Publication Information
ISSN: 2641-712X
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Evaluation of Bacterial Asthmatic Treatment Strategies: Targeting Toll Like Receptor4-Myeloid Differentiation-2 Complex Inhibition
Mr. Devesh Kumar1*, Mr. Dushyant Kumar Mishra2, Ms. Nishu Gautam3, Mr. Nishikant Gaur4
1, 2, 3Assistant Professor- United College of Engg. And Research (Pharmacy) KP-III, Greater Noida, Uttar Pradesh-201310
4Research Scholar- IEC College of Engg. And Technology KP-III, Greater Noida, Uttar Pradesh -201310
Received Date: April 17, 2023; Accepted Date: April 28, 2023; Published Date: May 04, 2023;
*Corresponding author: Devesh Kumar. Assistant Professor- United College of Engg. And Research (Pharmacy) KP-III, Greater Noida, Uttar Pradesh-201310, Contact No-8394999198. Email: rdev3322@gmail.com
Citation: Kumar D, Mishra D K, Gautam N, Gaur N (2023) Evaluation of Bacterial Asthmatic Treatment Strategies: Targeting Toll Like Receptor4-Myeloid Differentiation-2 Complex Inhibition. Int Jr Pharma Scie and Chem: IJPSC-111.
DOI: 10.37722/IJPSC.2023201
Abstract
Bacterial asthma exacerbation evaluated on Ovalbumin animal model induced by lipopolysaccharide and evaluated by TLR/MD2 complex antagonist. Asthma is a bronchial disorder that causes by shortens also; expand as well as creating additional bodily fluid in the airway tube. The model was performed on rats given ovalbumin, LPS and antagonist compound curcumin and study protocol results was confirmed by leukocyte level, body mass measurement and oxidative stress biomarkers level in blood serum. The curcumin has shown positive treatment results over animal samples such as leukocyte infiltration rate reduced, improvement in body weight as well as significant reduction in oxidative stress markers in treatment groups. According to results curcumin has shown therapeutic potential in the treatment of bacterial asthma management in ovalbumin asthma animal model. This compound can be a best treatment option for airways inflammation.
Keywords: Asthma, Exacerbation; LP; PAMP; TLRs; TLR-4
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are highly complex. It is the part of the syndromes many different various mediators, incendiary cytokines, and effects on the windpipe that are both intense and constant. We currently comprehend that these alterations could vary between patients because of hereditary weakness variety. For the many people, asthma is a constant irritation and some may find a difficult issue that holds them back from conveying about your day to day existences and may be cause a possibly deadly asthma assault. Though asthma may be recuperated, the condition's signs can be controlled. Considering that asthma assaults could change, it's pivotal to keep record of the clinical indications close by your doctor and alter your prescriptions(Barnes and Drazen 2002).
Exacerbations of asthma may be serious and necessitate medical intervention, including a trip to the emergency room, hospitalization, or an unplanned appointment with a physician(Moorman, Akinbami et al. 2012). Youngsters (63% of all asthmatic ED visits) are caused by children. Children in Australia visit the emergency room for asthma exacerbations at such a rate of 35 to 240 sessions per 100,000 people(Schatz, Sorkness et al. 2007).
Wintertime is when the climax happens. Asthma-related visits can vary by as much as 100% week to week. Such estimates show specifically targeted transmission patterns inside the community considering the essential part that rhinovirus plays in asthma exacerbations in kids and represent heterogeneity in the infections triggering asthma attacks (Baxi and Phipatanakul 2010).
Exacerbations of severe asthma can be equally fatal. Although the majority of deaths from asthma in terms of numbers happen in older generations, young people die from asthma more frequently than any other age category. Asthma mortality rates increase during the season, which is in line with the seasonal increase in influenza infection, which would be linked to extremely severe asthma exacerbations(Sharpe, Bearman et al. 2015).
Bacterial & Viral Asthma
Chlamydophila pneumoniae or Mycoplasma pneumoniae-associated infectious respiratory disease has become a significant clinical issue in healthy individuals with long-term respiratory issues. Although the precise role of atypical bacteria in the development and manifestation of asthma is unidentified, a growing body of basic and clinical research implicates these microorganisms as potential asthma triggers(Lambrecht and Hammad 2015).
