Publication Information
ISSN 2691-8803
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Medroxyprogesterone Acetate and Ethodolac Exposure durıng Pregnancy
Elif ONAT1*, Gülistan TÜRKER2
1Department of Medical Pharmacology and Clinical Pharmacology, School of Medicine, Adıyaman University, Adıyaman, 02040, Turkey
2Department of Medical Pathology, Firat University Hospital, Elazığ, 23119, Turkey
Received Date: December 01, 2022; Accepted Date: December 13, 2022; Published Date: December 20, 2022;
*Corresponding author: Assistant Professor ELİF ONAT. Department of Medical Pharmacology and Clinical Pharmacology, School of Medicine, Adıyaman University, Adıyaman, 02040, Turkey. Email: eonat@adiyaman.edu.tr
Citation: ONAT E, TÜRKER G (2022) Medroxyprogesterone Acetate and Ethodolac Exposure durıng Pregnancy. Adv Pub Health Com Trop Med: APCTM-171.
DOI: 10.37722/APHCTM.2022603
Abstract
This case is a report describing the development of omphalocele in the fetus as a result of the use of medroxyprogersterone acetate (MPA) and etodolac during pregnancy. The 25-year-old female patient used MPA for 12 days from the 3rd week of pregnancy and for 12 days from the 5th week of pregnancy. At the same time, the patient used etodolac for 5 days while her pregnancy was 3 weeks and 5 days old. The patient was informed by us that MPA is the US Food and Drug Administration (FDA) pregnancy risk category X. Therefore, it was recommended to terminate the pregnancy of the patient by the Gynecology and Obstetrics Clinic.It was observed that omphalocele developed in the fetus in the curettage material taken. Although firm conclusions cannot be drawn, this case report may add to existing data on the use of MPA and etodolac in pregnancy.
Introduction
Children of mothers exposed to MPA tablets during the first trimester of pregnancy may have an increased risk of hypospadias, clitoral enlargement and labial fusion. Studies in animals have shown that progestogens, including MPA, can have an adverse effect on the developing fetus, including teratogenicity and fetotoxicity. Other animal studies have shown that high doses of progestogens can cause masculinization of the female fetus. A significant increase in polysyndactyly and chromosomal abnormalities was observed in infants of those using the injection, this is most pronounced in women under 30 years of age. Babies born as a result of unwanted pregnancies occurring 1-2 months after injection with this drug may have an increased risk of low birth weight and neonatal death (1-17).
A registry-based study from Israel analyzed 265 pregnancies exposed to etodolac and observed no increased risk of major malformations. In animal studies, skeletal anomalies (eg polydactyly, oligodactyly, syndactyly, non-ossified phalanges, and synostosis of metatarsals) have been observed, but a direct drug or dose response relationship has not been established. Nonsteroidal anti-inflammatory drugs (NSAIDs) can impair women’s fertility; Discontinuation of NSAID therapy should be considered in women who have difficulty conceiving or are investigated for infertility. Use of the NSAID before 20 weeks of pregnancy should be based on a benefit-risk assessment. Medical literature has reported low levels of amniotic fluid with NSAID use for periods ranging from 48 hours to several weeks. Complications of prolonged oligohydramnios include extremity contractures and delayed lung maturation (18-21).
When the current literature on the use of MPA and etodolac in pregnancy is examined, it is seen that this case report is a human pregnancy outcome data showing that developed omphalocele in the fetus as a result of exposure to these drugs in the first trimester of pregnancy.
Case Report
The 25-year-old female patient with an unplanned pregnancy and a history of two live births used MPA for 12 days starting from the 3rd gestational week and 12 days from the 5th gestational week. At the same time, the patient used etodolac for 5 days while her pregnancy was 3 weeks and 5 days old. As a result of the literature review of the patient who was referred to us from the Gynecology and Obstetrics Clinic, it was found that MPA 6was FDA pregnancy risk category X and etodolac was FDA pregnancy risk category C. Curettage was recommended to the patient by the Gynecology and Obstetrics Clinic due to the possible risks of MPA on pregnancy. It was observed that omphalocele developed in the fetus in the curettage material taken. The pathology report of the patient is presented below.
