that of plants irrigated with the other treatment solutions. In average, plants treated with 1:1 + NI increased total biomass in 25%, leaf biomass in 33%, and stem biomass in 29%. Significant inter-treatment differences were also found with 1:1 versus 3:1 solutions in total leaf biomass; vegetative growth was higher with the 1:1 treatment solution. Aerial part:root ratio significantly increased in plants irrigated with the 1:1 + NI solution in comparison with the other two treatments (Table 1).
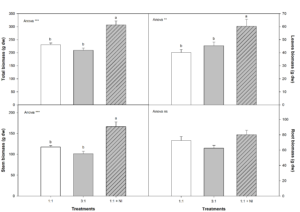
Figure 3: Leaf, stem, and root dry weight and total biomass growth parameters for almond plants irrigated with one of three possible treatment solutions (NO3-/NH4+ ratio 1:1, 3:1, or 1:1 + NI)*. In ANOVA: ‘ns’ indicates non-significant difference for a 95% confidence interval; ** and *** denote significant differences for P < 0.01 and 0.001, respectively. For each variable, lowercase letters denote significant differences between the treatments, established by Duncan's multiple range test (n= 4).
Table 1: Growth parameters (PA:R rate, height, and stem diameter) measured in greenhouse-grown Avijor almond plants irrigated with the one of three possible treatment solution (NO3-/NH4+ ratio 1:1, 3:1, or 1:1 + NI).
Treatment
PA:R ratio
Height (cm)
Stem diameter (mm)
NO3-/NH4+ ratio 1:1
2.24 b
84.5
18.7
NO3-/NH4+ ratio 3:1
2.40 b
82.8
17.5
NO3-/NH4+ ratio 1:1 + NI
2.89 a
76.6
17.9
ANOVA
*
ns
ns
In ANOVA: ‘ns’ indicates a non-significant difference for a 95% confidence interval and * significant differences for P < 0.05. For each variable, lowercase letters denote significant inter-treatment differences, established by Duncan's multiple range test (n= 4).
Physiological parameters
No significant inter-treatment differences were observed for any of the parameters analysed. Mean values for ACO2, gs, Ci and Ca were 2.6 µmol m-2s-1, 41 mmol m-2 s-1, 0.64 (nondimensional), and 3.36 (µmol CO2 mmol-1 H2O), respectively. Regarding chlorophyll fluorescence SPAD, Fv’/Fm’, Pq, and, PSII units were measured. No significant differences were found between the different treatments (ANOVA) (means of 0.78, 0.89, and 0.7 for NO3-/NH4+ ratio 1:1, 3:1, and 1:1 + NI, respectively). SPAD units were higher with treatments solutions 3:1 and 1:1 + NI in comparison to treatment solution 1:1 (Figure 4, Table 2).

Figure 4: Gaseous exchange parameters: (1) Net rate of photosynthetic CO2 = ACO2 assimilation, 2) Stomatal conductance = gs, 3) Substomatal and environmental CO2 relationship = CiCa, and physiological water use efficiency = pWUE), measured in greenhouse-grown plants cultivated with one of three possible treatment solutions (NO3-/NH4+ ratio 1:1, 3:1, or 1:1 + NI). In ANOVA: ‘ns’ indicates a non-significant difference for a 95% confidence interval; * and ** denote significant differences for P < 0.05 and 0.01, respectively. For each variable, lowercase letters denote significant inter-treatment differences, established by Duncan's multiple range test (n= 4).
Table 2: Chlorophyll concentration (measured in mean leaves). Chlorophyll fluorescence parameters (1) Efficiency of the antennas = Fv’/Fm’, 2) photochemical quenching = Pq, and 3) PSII photochemical efficiency = FPSII), measured in greenhouse-grown plants treated with one of three possible treatment solutions (NO3-/NH4+ ratio 1:1, 3:1, or 1:1 + NI).
Treatment
Chl (SPAD)
Fv’/Fm’
Pg
FPSII
NO3-/NH4+ ratio 1:1
31.1 a
0.779
0.911
0.710
NO3-/NH4+ ratio 3:1
36.0 b
0.780
0.891
0.695
NO3-/NH4+ ratio 1:1 + NI
36.0 b
0.778
0.879
0.684
ANOVA
*
ns
ns
ns
In ANOVA: ‘ns’ indicates a significant difference for a 95% confidence interval (n= 4).
