Publication Information
ISSN 2692-1529
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Managing Fish Diseases of Clarias Gariepinus in Aquaculture in Nigeria
Foluke Omotayo Areola1*; Oludotun Olubusola Oladele2; Oladele Ige Osanyinlusi 3; Olasupo Michael Alatise4
1Department of Fisheries, Lagos State University.
2Faculty of Veterinary Medicine, Department of Veterinary Medicine, Surgery & Radiology, University of Jos.
3Department of Agricultural Economics, University of Ibadan.
4Layolat Fish Farm, Kaduna.
Received Date: December 14, 2023; Accepted Date: December 25, 2023; Published Date: January 11, 2024.
*Corresponding author: Foluke Omotayo Areola, Department of Fisheries, Lagos State University. Phone No.: +2348033205882, Email: foareola@gmail.com
Citation: Areola FO, Oladele OO, Osanyinlusi OI, Alatise OM [2024] Managing Fish Diseases of Clarias Gariepinus in Aquaculture in Nigeria. Jr Aqua Mar Bio Eco: JAMBE-125.
DOI: 10.37722/JAMBE.2024203
Abstract
Fish diseases are as old as the beginning of Clarias gariepinus aquaculture in Nigeria. It did not pose so much threat at that time. The proliferation of fish farms by private investors, after the New Partnership for African Development (NEPAD) Fish for all Summit in 2005, the intensive culture of fish, and increase in the production of Clarias spp. brought its diseases to the fore. The lack of proper farm management practices which included slack of strict observance to Hazard Analysis of Critical Control Points [HACCP], led to an upsurge in disease occurrences. Investors were quick to seek solutions to safeguard their investments, hence the use of antibiotics and chemicals such as malachite green, potassium permanganate and copper sulphate. The identification, treatment, and documentation of fish diseases by Veterinary doctors working with fisheries officers commenced, and awareness was created amongst fish farmers. This led to the gradual establishment of a disease reporting system. It was found that the disease occurrence was more because of poor farm management especially water management, unconscious use of adulterated feeds and poor genetic fish strains which made the fish easily susceptible to infections. FAO had recommended the use of salt in Clarias gariepinus fish farming and identified the chief cause of fish diseases as Aeromonas hydrophila. A severe attack by Aeromonas hydrophila thrives in heavily polluted water. The bacterium has been identified as the single most causative factor of mortality in aquaculture, and it dies at 1.5% saline solution. The preventive and curative use of saline solution for the control of fish diseases depend on the percentage saline solution from 0.5% to 2%; exposure time of some seconds to 60 minutes; the size of the fish from fry to adult fish; and the degree of infection. This helps control bacterial, fungal and some parasitic diseases in Clarias spp.
Keywords: Aquaculture, fish disease, management, saline solution.
Introduction
Aquaculture is one of fastest growing food industries in the world and fish is an important source of animal protein in many countries of the world. In 2014, FAO [1] reported that per capita consumption of fish has been increasing from an average of 9.9 kg in the 1960s to 19.2 kg in 2012. One of the fish species that has been widely cultured in African countries is African catfish. Clarias gariepinus [African catfish] is a notable fish species in Nigeria with unique characteristics of fast growth, resistance to disease and handling stress. It has also been reported by FAO [2] that Nigeria is the world’s largest producer of African catfish. It represents 90% of Nigeria’s fish farming. While fish makes up 40% of animal protein intake in Nigeria, 10% of it is from catfish [2]. Its farming has gained prominence due to its contributions to livelihood sustenance and protein supply [3]. However, African catfish farmers may suffer loss in their bid to make a living from commercial catfish production if the issue of disease is ignored.
Introduction and spread of infectious diseases of fish limit aquaculture productivity which compromise nutrition security and fish farming livelihoods in Asia and Africa [4]. Disease is defined as any abnormality in structure or function displayed by living organisms through a specific or non-specific sign [symptom] [5]. A disease can be regarded as non-infectious or infectious. The latter simply refers to an infection by a pathogenic organism that results in disease. Other non-infectious factors such as nutrition, environment, and genetics, can cause a disease [or abnormal] condition [6]. Irrespective of the aquatic species that an aquaculture venture produces, there are significant risks to success due to the constant threats of a disease outbreak [6]. It can cause serious problems on fish farms which may destroy fish stocks or make products unmarketable [7].