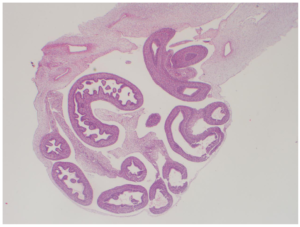
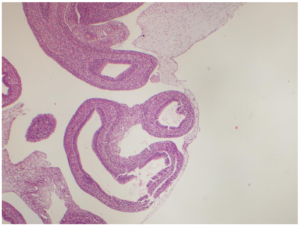
Pathology Report
- Macroscope:
Fetus
The fetus weighing 11 grams, with a head-rectum length of 3.2 cm, anus-heel length of 1 cm, a heel-toe length of 0.5 cm, an umbilical cord 7.5 cm in length and 0.8 cm in width was detected with formol. According to the external genital organ, it was observed that the organs were herniated on the umbilical cord and within a 0.5 cm diameter sac. It was observed that the skull was open from the frontal region and neural structures were protruding from this opening. It is a fetus whose gender cannot be determined and whose lower and upper extremities are in normal appearance and mouth opening is formed. Nose and rectal opening could not be clearly identified. A 0.5 cm hematoma area was observed on the umbilical cord.
Placenta
Placenta material with formol fixation and a weight of 30 gr and a size of 6.5×4.5×2.2 cm, membranous on one side and spongy surface on one side was seen. When the gray-maroon colored placenta was examined with serial sections, bleeding areas were seen.
Endometrial curettage
Samples of approximately 60 cc of bloody curette material with formol fixation were followed.
- Diagnosis:
Fetus
- -Immature Organ Findings
- -Akrania
- -Omphalocele
- -A fetus compatible with the 9-10 .maturation week and the gender is not determined
- -Umbilical cord; 2 arteries, 1 vein
Plasenta
- Villoz-perivillous fibrin accumulation.
Endometrıum, Curetage
- Degenere, necrotic, inflammatory decidua.
Discussion
MPA is the methylacetoxy derivative of progesterone. It has an indirect and also a direct antiestrogenic effect due to the inhibition of gonadotropin secretion. It also shows antiandrogenic activity. Progestins, MPA, and megestrol acetate are used in high doses to treat endometrial cancer and metastastic breast cancer. Virilization of the genitals of female babies whose mothers were treated with other synthetic progestins during pregnancy has been repeatedly reported (22,23) and has been described at least twice after maternal MPA treatment during pregnancy (24,25). There have been occasional reports of hypospadias in boys whose mothers were treated with synthetic progestins during pregnancy (22,23). This was reported three times after maternal MPA treatment (26,27,28) and after high-dose treatment with megestrol acetate (29). Adverse effects of intrauterine exposure on fertility in adulthood have not been observed so far. According to large long-term studies with medroxyprogesterone depot preparations, development towards adolescence seems age appropriate (30).
The anti-inflammatory activity of NSAIDs is based on inhibition of the cyclooxygenase (COX) enzyme, resulting in inhibition of prostaglandin synthesis. Etodolac is an indolecetic acid derivative. Although it has been suggested that it is not superior to other indollacetic acid derivative drugs in terms of efficacy and side effects, it has been found that it inhibits COX-2 highly selectively, therefore it does not impair the gastric mucosa and inhibit platelet function. A registry-based study from Israel analyzed 265 pregnancies exposed to etodolac and no increased risk of major malformations was observed (31). The same study included 128 exposures of indomethacin without the risk of major malformation. There are no other studies on the first trimester; however, there are no reports indicating the risk of malformations associated with the use of etodolac or indomethacin.
To our knowledge, this case is the first human report on the development of omphalocele in the fetus due to the use of MPA and etodolac in the first trimester of pregnancy. It is always difficult to establish causation and draw conclusions from case reports, especially when assessing drug exposure during pregnancy. However, this case may be considered as a contribution to the limited safety data available from epidemiological studies.
Conflict of Interests: The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Pardthaisong T, Yenchit C, Gray R “The long-term growth and development of children exposed to Depo- Provera during pregnancy or lactation.” Contraception 45 (1992): 313-24.
- Jaffe B, Shye D, Harlap S, Baras M, Lieblich A “Aggression, physical activity levels and sex role identity in teenagers exposed in utero to MPA.” Contraception 40 (1989): 351-63.