Mycoplasma pneumonia penetrates ciliated tracheal epithelial cells through a distal part, leading epithelial death and ciliary distortion. M pneumoniae has been associated to existing wheeze, exacerbations of chronic asthma, including long-term respiratory symptoms decline, indicating that it may play a significant role in asthma(Johnson and Ownby 2017).
Chlamydophila pneumonia commonly known as a "atypical" bacterial pathogen, is a frequent cause of bronchitis and atypical pneumonia, can cause persistent infections, and has been linked to subsequent wheezing sickness, similar to M pneumoniae. C pneumoniae also causes exacerbations of preexisting asthma, according to case studies such as Allegra and colleagues', who found that 10% of a cohort of seventy patients presenting with asthma exacerbation were found to be rapidly infected with C pneumoniae by serology(Kraft 2000).
Airway hyperresponsiveness when airways remain extremely responsive and sensitive to inhale constrictor agonists like histamine, methacholine, or cold air, this is the pathophysiological characteristic of asthma(Postma 2007). It is described as the simplicity or sensitivity, with the windpipe react to a stimuli, including the sensitivity and reactivity to a certain stimulus, like lipopolysaccharide and its derivatives. As we know the lipopolysaccharide an infection inducer. LPS is recognised by the toll-like receptor 4 (TLR-4) on rodent immune system. Complementary proteins CD14 and MD-2 promote to identification(Kearney, Dziekiewicz et al. 2015). The intermediate molecule myd88 interacts when TLR-4 is activated MD a first-responder polypeptide 88. The myd88-dependent upregulating activates nuclear factor kappa-B (NF-ƙB), which is regulates the activation of aimed genes that produce pro-inflammatory cytokines. Excess inflammation inside the organ might result from receptor over activity. Overproduction of cytokines and chemokines produced cellular inflammation, through the Nrf/NF-ƙB signaling pathway ovalbumin produced the oxidative disbalance and activation the activity of the oxidative markers(Brannan and Lougheed 2012).

Figure 1: Mechanism of hyper responsiveness.
Material & Method
Experimental Animals
210–310 g male Wistar rats will be used. Animals will be kept in plastic boxes with unrestricted accessibility to feed or liquid in a monitored atmosphere with a 12-hour light/dark cycle and a temperature of 21.2 C (55.5% relative humidity). 6 animals were present in each intervention class. The animal experiments were performed by Institutional Animal Ethics Committee (RVNI/IAEC/2021/17) and by the animal regulatory body of the government CPCSEA guidelines.
Grouping of Animals
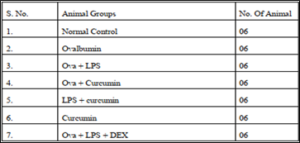
Drugs and treatment
- Inducer drug:
- Ovalbumin was purchased from sigma Aldrich (138831-86-4), dose 150 µg, i.p route for sensitization and 50µg, i.n route for challenge.
- Lipopolysaccharide was purchased from sigma Aldrich (297-473-0) dose 20µg, i.p. route for sensitization and 4 µg, i.n. route for challenge.
- Treatment drug: Curcumin was purchased from sigma Aldrich (458-37-7). Dose 0.60µg/ml, oral route.
- Standard drug: Dexamethasone was purchased from sigma Aldrich (50-02-2). Dose 1 mg/kg, i.p. route.
Sensitization and challenging procedure

A mixture of ovalbumin and Al(OH)3 gel (2 mg of dry gel + 150 mg of ovalbumin in 0.5 ml of phosphate buffer saline solution as indicated in the following) was used to sensitize the rats to ovalbumin by administering it intraperitoneally on the 1st and 7th day. Similarly, the rats were intranasally treated with the same amount of 0.9% phosphate buffer saline solution or 150 mg of OVA alone (OVA group), or along with 4mg of LPS in a total volume of 20mg (OVA-LPS group). On days 14 to 21, all rats received the 1 hour exposures to an aerosolized 1 percent OVA solution (or a 0.9 % PBS solution for the control animals). The asthmatic rats were subsequently given the same treatment for the LPS-curcumin group, OVA-curcumin group, and curcumin alone. Then the rats anaesthetized and the sample was taken for biochemical and other analysis 24 hours after their final challenge.