Fish disease is a critical constraint to aquaculture development and its sustainability because it increases production cost [8]. Nutritional disease is one of the most devastating threats to aquaculture production [8]. Fish health management has become important operation for preventing outbreak of diseases and improving fish productivity. Disease develops in aquatic ecosystem when stressors cause an imbalance between host [fish], pathogen, and environment [9].
Fish diseases were classified into 6 namely: protozoan, helminth, crustacean, bacterial, fungal, and environmental/genetic related diseases [10]. Fish diseases were also classified into bacterial, viral, fungal, parasitic, nutritional, and environmental diseases [9]. In their study, some researchers [11, 12] identified bacterial, fungal, and parasitic as common sources of fish diseases. Also, other researchers [13] in their study of parasitic infestations on African catfish in Nigeria found that four parasitic species were encountered including 2 protozoans [Ichthyophthirius multifilis, Trichodina spp.] and 2 Helminthes [cestodes]. African catfish disease that is dietary related was also identified [14]. It is a broken-skull disease in African catfish which comes because of dietary deficiency of ascorbic acid. This disease is characterized by an inflammation of the skull, destruction of accessory respiratory organs and a lateral skull fracture.
Fish lives in complex environment which makes them vulnerable to diseases. In aquaculture, health management of fish is critical to sustainable production of healthy fish. Environmental and management factors such as under-feeding, poor pond environment, high stocking density, over-feeding, and use of excess additional inputs stress fish and make them more susceptible to diseases [10]. Low genetic immunity of fish seed, malnutrition [e.g. under-feeding], deteriorated water quality, biosecurity lapses, contaminated fish feed, and presence of molluscs in fishery habitat are general causes of fish diseases. Rapid changes in temperature and pH, suspended solid loads, high stocking density, insufficient dissolve oxygen, hydrogen sulphide, poor nutrition and poor handling as factors contributing to fish diseases [9]. It was shown that the reliance of fish farms on wild broodstock is another factor that predisposes aquaculture industry to diseases especially at farm level [15]. It was also reported that poorly managed aquaculture farms often face greater risks of fish disease [4]. Among the major causes of infectious disease outbreaks in fish farms are pathogenic bacteria of the genus Aeromonas [3]. The high mortality reported in Clarias gariepinus in this case [3] was caused by Aeromonas caviae. Aeromonas hydrophila thrives in heavily polluted water.
Aeromonas hydrophila is one of the 36 species in the genus Aeromonas [16]. The disease-causing bacterium Aeromonas hydrophila, is ubiquitous, present in water, soil and has even been found in bottled water in Saudi Arabia [17]. It is salt tolerant up to 1.5%, and susceptible to many antibiotics. The bacteria are present in the stomach of the fish but do not become lethal until stress comes in [18]. It enters the fish through the oral routes or wounds.
This pathogen affects all ages of fish and mortality in catfish hatchery can be up to 100%, within 3-4 days from onset of clinical signs. Affected fishes are usually seen to be lethargic, anorexic and in severe cases may be seen hanging by the side of the pond. or in lateral recumbency when seen on the floor of fish tanks. Fishes often exhibit exophthalmia, erythema and ulceration of the skin and may have distended abdomen filled with ascitic fluid. The liver may be slightly greenish or have a light chocolate color appearance with necrotic patches. The kidney often may be inflamed and friable.
Other species of Aeromonas known to be pathogenic to fish are A.veronii, A. sobria, A. piscicola, A. schubertii, A. jandaei [19]. Aeromonas sobria has been shown to be associated with a condition called Arborescent organ necrosis syndrome in African catfish [20]. Severe outbreak in catfish caused by Aeromonas caviae was also reported [3].
In farming of freshwater fish species like African catfish, the use of common salt [NaCl] has been of immense help in managing some disease conditions. Saline bath of fish has been shown to be effective against some external parasites, also through the presence of chloride ions it counters the uptake of nitrite [which is toxic], promotes formation of slime coating [which is protective], assists in healing of injuries and improves gill function [21]. It has been reported that salinity at concentration between 0.1% to 3% can induce stress on old culture of the bacterium Aeromonas hydrophila [22], hence, bringing about tendency to reduce its population in the aquatic environment. With adequate biosecurity measures in fish farms and routine use of saline bath, there will be reduced need for antimicrobials on the fishes.