- Resseguie LJ, Hick JF, Bruen JA, Noller KL, O’Fallon WM, Kurland LT “Congenital malformations among offspring exposed in utero to progestins, Olmsted County, Minnesota, 1936-1974.” Fertil Steril 43 (1985): 514-9.
- “Product Information. Provera (medroxyprogesterone).” Pharmacia and Upjohn, Kalamazoo, MI.
- Katz Z, Lancet M, Skornik J, Chemke J, Mogilner BM, Klinberg M “Teratogenicity of progestogens given during the first trimester of pregnancy.” Obstet Gynecol 65 (1985): 775-80.
- Jaffe B, Harlap S, Baras M, Gordon L, Lieblich A, Magidor S, Sanchez M “Long-term effects of MPA on human progeny: intellectual development.” Contraception 37 (1988): 607-19.
- Heinonen O, Slone D, Shapiro S; Kaufman DW ed. “Birth Defects and Drugs in Pregnancy.” Littleton, MA: Publishing Sciences Group, Inc. (1977): 297.
- Pardthaisong T, Gray RH “In utero exposure to steroid contraceptives and outcome of pregnancy” Am J Epidemiol 134 (1991): 795-803.
- “Product Information. depo-subQ provera 104 (medroxyPROGESTERone (medroxyprogesterone)).” Pfizer U.S. Pharmaceuticals Group, New York, NY.
- Yovich JL, Turner SR, Draper R “Medroxyprogesterone acetate therapy in early pregnancy has no apparent fetal effects.” Teratology 38 (1988): 135-44.
- Jordan A “Toxicology of depot medroxyprogesterone acetate.” Contraception 49 (1994): 189-201.
- “Product Information. MedroxyPROGESTERone Acetate (medroxyPROGESTERone (medroxyprogesterone)).” Greenstone LLC, Peapack, NJ.
- Gray RH, Pardthaisong T “In utero exposure to steroid contraceptives and survival during infancy” Am J Epidemiol 134 (1991): 804-11.
- Schwallie PC “The effect of depot-medroxyprogesterone acetate on the fetus and nursing infant: a review.” Contraception 23 (1981): 375-86.
- Cerner Multum, Inc. “Australian Product Information.” O.
- “Product Information. Depo-Provera (medroxyprogesterone).” Pharmacia and Upjohn, Kalamazoo, MI.
- Cerner Multum, Inc. “UK Summary of Product Characteristics.” O.
- US Food and Drug Administration “FDA recommends avoiding use of NSAIDs in pregnancy at 20 weeks or later because they can result in low amniotic fluid.
- “Product Information. Etodolac ER (etodolac).” Taro Pharmaceuticals U.S.A. Inc, Hawthorne, NY.
- “Product Information. Lodine (etodolac).” Wyeth-Ayerst Laboratories, Philadelphia, PA.
- Cerner Multum, Inc. “UK Summary of Product Characteristics.” O 0
- Schardein JL. Congenital abnormalities and hormones during pregnancy: a clinical review. Teratology 1980; 22: 251-70.
- Schardein JL. Chemically Induced Birth Defects, 3rd edn, New York: Marcel Dekker 2000.
- Burstein R, Wasserman HC. The effect of Provera on the fetus. Obstet Gynecol 1964; 23: 931-4.
- Eichner E. The clinical uses of Provest. Int J Fertil 1963; 8: 673-80.
- Harlap S, Prywes R, Davies AM. Birth defects and oestrogens and progesterones in pregnancy.Lancet 1975; 1: 682-3.
- Aarskog D. Clinical and cytogenetic studies in hypospadias. Acta Paediatr Scand 1970; 203:7-62.
- Goldman AS, Bongiovanni AM. Induced genital anomalies. Ann NY Acad Sci 1967; 142: 755-67.
- Farrar DJ, Aromin I, Uvin SC et al. Hypospadias associated with the use of high dose megestrol acetate in an HIV infected woman. Genitourin Med 1997; 73: 226.
- Pardthaisong T, Yenchit C, Gray R. The long-term growth and development of children exposed to Depo-Provera during pregnancy and lactation. Contraception 1992; 45: 313-24.
- Daniel S, Matok I, Gorodischer R et al. Major malformations following exposure to nonsteroidal antiinflammatory drugs during the first trimester of pregnancy. J Rheumatol 2012; 39: 2163-9.