Parameters evaluation
Serum MDA activity
Thiobarbituric acid (TBA), a fluorescence-producing substance, was utilised to derivatize MDA. The MDA-TBA pair was then examined using the earlier discussed HPLC-fluorescence method. The application of HPLC extraction of MDA-TBA pair in the latest investigation reduced influence from TBA-reactive compounds (TBARS) and enhanced specificity and accuracy of MDA testing when comparison to the traditional colorimetric analytical approach for MDA-TBA pair detection(Moselhy, Reid et al. 2013).
Started with TBA complexation for the free MDA measurement by combining a sample with phosphoric acid and TBA solution. After that, the combination was allowed to create MDA-TBA2 by being incubated at 80 °C for 1 hour. Free and total MDA performed the same TBA derivatization procedure, but total MDA was measured after an additional alkaline hydrolysis reaction. For the serum samples, the alkaline hydrolysis technique involves incubating the sample with 1N NaOH at 60 °C for 30 min, then combining it with 1N HCl (Karadogan, Beyaz et al. 2022).
However, the EBC samples' hydrolysis process was bit better and went as follows. After 30 minutes of incubation with 5N NaOH and 60 °C, 5N HCl was added to an aliquot of EBC. The resulting mixture was introduced into the HPLC apparatus after TBA complexation, where the MDA-TBA2 pair was separated.
The HPLC system's mobile phase contained KH2PO4 solutions and methanol. The solution's pH was then raised to 6.8 before being filtered through nylon membrane. The mobile phase's flow rate was established. A fluorescence sensor used to detect emission, stimulated wavelength was used to measure the MDA-TBA2 pair. The analytical accuracy, extraction recoveries, and technique detection limits were 1.8 nM, 75.9 %, and 20% of total, respectively (calculated as comparative standard variance from eight replicate injections) (Moselhy, Reid et al. 2013).
Serum GPx activity
A linked experiment employing a commercial kit to measure glutathione peroxidase activity was used to quantify the GPx activity spectrophotometrically in triplicate(Cellat, Kuzu et al. 2021).
Initially, 50 L of glutathione reductase and 50 L of glutathione were poured into 96 wells of the dish containing 50 L of serum and incubated at 38⁰ C for 11 minutes.
Depending on a colorimetric assay with a baseline ranges of detection of 0.5 Mu/mL. After that, 50 mL of NADPH and 50 mL of butyl-hydroperoxide were added as substrates to start the reaction.
The kinetic variations of wavelength (reduces) at 340 nm were monitored after incubation at 37oC for 10 min. A standard calibration curve was used to estimate the GPX activity (U/L) (Tabatabaei, Babaee et al. 2020).
Serum Catalase activity
The following are the experimental ideal conditions:
At 37°C for 60 seconds, 0.25 ml of sample was incubated with 1.5 ml of substrate (63 pmol of H2O2 in 61 mol/l sodium-potesium PO4 buffer, pH 7.4).
Up to 110 kU/l, serum catalase action is linear. If the catalase activity was more than 110 kU/l.
The experiment was restarted after diluting the serum with PO4 buffer. Below these conditions, units of catalase disintegrates 1/mol of h2o2/ 1 min(Al-Kinani and Sayyah 2016).
Spectrophotometrically assay of hydrogen peroxide
With 1.5 ml of 33.0 mmol/1(NH4)6, the catalytic process was paused, and the yellow molybdate and H2O2 pair was detected at 406 nm verses blank 3.
![]()
- In blank 1, 1.5 ml of substrate, 1.5 ml of molybdate, and 0.3 ml of serum were present.
- In blank 2, 1.5 ml of substrate, 1.5 ml of molybdate, and 0.3 ml of buffer were present.
- In blank 3, 1.5 ml of buffer, 1.5 ml of molybdate, and 0.3 ml of buffer were present (Yunis 1969).
GSH level
The GSH-DTNB response delivers the TNB chromospheres, with the greatest absorbance at 413 nm, and an oxidized glutathione-TNB adduct, which act as the reason for the test (GS-TNB)(Dias Araujo Mazzari 2017).
How much GSH in the example straightforwardly influences how rapidly TNB structures when recognized at 412 nm.
From that point forward, GR lessens the disulfide item (GS-TNB) within the sight of NADPH, reusing GSH back into the interaction.
How much glutathione estimated advised the whole measure of altogether brought down and oxidized glutathione in the example ([GSH]total = [GSH] + 2 [GSSG]) in light of the fact that GR changes over the GSSG delivered into 2GSH(Kodama, Kishimoto et al. 2017).