Plate 1: Marked ascites [black arrow] caused by Aeromonas hydrophila in moribund catfishes.

Material and Methods
The study area is Nigeria. This study was conducted to empirically examine how diseases affecting African catfish [Clarias gariepinus] have been managed among fish farmers in Nigeria. It is a case study among African catfish farmers. The respondents were purposively selected based on the experience of the occurrence of diseases related to Aeromonas spp on their farms. We employed a qualitative approach using an Interview Guide [IG]. Individual farm owners that agreed for the interview were used for the study. This involves thematic approach was used to explain issues of discussion. Information was also sought from Veterinarians to get records which indicate incidence of confirmed disease outbreaks involving Aeromonas species.
Findings
Why the use of saline solution?
It has the capacity to kill bacteria by osmosis. Changing fresh water to saline can result in death of Aeromonas hydrophila at 1.5% concentration. Saprolegnia spp dies at NaCl concentration above 12,000μg/ml, i.e. 1.2% [23]. Saline solution has no residue, it is natural. It improves the immunity of the fish; it serves as anti-stress. FAO equally recommends its use[1]. A two percent saline is okay for sterilization of farm equipment.
Why not antibiotics?
Antibiotic is bacteria specific. It has a residue which makes smoked fish rejected in EU markets apart from poor processing.
Procedure for Saline Solution Production
One kg of salt [NaCl] is dissolved in 50 litres of water. This gives a 2% saline solution. Addition of 750 grams NaCl that is 0.75 kg in 50 liters of water is 1.5% and 500 grams of salt in 50 liters of water gives 1% saline solution. Using the biology of Clarias that can tolerate saline solution up to 5% for some two minutes, due to its good kidneys and osmoregulation and the bacteria, Aeromonas hydrophila that dies at 1.5% saline. This is the key to the salt treatment.
Treatment method
Fish tank should be washed properly using a brush. Rinse the fish until the water is clean. Prepare 2% saline solution and immerse the adult fish totally for a period of 60 minutes. This can be done for three consecutive days at 60 minutes daily. For earthen ponds, empty all the water, clear all the biological debris at the pond bottom and flush the pond well until what you get is clean water. Estimate the volume of water that can immerse all the fish, then prepare two percent solution for the treatment. This can be done for three consecutive days. After treatment, discharge the saline solution if it is dirty or you build up if not and introduce feed gradually after 45 minutes.
For breeders: Fry and Fingerling Treatment Percentage.
Use a 1.5% saline solution only for fry and fingerlings. A two percent saline solution is good enough for sterilizing equipment in the hatchery. The use of copper sulfate, formaldehyde, potassium permanganate, chloroform, malachite green etc., are not without major risks. Unknown to many breeders, some of these chemicals are injurious at 100 parts per million. It is advisable that all breeders should prepare qualitatively 1, 1.5%, and 2% saline solution for use in their facility. Care must be taken as salts are of different salinity. Table salt is of higher salinity than industrial. However, the difference is not so significant, but it exists.
For fingerlings and fry, once in three-/four-day fingerling treatment. Fingerlings have not fully developed the arborescent organ, that is, the false lungs by which they can breathe when out of water, so they remain in water all through the treatment. Carefully wash tanks. They will swim away from brush stroke. Maintain the steady flow through until the water is clean. Wash the tank thoroughly. For example, if 50 litres are enough to immerse the fingerlings, leave them in 30liters of water, dissolve 750g of salt in 20liters in a bowl, and gradually, add or sprinkle the saline solution into the tank. Immerse them in water for 20-30mins. Repeat for 3days. Introduce feed after 5 minutes. The nursery tanks, discharge pipes, etc. must be cleaned daily.
