Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Lethal Effect of Metarhizium brunneum and Oxalic Acid on Varroa destructor Under Laboratory Conditions
Fadime Yetis1, Jennifer Han2, Nick Neager2, Walter S. Sheppard2*
1Department of Entomology, Republic of Turkey Ministry of Agriculture and Forestry Biological Control Research Institute
2Department of Entomology, Washington State University, Pullman WA 99164-6382, USA
Received Date: July 14, 2023; Accepted Date: July 21, 2023; Published Date: July 27, 2023;
*Corresponding author: Walter S. Sheppard, Department of Entomology, Washington State University, Pullman WA 99164-6382, USA. Email: shepp@wsu.edu
Citation: Yetis F, Sheppard W S, Han J, Neager N (2023) Lethal Effect of Metarhizium brunneum and Oxalic Acid on Varroa destructor Under Laboratory Conditions. Adv Agri Horti and Ento: AAHE-183.
DOI: 10.37722/AAHAE.2023201
Abstract
Honey bees, Apis mellifera L., are beneficial insects for human nutrition and agriculture. They are important crop pollinators; contributing $15-20 billion to the agricultural economy in the United States. Many factors can lead to a decline in these utilitarian insects, and Varroa destructor, an ectoparasitic mite (Anderson & Trueman), is one of the most important worldwide. This pest, feeds on fat body tissue of honey bees, and cause collapse of untreated colonies within two years. Although beekeepers have used acaricides to control these mites, they continue to face resistance problem to the chemicals. Emerging acaridae resistance in varroa mites have compelled beekeepers to explore alternative ways to control varroa mites. In this study, laboratory assays were performed to test the virulence of Metarhizium brunneum and oxalic acid to V. destructor. Different concentrations of M. brunneum (1 x 108, 1 x 107, 1 x 106, 1 x 105, and 1 x 104) and 1.5% oxalic acid were mixed and applied to V. destructor using a Potter Precision Spray Tower machine. The result indicates that mite mortality observed optimum, 90.2%, at the mixture solution with 1 x 108 concentration of M. brunneum. The mortality was similar at with mixture solutions with 1 x 107 and 1 x 106, 75.2% and 67.3 at lower mixture solutions with 1 x 105 and 1x 104 concentrations; the mite mortality recorded 40.3% and 32.7%. The mortality result was 60.5% at 1.5% oxalic acid solution without entomopathogenic fungi.
Keywords: Biologic Control; Entomopathogenic Control; Honey Bees; Metarhizium Brunneum; Oxalic Acid; Varroa Destructor
Introduction
The Varroa mite (Varroa destructor) is one of the most significant pests of Western honey bee (Apis mellifera L.) and a threat to entire beekeeping industry. Varroa mite has spread rapidly worldwide and has caused considerable damage to colonies of honey bee (Morse and Laigo, 1969). Spreading of V. destructor to Europe and North America caused agricultural losses due to a pollination crisis (Piou et al., 2016). Varroa mites feed on fat body tissues of the honey bee (Ramsey et al. 2019), and can survive on larval, pupal, and adult bees (Roth et al., 2020). The effect of varroa mites on honey bees’ population, the direct effects on young bees, and the condition as a confirmed vector of viruses show the role varroa mites on honey bees (Ramsey et al. 2019). The damage of the Varroa parasite remains worldwide (Elzen and Westervelt 2002).
The Aim of Control of Varroa destructor
There are several reasons to keep V. destructor under control. The balanced host-parasite relationship is lacking for honey bee and the mite. There is an increase in costs on regular treatments for beekeeping and risks of chemical residues in products of honey bees (Rosenkranz et al., 2010). In 2010, Guzman-Novoa suggested that if a colony untreated for V. destructor, high mite infestation could kill or severely weaken colonies entering the winter, and the presence of varroa mites in a colony was found to be the primary cause of winter deaths.
To manage V. destructor, beekeepers have used some different mechanical control methods. Removing drone brood, pollen traps, screened bottom boards, and inert dust is mechanical control methods used (Calderone, 2005). In addition, there are many commercial methods are available to control varroa mite infestation in beehives (Zhou et al., 2004). In these methods, available synthetic pyrethroids include tau-fluvalinate (Apistan®) and flumethrin (Bayvarol®). Organophosphate pesticides, such as coumaphos (CheckMite®) which is also in synthetic pyrethroid. These products are preferred because of low cost, ease of application, long-term activity, and high efficiency (Gregory et al., 2018).