Analysis of minerals in leaves and roots
Regarding the nutritional status of the leaves, significant inter-treatment differences for K and N were found after five months of irrigation with the treatment solutions (Table 3). Highest accumulation of N and K was seen in the leaves of plants irrigated with treatment solution NO3-/NH4+ ratio 1:1 + NI (N = 2.71 g 100 g-1 of dry weight and K = 2.18 g 100 g-1 of dry weight), although these values were non-significant for treatment solutions 3:1 and 1:1.
Significant differences were determined in Cu levels; in plants irrigated with treatment solution 3:1 the concentration of this micronutrient was higher in comparison with treatment solutions 1:1 and 1:1 + NI (Table 3). On the other hand, no significant inter-treatment differences were observed in the concentrations of root macro and micronutrients (Table 4), except for P and N, which were higher with treatment solution 3:1.
Table 3: Macronutrient (g 100 g-1 dry weight) and micronutrient (ppm) concentrations in greenhouse-grown Avijor almond leaves irrigated with one of the three possible treatment solutions (NO3-/NH4+ ratio 1:1, 3:1, or 1:1 + NI)
Macronutrients (g 100 g-1 dry weight)
Treatment
Ca
K
Mg
Na
P
N
C
NO3-/NH4+ ratio 1:1
1.60
2.11 a
0.548
0.140
0.271
2.51 b
43.8
NO3-/NH4+ ratio 3:1
1.71
1.93 b
0.565
0.138
0.295
2.68 a
44.0
NO3-/NH4+ ratio 1:1 + NI
1.66
2.18 a
0.538
0.123
0.295
2.71 a
43.9
ANOVA
ns
*
ns
ns
ns
***
ns
Micronutrients (ppm)
Treatment
B
Cu
Fe
Mn
Zn
NO3-/NH4+ ratio 1:1
21.4
4.35 b
53.5
71.4 b
17.4
NO3-/NH4+ ratio 3:1
23.0
5.91 a
56.1
80.3 a
19.4
NO3-/NH4+ ratio 1:1 + NI
19.2
4.89 b
51.5
84.8 a
20.6
ANOVA
ns
**
ns
***
ns
In ANOVA: ‘ns’ indicates a non-significant difference for a 95% confidence interval; *, ** , and *** depict significant differences for P < 0.05, 0.01, and 0.001. For each variable, lowercase letters denote significant inter-treatment differences, established by Duncan's multiple range test (n= 4).
Table 4: Macronutrient (g 100 g-1 dry weight) and micronutrients (ppm) concentrations in greenhouse-grown Avijor almond roots irrigated with one of the three possible treatment solutions (NO3-/NH4+ ratio 1:1, 3:1, or 1:1 + NI).
Macronutrients (g 100 g-1 dry weight)
Treatment
Ca
K
Mg
Na
P
N
C
NO3-/NH4+ ratio 1:1
1.15
0.633
0.219
0.094
0.238 b
1.70 b
47.0
NO3-/NH4+ ratio 3:1
1.15
0.633
0.208
0.090
0.302 a
1.84 a
46.5
NO3-/NH4+ ratio 1:1 + NI
1.06
0.629
0.201
0.092
0.268 b
1.71 b
46.8
ANOVA
ns
ns
ns
ns
***
*
ns
Micronutrients (ppm)
Treatment
B
Cu
Fe
Mn
Zn
NO3-/NH4+ ratio 1:1
30.3
22.4
818.4
78.7
25.4
NO3-/NH4+ ratio 3:1
32.1
25.1
781.4
93.4
30.7
NO3-/NH4+ ratio 1:1 + NI
28.2
23.8
732.5
84.1
27.6
ANOVA
ns
ns
ns
ns
ns
In ANOVA: ‘ns’ indicates non-significant differences for a 95% confidence interval
Discussion
In this study, we compare the physiological and vegetative behaviour of Avijor almond plants treated by fertigation with three nutrient solutions. The solutions have different NO3-:NH4+ rate and include or not DMPSA. We conclude that this NI has beneficial effects on almond plants and the environment. Thus, introduce DMPSA in almond orchards may be a good strategy.