Fry treatment: This is a delicate treatment. They don't swim away from brush strokes, and they must be left inside water as there are no false lungs. Carefully and slowly discharged water, wash the areas not inhabited by the fry, allow them to occupy a small portion inside the water. Estimate the volume of water that can contain them. For example, 50 liters, leave them in 30 litres of water. Dissolve 750grams of salt into 20liters of water. Gradually add or sprinkle the water. Leave them inside for about two to five minutes. Repeat for three days. It is very important to clean tanks daily and discharge pipes also.
Prophylaxis: Giving fry and fingerlings 1.5% saline solution once in three or four days, boosts their immunity. They are hardier and are more active. Inbred fry or fingerlings cannot tolerate even 1% saline solution. Prepare a small quantity of 1.5% saline solution. Put some of your fry or fingerlings and observe. Prophylactic treatment for adult fish is once in a week.
A breakdown of how the saline bath is done for different classes of fish is shown in table 1.
Table 1: Treatment guideline of African catfish in saline water based on their stages of development.
Size
Percentage saline
Time in minutes
Period in days
Adult fish
2
60minutes
3days
Juvenile to Jumbo
2
30-40minutes
3days
Fingerling
1.5
20-30minutes
3days
Fry
1.5
2-5minutes
3 days when they are sick
Passive surveillance data from Veterinary diagnostic laboratories
The information on the common bacterial diseases diagnosed in the laboratories was provided by one of the authors who had worked in the organization. Cases were presented by fish farmers between January and December 2018. All information was obtained from the records of the laboratories. Tables 2 and 3 provide a breakdown of the information.
Results and Discussion
Note that Table 1 indicates information on the treatments of African catfish based on their stages of development. When the fish are fully immersed, and there is room for movement, if it is noticed that they are getting tired, or the foam is much before the stipulated time, the saline solution could be drained, or fresh water could be added quickly to dilute and run off the dirty water. Time of immersion is equally depended on the degree of infection observed by several sores on the adult fish. Extra care is very important.
Table 2: Cases of fish bacterial disease outbreaks diagnosed in 2018
Month
Aeromonas spp
Vibrio spp
Salmonella spp
Staphylococcus spp
Streptococcus spp
Escherichia coli
Bacillus spp
Klebsiella spp
Citrobacter spp
January
10
5
3
1
0
0
0
0
0
February
8
5
1
0
0
0
0
0
0
March
17
8
1
0
0
0
0
0
0
April
3
1
0
0
0
0
0
0
0
May
1
2
0
1
0
0
0
0
1
June
4
1
1
0
0
0
0
2
1
July
2
4
2
0
0
0
0
0
0
August
3
2
1
0
0
0
1
0
0
September
2
4
2
1
0
0
0
0
0
October
0
1
0
0
0
0
0
0
0
November
3
3
0
1
0
1
0
0
0
December
6
1
1
0
1
0
0
0
0
59
37
12
4
1
1
1
2
2
Month
Number of cases of bacterial diseases of fish
Total population of fish on all farms attended to in a month
January
19
157,000
February
14
273,000
March
26
383,800
April
4
70,000
May
5
152,000
June
9
276,250
July
8
183,900
August
7
85,000
September
9
120,250
October
1
125,000
November
8
95,000
December
9
80,000
Total
119
2,001,200
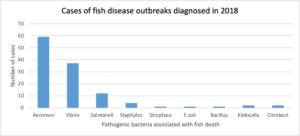
Tables 2 and 3 show data on passive surveillance done on bacterial diseases of fish in 2018 by a private Veterinary Diagnostic Laboratory [Animal care®] with offices in five locations [Ogun, Lagos, Oyo, Delta states and Abuja] in Nigeria. Cases were mainly of Catfishes like Clarias gariepinus and hybrid of Clarias and Heterobranchus spp. Out of a total of 119 cases of bacterial diseases, 59 [49.57%] were caused by Aeromonas spp. This is significant as it is almost half the total number of cases received in the laboratories.