Although using chemicals are cheap and effective, they cause resistance problem on varroa mites. The first report about fluvalinate-resistant was published in Italy in the 1990s (Lodesani et al. 1995) and in 1995, a resistance in varroa mites for fluvalinate was observed in southern Europe (Conte et al., 2010). In 1995, Milani reported several miticides, including fluvalinate, flumethrin, and amitraz, causing some significant problems in beekeeping. The problems included developing resistance against pesticides in varroa mites, chemical residues in honey and wax, and difficulty to effectively treat reproductive varroa mites in brood cells. Contamination of hive products, especially honey and wax with chemicals has stimulated new idea to find safer ways to control varroa mites (Damiani et al., 2009) and researchers have analyzed different non-chemical ways to control the V. destructor.
Many new applicable models and methods have been developed to manage V. destructor in the fields (Dussaubat et al., 2012). Oxalic acid, formic acid and thymol are additional compounds that have been found useful in the control of V. destructor (Aboushaara et al., 2017). As biological control agents, Hirsutella thompsonii, M. brunneum (formerly M. anisopliae) (Behle et al., 2013) (Kanha et al., 2002) and Beauvaria bassiana (Meikle et al., 2007) have been tested to control the mites. M. brunneum is one of the entomopathogenic fungi, that is wedely used in biological control programs (Jaber et al.,2017).
Using Metarhizium brunneum to Control Varroa destructor
Metarhizium, a genus of entomopathogenic fungi in the Clavicipitaceae family, has long been known for its biological control potential against arthropods (Bischoff et al. 2009). This entomopathogenic fungi can interfere through direct penetration of the cuticle of sensitive hosts with the initial and potentially decisive interaction between the fungal sport and the insect epicuticle (Ortiz-Urquiza & Keyhani, 2013). The fungi are commonly observed in nature, in the soil, in the rhizosphere of the plants, or on arthropod cadavers as a saprophyte (Schrank & Vainstein, 2010).
- brunneum is used to control many pests in agriculture (Sandhu et al., 2001). According to literature, M. brunneum was tested on Ixodes scapularis, which is commonly known as the deer tick or black-legged tick. In 2017, Fischhoff et al. used Met52®, a commercial strain of M. brunneum, to reduce the abundance of I. scapularis from Lyme disease-causing bacteria control, caused by the bite of an infected tick, and other tick-borne pathogens. Behle et al., (2013) tested the efficacy of M. brunneum microsclerotia against nymphs of Ixodes scapularis (Acari: Ixoididae). Furthermore, Behle et al. (2015) reported that Japanese beetle (Popillia japonica) Newman (Coleoptera: Scarabaeidae) larvae were susceptible to infection by M. brunneum.
Hamiduzzaman et al. (2012) reported M. brunneum is non-toxic to humans, can be mass-cultured, and occurs naturally in the environment. In 2017 Taibon et al. tested honey samples, had been used M. brunneum against V. destructor in tested same hives. The study result showed that the samples were residue-free. Therefore, M. brunneum is not harmful to the health of people.
Using Oxalic Acid to Control Varroa destructor
Treatment with oxalic acid (OA) is another control method against Varroa destructor. OA is a natural component of honey (Akyol & Yeninar, 2008). There are two formulations of oxalic acid, anhydrous (C2H2O4) (CAS # 144-62-7) and dihydrate (C2H2O4 2H2O) (CAS # 6153-56-6) (Aliano, 2008). Use of OA does not cause residue problems, and a 5% dose of this acid can be tolerated by honey bees (Rademacher and Harz, 2006, Bogdanov et al., 2002). More than 50 references on OA use in European countries have been summarized (Rademacher and Harz, 2006).
As an alternative to specific acaricide control agents, oxalic acid has been found highly effective in the treatment of colonies without brood (Gregorc & Planinc, 2012). Although OA is highly toxic to V. destructor, it exhibits little toxicity to honey bees (Milani 2001). Use of OA is easy, cheap, and safe for beekeepers, and it is particularly useful in broodless colonies (Rademacher et al., 2006, Mutinelli et al., 1997). Milani (2001) applied OA to varroa mites in laboratory conditions, and he observed 100% efficient on mite mortality. Most of the research on the development of OA as a treatment in bee colonies was carried out using three different application form in EU countries and Switzerland. These application forms include trickling (using a syringe or a similar applicator), evaporation (evaporating of crystals, gelatin capsules or tablets forms of oxalic acid dihydrate with different types of evaporators) and spraying (spraying oxalic acid dihydrate solution onto the bees (Rademacher & Harz, 2006).