The analysis of drainage chemical composition shows NI reduces the level of nitrates in leachates, limiting their discharge into the environment. During the experiment, we irrigated the trees with 0.6 L of the nutrient solution and obtained 15% of drainage from that volume. Total drainage volume obtained during the four months of experimentation for each of the treatments was around 14.4 L. This implies that the amount of leached nitrate is 14.7 g, 10.44 g, and, 9.3 g for treatments solutions NO3-/NH4+ ratio 1:1, 3:1, and, 1:1 + NI, respectively. Based on this estimate, this is, considering the irrigation supply of nitrates to the plant and nitrates leached in the drainage, we confirm the NI reduces nitrate lixiviation in 11% and 37% with standard 3:1 fertilization or with nutrient solutions without NI.
We used the same N concentration in the three treatment solutions tested (NO3-/NH4+ ratio 1:1, 3:1, and, 1:1 + NI) and ammonium concentration was similar in the case of treatment solutions 1:1 and 1:1 + NI. Based on this, our results seem to indicate less degradation of ammonium to nitrate with the presence of the NI, as shown by lower nitrate values for treatment solution 1:1 + NI in comparison with solutions 1:1 and 3:1, and higher levels of ammonium in plant irrigated with the 1:1 + NI solution. In an in vitro study, Cui and others incubated soil samples under different conditions [16]. The authors showed that the use of a combination of NIs nitrapyrin (CP) + 3,4-dimethylepyrazole phosphate (DMPP) or CP + dicyandiamide (DCD) on a soil similar to the one used in our experiments (high pH and low in organic matter), also reduced nitrate concentration and the nitrification rate, and increased ammonium content. However, for acid soils with high inorganic matter, the best NI combination was CP + DCD. These results show that to have low nitrification rate using inhibitors, it is necessary to optimize their use considering the soil´s characteristics. Besides soil´s pH, soil organic matter and soil texture also affect the effectiveness and persistence of NIs [17-19]. Moreover, although in our study no measurements were taken of N2O emissions to the atmosphere, their reduction is another beneficial effect associated to the use of NIs in agriculture. Recio and others [20] observed that the use of the NI DMPSA reduced N2O emissions in 73% in a wheat crop.
Besides the environments benefits derived from the use of an NI, another positive effect is the stimulation of vegetative growth. Leaf and stem biomass is higher in plants treated with the NO3-/NH4+ ratio 1:1 + NI solution (Figure 3) in comparison with plants treated with 1:1 and 3:1 solutions. The differences in vegetative growth are not due to differences in plant nutritional status or changes in ACO2 or WUE physiological parameters, as we hardly observe inter-group differences in these variables (Figure 4). Ferrández and others established a range of normality for almond tree leaf N concentration of 1.84% to 2.63%. Thus, in our study, N values are within the range of normality -not a limiting factor-, same as for the rest of nutrients. For almond trees, higher concentrations of ammonium in the soil (1:1 + NI) may have favoured more efficient CO2 assimilation by the plants in the form of biomass, with the consequent stimulation of vegetative growth, which may explain inter-treatment differences. For the rest of the treatments, the respiration rate of the plants may have increased, thus loosing effectiveness for incorporating CO2 to their biomass. Moreover, plants treated with solution 1:1 + NI absorb the highest amounts on N from the soil, confirmed by highest leaf N concentration (mg/kg) and leaf and stem biomass. Thus, treatment solution 1:1 + NI proved to be the most efficient when it comes to plant N absorption. Its effectiveness in comparison with the other two tested solutions may be due to ammonium absorption by the roots and its assimilation to glutamine without previous conversion from nitrate to ammonium [4].
As in horticultural plants, some tree species prefer nitrate to ammonium, while the contrary occurs in other species. In a study by Yan and Ma [21], the authors found that Cunninghamia lanceolata prefers NO3− over NH4+, while Pinus massoniana and Schima superb prefer NH4+ a NO3−. Studies on the preferences of agronomic crops by nitrate of ammonium, will be of help towards effectively managing N fertilization and use of NIs, regarding both crop performance and environmental issues. A meta-analysis by Yan and others [22] revealed increased growth in all seed plants with NH4+ than with NO3-, and that this positive effects mainly occur in trees and forbs but not in grass, which responded marginally less to NH4+ than to nitrate.
To the best of our knowledge, to date no studies have analysed the preferences of almond trees for nitrate or ammonium. Further studies should confirm our findings.