Effect of diseases in aquaculture has strong implications on farmer’s productivity, profit [increasing cost of production], and food and nutrition security. Disease outbreak is reported to be the single-greatest challenge and limit to growth in the aquaculture industry [24]. According to a report by International Bank for Reconstruction and Development (IBRD) and World Bank [15], disease outbreaks cause an estimated $6 billion in losses for the aquaculture industry every year. Diseases, therefore, pose a major threat to aquaculture leading to about 10-15% of production cost when it occurs thereby reducing the profit level in aquaculture production [11]. The consequences of fish diseases include the loss of productivity or harvest, rejection of aquaculture products, the collapse of aquaculture ventures which threatens the sustainability of the entire industry [5]. Parasitic infections in African catfish hinder high fish productivity in farms [13]. Broken-skull disease often results in high mortality in African catfish thereby causing economic loss to farmers [14].
Various solutions have been highlighted in literature in managing fish disease. To ensure management of fish diseases, good management practices at all operational stages of aquaculture and prophylactic measures [such as maintaining water alkalinity, water depth, avoiding over feeding, etc.] must be employed since isolating diseased fish is difficult in fish management [10]. Prophylactic approach and chemical treatments are principles of fish disease control [9]. A cost-effective strategy is to develop a regional breeding center for all producers in order to eliminate the use of wild broodstock [15]. It was suggested that there should be strong national aquatic animal health policies. The use of rapid diagnostic tools for early detection of fish pathogens can help hatchery and nursery operators as well as farmers to take timely action to reduce the spread of disease [4]. It was further stated that at the farm level, implementing better management practices will significantly reduce disease-related losses.
Conclusion
The use of saline solution in prophylaxis against bacterial infection in catfish is very important. This will help in limiting the use and avoid the abuse of antibiotics which has become a norm amongst fish farmers who may be ignorant of the risk of build-up of antibiotic residues in edible tissues of fish. Another risk is that of antimicrobial resistance which is currently a global threat to both man and animals [26]. Use of saline solution with strict adherence to biosecurity measures and standard good management practices are key to maintaining a profitable fish farming business.
Acknowledgment: Special thanks to the Management of Animal care services konsult Nig. Ltd for the use of information from their company records.
References
- FAO [2014] The State of World Fisheries and Aquaculture 2014. Rome. 223 pp. http://www.fao.org/3/a-i3720e.pdf
- FAO [2023] FISH4ACP Unlocking the potential of sustainable fisheries and aquaculture in Africa, the Caribbean and the Pacific. https://www.fao.org/3/cb4127en/cb4127en.pdf
- Oladele, O. O., Ameji, N.O., Gurumyen, G. Y., Adanu, W. A., Kolade, T. T., Agbato, O. A. and Lombin, L. H. [2021] Mortality of Clarias gariepinus caused by Aeromonas caviae and nitrite toxicity in a fish farm. Sokoto Journal of Veterinary Sciences, 19[2]: 138 - 144. http://dx.doi.org/10.4314/sokjvs.v19i2.10
- Mohan C.V., Delamare-Deboutteville J, Beveridge M.C.M. and Marwaha N. [2021] Reducing disease risks in fish through better detection, management and prevention. Penang, Malaysia: CGIAR Research Program on Fish Agri-Food Systems. Program Brief: FISH-2021-07. https://digitalarchive.worldfishcenter.org/bitstream/handle/20.500.12348/4833/a4fd37f3aba7ab43f32fabc627508032.pdf?sequence3=
- Lavilla, C. R. [2011] Chapter One: Disease development. https://repository.seafdec.org.ph/bitstream/handle/10862/724/hma01-01chap.pdf;jsessionid=72C2D7027E2534A5D2D113B403CBFE83?sequence=1
- World Aquaculture Society [2022] The many challenges of disease management in aquaculture. Wiley DOI: 10.1111/jwas.12936
- Sadler, J. and Goodwin, A. [2007] Disease Prevention on Fish Farms. SRAC Publication No. 4703. https://fisheries.tamu.edu/files/2013/09/SRAC-Publication-No.-4703-Disease-Prevention-on-Fish-Farms.pdf
- Shefat, S.H.T. and Karim, M.A. [2018] Nutritional Diseases of Fish in Aquaculture and Their Management: A Review. Acta Scientific Pharmaceutical Sciences [ISSN: 2581-5423] Volume 2 Issue 12 December 2018.