The goal of the current study was to have an idea about whether mortality of Varroa mites could be increased through the co-application of OA and M. brunneum at different concentrations. Results from this research compared an entomopathogenic fungus with a pure organic acid as possible control measures against V. destructor under laboratory conditions.
Material and Methods
Collecting Varroa destructor
To collect Varroa mites from research beehives in Pullman, Washington, the powdered sugar (Great Value Confectioners, 32 oz) method was used. The used mites for research were collected from the frames with open brood selected in beehives. With the collecting method, the bees were shaken into a container from the frames to have bees with mites by using a funnel, and the bees were poured into a glass jar and powdered sugar was added. The bees were shaken with powdered sugar in the jar half minutes, and then they were poured into the white strainer. This way helps no injuries on the bees. The collected mites were carried to the laboratory in glass vials. At the same time, two late instar honey bee larvae were collected from the frames as a food source for the Varroa mites.
Preparing of concentrations of the material
The stock culture of the fungi (Metarhizium brunneum F52), which is diluted in a solution of 0.05% Tween 80 (Acros Organics AC278632500 Tween 80 (250 mL) CAS 9005-65-6) to provide the concentrations needed for bioassays, was used to prepare the concentrations of fungi. By using hemocytometer, the conidia concentrations of M. brunneum were calculated with A compound microscope at 4x magnification. Thus, the concentrations were arranged as 1 x 104, 1 x 105, 1 x 106, 1 x 107, and 1 x 108, and these conidial suspensions were mixed with 1.5% oxalic acid. Each solution weas prepared 1000 μl (500 μl from each conidial suspension and 500 μl 1.5% oxalic acid).
The concentrations were sprayed to petri dishes (VWR 100 x 15mm), which have Difco™ Potato Dextrose Agar (PDA) (Kartoffeldextrose-Agar) agar and then incubated at 35°C for 24 h to test conidial viability for each concentration. Lactophenol cotton blue stain was added to each petri dish to fix and stain fungi into conidia. By glass slide, the droplets were covered, and the germinated spores calculated under the microscope. The same way was used to test spray density of the Potter Precision Spray Tower machine (Burkard Scientific, UK). Each 1000 μl solution were sprayed on petri dishes with Difco™ Potato Dextrose Agar (PDA) agar and again lactophenol cotton blue stain was added to stain the conidia.
Applying solutions on Varroa destructor
Before applying the fungi and oxalic acid solutions to varroa mites, 20-35 Varroa mites were apportioned into each of six glass petri plates containing a wet Whatman filter paper (90 mm diameter) (Whatman International, Maidstone, England). For each set of mites, 1000 µl of the six suspensions (the five concentrations of mixture solutions and the pure 15% oxalic acid) was applied. By using the Potter Precision Spray Tower with 1.0 kg cm−2 pressure and a 0.25 mm orifice diameter nozzle, the concentrations of fungi and oxalic acid were distributed and 1000 µl pure 15% oxalic acid on the V. destructors. Moreover, 1000 µl 0.05% Tween 80 was applied in the same machine for control groups.
After applying the suspensions, ~20-30 mites were transferred into 24 well tissue culture plates. To provide food for the mites, two late instar honey bee larvae were placed onto the 24 well tissue culture plates. Every two days thereafter, the larvae food was changed.
Determination of the effectiveness of solutions on Varroa mites
The prepared 24-well tissue culture plates were placed at 35 °C in a Percival Scientific incubator (SHEL LAB, SRI21D, USA), and observed the death of mites. The dead mites onto these plates were daily collected from the plates, and before transferring petri dishes with PDA agar, the dead mites were sterilized in 95% ethanol. The transferred dead in agar were kept at 25 °C for one week and observed the presence of external fungi on dead mites in petri dishes.
Besides, after spraying 15% oxalic acid without M. brunneum on the mites, death times of the varroa mites were observed and recorded. This recording helped to compare the effectiveness of other materials to kill the varroa mites under laboratory conditions.