Conclusions
DMPSA applied by fertigation has several beneficial effects, such as lower lixiviation to the environment and greater vegetative growth of Avijon almond plants. Further works should clarify the effect nitrate:ammonium rate has on plant physiology and the mechanism of action of NIs in soils when designing nitrogen fertilization programs to avoid nitrate lixiviation and benefit the crops.
Acknowledgements
CEBAS and UMH researchers would like to express their gratitude to EuroChem Agro Iberia for funding this study, as well as for the supply of the fertilizers and the NI DMPSA.
References
- Rütting T, Aronsson H, Delin S (2018) Efficient use of nitrogen in agriculture. Nutr Cycl Agroecosyst, 110:1-5.
- Asociación Nacional de Fabricantes de Fertilizantes (2022) Información sectorial. Evolución del consumo. Madrid Spain.
- MAPA (2021).
- Hachiya T, Sakakibara H (2017) Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. Journal of Experimental Botany, 68, Issue 10, 1 May 2017:2501-2512.
- Kanter DR (2018) Nitrogen pollution: a key building block for addressing climate change. Climatic Change, 147:11-21.
- Norton J, Ouyang Y (2019) Controls and Adaptive Management of Nitrification in Agricultural Soils. Frontiers in Microbiology, 10.
- McCarty GW (1999) Modes of action of nitrification inhibitors. Fertil. Soils, 29:1-9.
- Huérfano X, Fuertes T, Fernández K, Estavillo JM, González C, et al. (2016) The new nitrification inhibitor 3,4-dimethylpyrazole succinic (DMPSA) as an alternative to DMPP for reducing N2O emissions from wheat crops under humid Mediterranean conditions. European Journal of Agronomy, 80:78-87.
- Burzaco JP, Ciampitti IA, Vyn TJ (2014) Nitrapyrin impacts on maize yield and nitrogen use efficiency with spring-applied nitrogen: field studies vs. meta-analysis comparison. J, 106:753-760.
- Qiao CL, Liu LL, Hu SJ, Compton JE, Greaver TL, et al. (2015) How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Change Biol, 21:1249-1257.
- Thapa R, Chatterjee A, Awale R, McGranahan DA, Daigh A (2016) Effect of enhanced efficiency fertilizers on nitrous oxide emissions and crop yields: a meta-analysis. Soil Sci. Soc. Am. J, 80:1121-1134.
- Abalos D, Jeffery S, Sanz-Cobena A, Guardia G, Vallejo A (2014) Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Ecosyst. Environ, 189:136-144.
- Menéndez S, Barrena I, Setien I, González-Murua C, Estavillo JM (2012) Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biol. Biochem, 53:82-89.
- Guardia G, Cangani MT, Sanz-Cobena A, Junior JL, Vallejo A (2017) Management of pig manure to mitigate NO and yield-scaled N2O emissions in an irrigated mediterranean crop. Ecosyst. Environ, 238:55-66.
- Food and Agriculture Organization. (2021) Data on agricultural production.
- Cui L, Li D, Wu Z, Xue Y, Xiao F, et al. (2021) Effects of Nitrification Inhibitors on Soil Nitrification and Ammonia Volatilization in Three Soils with Different pH. Agronomy, 11:1674.
- Lu YF, Zhang XN, Jiang JF, Kronzucker HJ, Shen WS, et al. (2019) Effects of the biological nitrification inhibitor 1,9-decanediol on nitrification and ammonia oxidizers in three agricultural soils. Soil Biol. Biochem, 129:48-59.
- Zhou ZF, Zhang ZY, Wang MX, Liu YM, Dai JS (2018) Effect of the nitrification inhibitor (3,4-dimethylpyrazole phosphate) on the activities and abundances of ammonia-oxidizers and denitrifiers in a phenanthrene polluted and waterlogged soil. Environ. Safe, 161:474-481.
- Li YY, Chapman SJ, Nicol GW, Yao HY (2018) Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem, 116:290-301.
- Recio J, Álvarez JM, Rodríguez M, Vallejo A (2019) Nitrification inhibitor DMPSA mitigated N2O emission and promoted NO sink in rainfed wheat. Environmental Pollution, 245:199-207.
- Yan X, Ma X (2021) Responses of root morphology and seedling growth in three tree species to heterogeneous supplies of ammonium and nitrate. Forest Ecology and Management,
- Yan L, Xu X, Xia J (2019) Different impacts of external ammonium and nitrate addition on plant growth in terrestrial ecosystems: A meta-analysis. Sci Total Environ. 686:1010-1018.