- Das, P. and Das, S. S. [2015] Fish Health Management in Aquaculture. Handbook on Pig and Fish Husbandry Practices. ICAR-Central Inland Fisheries Research Institute. file:///C:/Users/user/Downloads/2015_FISH%20HEALTH_PIG-FISH.pdf
- Sector Network Natural Resources and Rural Development Asia [SNRD Asia] [2023] Fish Diseases in Aquaculture. https://snrd-asia.org/wp-content/uploads/2023/07/1.-Fish-Diseases.pdf
- Sahoo, P. K, Das, B. K. and Mishra, S.S. [2016]. Disease Management in Aquaculture. ICAR-Central Institute of Freshwater Aquaculture. https://cifa.nic.in/sites/default/files/Disease_management.pdf
- Sahoo, S. K., Kumar, R., Tiwari, P.K., Pillai, B.R. and Giri, S. S. [2018] Training manual on mass breeding and culture technique of catfishes, SAARC Agriculture Centre, Dhaka Bangladesh, xxxp.
- Udechukwu, C. U., Panda, S. M., Danladi, S. I. and Bello, F. A. [2018] Parasites associated with Clarias gariepinus [African catfish] from dam, plastic and concrete ponds in Bauchi metropolis, Bauchi State, Nigeria. GSC Biological and Pharmaceutical Sciences, 02[02], 001–005.
- Eya, J. C. [1996] Broken-Skull Disease’ in African Catfish Clarias gariepinus is Related to a Dietary Deficiency of Ascorbic Acid. Journal of the world aquaculture society, 27[4]:1-6.
- International Bank for Reconstruction and Development [IBRD] and The World Bank [2014] Reducing Disease Risk in Aquaculture. World Bank group 2014. World Bank Report Number 88257-GLB. https://documents1.worldbank.org/curated/en/110681468054563438/pdf/882570REPLACEM00NAME0Reantaso0Melba.pdf
- Fernández-Bravo A., and Figueras M. J. [2020] An update on the genus Aeromonas: taxonomy, epidemiology and pathogenicity. Microorganisms8, 1–39. doi: 10.3390/microorganisms8010129, PMID:
- Peter J.S., Mohammed A.F and Ahmed R.A. [1986] Isolation of Aeromonas hydrophila from bottled water and domestic water supplies in Saudi Arabia. Journal of food protection Vol 49 issue 6 pp 471-476.
- Kim K-T, Lee S-H, Lee K-K, Han J.E, Kwak D. [2021] Enhanced Virulence of Aeromonas hydrophilaIs Induced by Stress and Serial Passaging in Mice. Animals.; 11[2]:508. https://doi.org/10.3390/ani11020508
- Austin, B and Austin, D.A. [2007] Bacterial fish pathogens- diseases of farmed and wild fish [4th] Springer-Praxis publishing, Chichester, UK. Pp 594.
- Oladele, O.O., Olufemi. B.E., Oladosu, G.A. Ajayi, O.L., Adediji, A.A. and Arasi, I.O. [2011] Arborescent organ necrosis syndrome in Catfish Clarias gariepinus [Burchell]: A case report. Journal of fish diseases vol 34. pp 801-804
- Shirlie S. [2021] Using salt to treat diseases in freshwater Aquariums. Available online @ https://www.thesprucepets.com/using-salt-in-a-freshwater-aquarium-1378797#:~:text=Place%20five%20to%20ten%20level,for%20five%20to%2030%20minutes. Retrieved in November 2023.
- Iqbal, K. J., Md Furkanur, R. M., Angela J. H. and Sang-Do H [2015] Effect of salinity and incubation time of planktonic cells on biofilm formation, motility, exoprotease production, and quorum sensing of Aeromonas hydrophila. Food Microbiol 49:142-51. doi: 10.1016/j.fm.2015.01.016.
- Esam H.A. [2009] Antifungal activity of sodium chloride on Saprolegnia diclina and Aphanomyces Acta Mycologica Vol. 44 [1]: 125–138.
- Hennig, J. and Jain, M. [2018] Aquaculture: Disease Management. Fish 2.0 Investment Sights.
- Mohammed M.A. [2022] Antimicrobial resistance and its spread is a global threat. Antibiotics 11[8]: 1082. doi: 3390/antibiotics11081082
[1] https://www.fao.org/3/AC378E/AC378E12.htm