Data analysis
V. destructor mortality was recorded daily, and the response of dose-mortality was analyzed by using the general linear model in R package. The research was on survival of varroa mites in response to different concentrations of mixture solutions (M. brunneum with 15% oxalic acid and pure 15 % oxalic acid, so with this statistical way, the average prevents dead of mites in the curves, and the percentage of mortality for each one was tested. The rationale this approach is to know the effect of the explanatory variables (day, doses of solutions), on the response variable (mite mortality).
Result
Daily mite mortality for mixed oxalic acid with 1 x 108 (500 µl fungi + 500 µl oxalic acid) was significantly different from other concentrations in mortality of V. destructor under laboratory conditions. It killed 90.2% varroa mites within 24 h (P < 0.05). The effectiveness of 1x107 and 1 x 106 conidia of M. brunneum on mortality of varroa mites were similar each other (Figure 1); 75.2% and 67.3% mites dead within 24 h. There was no big difference on the virulence of mites which treated with concentrations 1 x 105 and 1 x 104 of M. brunneum and oxalic acid. 40.4% and 32.7% virulence of varroa mites were observed within 24 h, respectively (p < 0.05). Also, the effect of oxalic acid (1000 µl) on mortality of V. destructor was slightly different from the lower concentrations of oxalic acid and M. brunneum; it caused 60.5% varroa mortality within 24 h. The mite virulence in control group within 24 h was 21.8% (Figure 1). 100% of mortality rates were caused by all the assayed isolates with control within 144 hours.
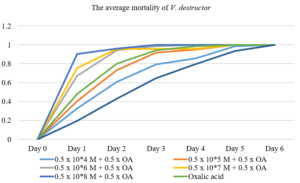
Figure 1: Dose-mortality curve for two different control agents (Meterhizium brunneum and oxalic acid). 105 (M +OA) 106 (M +OA) 107 (M +OA) 108 (M +OA) 147 147 147 147 40.4 ± 11.7 67.3 ± 11.7 75.2 ± 11.7 90.2 ± 11.7 17.344 - 63.5 44.163 - 90.4 52.098 - 98.3 67.146 - 113.3
Fungal isolate
na
Emmean ± SE
CL lower - CL upper
104 (M +OA)
147
32.7 ± 11.7
9.636 - 55.8
a= Total number of mites tested
Discussion
The results of the assay (Part 1: three different control agents) demonstrated that V. destructor is a suitable host for the entomopathogenic fungi, Metarhizium brunneum. The data indicate an optimal volume of varroa mite mortality under laboratory conditions. Applying M. brunneum and oxalic acid together helped to combine the effectiveness of agents with different concentrations on varroa mites. The high concentration of M. brunneum in the mixture solutions was more virulent for varroa because it killed the mites in a short time. The oxalic acid solution alone (without M. brunneum) showed moderate effect for mortality of varroa.
Under laboratory conditions, the result suggest that the mixture solutions (oxalic acid and M. brunneum) have an effective role on varroa mite mortality. Although there is no previous research about mixing oxalic acid and M. brunneum, the results reported here provide an indication that this approach to mite control should be further tested. Mixing oxalic acid solutions with M. brunneum with different concentrations of fungi and oxalic acid can contribute to reduction in mite numbers during in beehives. Integrating biological and soft-chemical control methodologies may reduce the likelihood of resistance development by Varroa, while still providing control efficacy greater than either treatment alone. Additional studies to refine the control procedures and work toward effective application protocols are warranted.
Besides, the study supported that oxalic acid killed varroa mites in 3 days in laboratory conditions. Aliano et al., (2006) observed natural mortality on varroa mites 9.7 ± 3.4% after 24 h in their bioassay. In addition, Aliano and Ellis (2009) reported that oxalic acid could be used in beekeeping program to reduce mite populations in package bees. They set up a trial to develop a protocol to reduce the mite population with oxalic acid in package bees. The oxalic acid solution was spread directly the honey bees, which include varroa mites. There was an effective controlling on varroa mites with minimal adult bee mortality. Milani (2001) reported 22% mortality in 42% R.H. and 30% mortality in 75% R.H. after 48 hours when he kept V. destructor for one hour in capsules treated with oxalic acid (1 µg/cm2). Toomemaa (2018) tested the synergetic effects of weak oxalic acid and thymol solutions with trickling method on V. destructor and honey bees, he reported 46.8 ± 4.4% effectiveness for oxalic acid and the author supported that a high efficiency and good bee tolerate were provided when trickling the colonies with a low dosage of OA / TH or OA (15 and 20 mL/card combo, respectively).
Acknowledgment: I thank Adekunle Adesanya for their help in the analysis.
References
- Aboushaara, H., Staron, M. Cermakova, T. (2017). Impacts of oxalic acid, thymol, a potassium citrate as Varroa control materials on some parameters of honey bees. TUBITAK 41:238-247.
- Akyol, E., & Yeninar, H. (2008). Controlling Varroa destructor (Acari: Varroidae) in honey bee Apis mellifera (Hymenoptera: Apidae) colonies by using Thymovar® and BeeVital®. Italian Anim. Sci. 7:237-242.
- Aliano, N. P., Ellis, M. D., Siegfried, B. D. (2006). Acute Contact Toxicity of Oxalic Acid to Varroa destructor (Acari: Varroidae) and Their Apis mellifera (Hymenoptera: Apidae) Hosts in Laboratory Bioassays. Econ. Entomol. 5:1579-1582.
- Aliano, N. P. (2008). An investigation of techniques for using oxalic acid to reduce varroa mite populations in honey bee colonies and package bees. The Graduate College at the University of Nebraska.
- Aliano, N. P., Ellis, M. D. (2009). Oxalic acid: a prospective tool for reducing Varroa mite populations in package bees. Exp Appl. Acarol. 48:303-309.
- Anderson, D. L., & Trueman, J. W. (2000). Varroa jacobsoni (Acari: Varroidae) is more than one species. Springer 24:165-89.
- Behle, R. W., Jackson, M. A., Flor-Weiler, L. B. (2013). Efficacy of a Granular Formulation Containing Metarhizium brunneum F52 (Hypocreales: Clavicipitaceae) Microsclerotia Against Nymphs of Ixodes scapularis (Acari: Ixoididae). Econ. Entomol. 106:57-63.
- Behle, R. W., Richmond, D. S., Jackson, M. A., & Dunlap, C. A. (2015). Evaluation of Metarhizium brunneum F52 (Hypocreales: Clavicipitaceae) for Control of Japanese Beetle Larvae in Turfgrass. Econ. Entomol. 108:1587-95.
- Bischoff, J. F., Rehner, S. A., & Humber, R. A. (2009). A multilocus phylogeny of the Metarhizium anisopliae Mycologi 101:512-530.
- Bogdanov, S., Charriere, J., Imdorf, A., Kilcheman, V., & Fluri, P. (2002). Determination of residues in honey after treatments under field conditions with formic and oxalic acid under field conditions. Apidologie 33:399-409.
- Calderone, N. W. (2005). Evaluation of drone brood removal for management of Varroa destructor (Acari: Varroidae) in colonies of Apis mellifera (Hymenoptera: Apidae) in the northeastern United States. Econ. Entomol. 98:645-650.
- Conte, Y. L., Ellis, M., & Ritter, W. (2010). Varroa mites and honey bee helt: Varroa explain part of colony losses? Apidologie 41:353-363.
- Damiani, R., Schoch, R. R., Hellrung, H., Werneburg, R., & Gastou, S. (2009). The plagiosaurid temnospondyl Plagiosuchus pustuliferus (Amphibia: Temnospondyli) from the Middle Triassic of Germany: anatomy and functional morphology of the skull. J. Linnean Soc.155:348-373.
- Dussaubat, C, Brunet, J L, Higes, M, Colbourne, J. K, Lopez, J, Choi, J H, ... Alaux, C (2012). Gut Pathology and Responses to the Microsporidium Nosema ceranae in the Honey Bee Apis mellifera. PLoS ONE
- Elzen, P. J. and Westervelt, D. (1999). Detection of coumaphos resistance in Varroa destructor in Florida, American Bee J. 142:291-292.
- Fischhoff, I. R., Keesing, F., & Ostfeld, R.S. (2017). The Tick biocontrol agent Metarhizium brunneum (=M. anisopliae) (strain F52) does not reduce non-target arthropods. PloS ONE
- Gregory, A., Alburaki, M., Sampson, B., Knight, P. R., & Adamczyk, L. (2018). Toxicity of Selected Acaricides to Honey Bees (Apis mellifera) and Varroa (Varroa destructor Anderson and Trueman) and Their Use in Controlling Varroa within Honey Bee Colonies. Insects 9, 55.
- Guzman-Novoa, E., Eccles, L., Calvete, Y., McGowan, J., Kelly, P. G., & Correa-Benitez, A (2010). Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada, Apidologie 41:443-450.
- Hamiduzzaman, M. M., Sinia, A., Guzman-Novoa, E., & Goodwin, P. H. (2012). Entomopathogenic fungi as potential biocontrol agents of the ecto-parasitic mite, Varroa destructor, and their effect on the immune response of honey bees (Apis mellifera). Journal of Inverteb. Pathol. 111:237-243.
- Jaber, L.R., & Ownley, B. H. (2017). Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? ELSIVIER.
- Lodesani, M., Colombo, M., & Spreafico, M. (1995). Ineffectiveness of Apistan treatment against the mite Varroa jacobsoni in several districts of Lombardy (Italy). Apidologie 26:67-72.
- Meikle, W. G., Marcadier, G., Holst, N., Nansen, C., & Girod, V. (2007). Duration and Spread of an Entomopathogenic Fungus, Beauveria bassiana (Deuteromycota: Hyphomycetes), Used to Treat Varroa Mites (Acari: Varroidae) in Honey Bee (Hymenoptera: Apidae) Hives. Oxford Academi 100:1-10.
- Milani, N. (1995). The resistance of Varroa jacobsoni Oud to pyrethroids: a laboratory assay. Apidologie 26:415-429.
- Milani, N. (2001). Activity of oxalic acid and citric acids on the mite Varroa destructor in laboratory assays. Apidologie 32:127-138.
- Morse, R. A., & Laigo, F. M. (1969). Apis dorsata in Philippines. Acad. Nat. Sci. Phil.1:1-96.
- Mutinelli, F., Baggio, A., Vapolongo, F., & Piro, R. (1997). A scientific note on oxalic acid by topical application for the control of varroosis. Apidologie
- Ortiz-Urquiza, A., & Keyhani, N. (2013). Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects 3:357-374.
- Piou, V., Tabart, J., Urrutia, V., Hemptinne, J-L., & Vetillard, A. (2016). Impact of the Phoretic Phase on Reproduction and Damage Caused by Varroa destructor (Anderson and Trueman) to Its Host, the European Honey Bee (Apis mellifera). PLoS ONE 11.
- Rademacher, E., & Harz, M. (2006). Oxalic acid for the control of varroosis in honey bee colonies - a review. Apidologie 37:98-120.
- Ramsey, S. D., Ochoa, R., Bauchan, G., Gulbronson, C., Mowery, J. D., Chen, A., Lim, D., Joklik, J., Cicero, J, M., Ellis, J. D., Hawthorne, D., vanEngelsdorp, D. (2019). Varroa destructor feeds primarily on honey bee fat tissue and not hemolymph. PNAS J. 116:1792- 1801.
- Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. J. Invertebr. Pathol. 103:96-119.
- Roth, M. A., Wilson, J. M., Tignor, K. R., Gross, A. D. (2020). Biology and Management of Varroa destructor (Mesostigmata: Varroidae) in Apis mellifera (Hymenoptera: Apidae) Colonies. JIPM 11, 36.
- Sandhu, S. S., Unkles, S. E., Rajak, R. C., & Kinghorn, J. R. (2001). Generation of benomyl resistant Beauveria bassiana strains and their infectivity against Helicoverpa armigera. Biocon. Sci. Technol. 11:245-250.
- Schrank, A., & Vainstein, M. H. (2010). Metarhizium anisopliae enzymes and toxins. ELSEVIER 56:1267-1274.
- Toomemaa, K. (2018). The synergistic effect of weak oxalic acid and thymol aqueous solutions on Varroa mites and honey bees. Apic. Res. 1:37-52.
- Zhou, T., Anderson, D.L., Huang, Z. Y., Huang, S., Yoa, J., Ken, T., & Zhang, Q. (2004). Identification of Varroa mites (Acari: Varroidae) infesting Apis cerana and Apis mellifera in China. Apidologie 35:645-65.