For simplicity of purpose and testing consistency, the speed of shift in absorbance (412nm min 1) is intended to be straight and is directly connected with the general measure of GSH. The direct condition or relapse bend is made from numerous GSH norms and used to work out how much an unidentified sample.
Because the ratio of GSH to GSSG is only 1:09 and because GSH oxidizes quickly, it usually changes this ratio for GSSG, it is difficult to examine GSSG in cell removal. In any scenario, it is possible to test GSSG right away to prevent the conversion of GSH to GSSG. To ensure the presence of GSSG, Griffith's approach is used in conjunction with the GSSG reductase reuse strategy. 2-Vinyl pyridine is applied to the cell extricates, and GSH is produced covalently as a result (however not GSSG). Triethanolamine destroys 2-vinyl pyridine in large quantities(Karadogan, Beyaz et al. 2022).
Procedure
- Set up the GSH and GSSG arrangements as per the directions, yet this test
utilizes 100 ml of every standard. - In a 1 ml cuvette, pour 700 ml of KPE cradle. In the event that is essential, trade
out 100 ml of the example for 100 ml of a bunch of tests that have been 2-vinyl
pyridine-treated and were made as per Step 2B. - Mix DTNB and GR in equivalent parts, then add 120 ml of this mixer.
- Allow GSSG 30 seconds to change over completely to GSH prior to adding 60
ml of - NADPH. - Modify the cuvette utilizing parafilm, set it in the cuvettes, and run the analysis
for two minutes at 412 nm. - At 0 minutes, 1 moment, and 2 minutes, note the assimilation. Ordinarily, the bend's direct line falls somewhere in the range of 1 and 2 absorbance degrees.
- Each example's GSH content is assessed utilizing a standard bend. To work out
the foundation rate, an example clear without GSH or test is utilized. - Normally, values are communicated as GSH counterparts, for example, nM or M
per gm-1 of tissues or nm/105 cells of proteins. Regularly, the qualities are deducted from absolute GSH-GSSG to estimate the GSH rate (Vural and Uzun 2000).
Result & Discussion
Effect of curcumin on the oxidative stress markers level
Ovalbumin induced asthma was evaluated in wistar rat by orally administration of estimated dose of curcumin, and then blood sample was collected through retro orbital plexus and then rats were sacrificed to evaluate the oxidative marker like MDA, GSH, GPx and CAT level.

The values are provided as mean SEM (n=6). The significance of the data was evaluated using a one-way ANOVA followed by Tukey's multiple comparison test. ###p < 0.001 significant, versus normal control (NC); @p < 0.05 significant versus Ovalbumin; **p < 0.01, ***p < 0.001 significant, versus Ova+LPS and ns is non- significant versus OVA+LPS.MDA: Malondialdehyde, GSH: Reduced glutathione, GPx: Glutathione peroxidase, CAT: Catalase
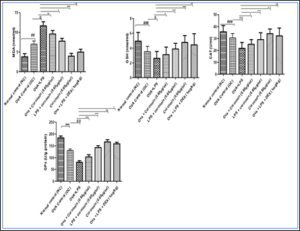
Figure 5.1: Effects of curcumin treatment on antioxidant parameters.
The values are provided as mean standard error (n=6). The significance of the values was evaluated using a one-way ANOVA followed by Tukey's multiple comparison test. ###p < 0.001 significant, versus normal control (NC); @p < 0.05 significant versus Ovalbumin; **p < 0.01, ***p < 0.001 significant, versus Ova+LPS and ns is non-significant versus OVA+LPS.
Effect of curcumin on rat’s TLC and DLC count
Ovalbumin induced asthma was evaluated in wistar rat by orally administration of estimated dose of curcumin, and then blood sample was collected through retro orbital plexus and then rats were sacrificed to evaluate the TLC, Eosinophils, Lymphocyte, Macrophages, Neutrophils level.
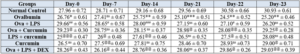
Table 5.2: Effects of curcumin treatment on Differential leukocyte count.
The values are provided as mean standard error (n=6). The significance of the values was evaluated using one-way ANOVA followed by Tukey's multiple comparison test. ###p < 0.001 significant, versus normal control (NC); @p < 0.05 significant versus Ovalbumin; **p < 0.01, ***p < 0.001 significant, versus Ova+LPS and ns is non-significant versus OVA+LPS.
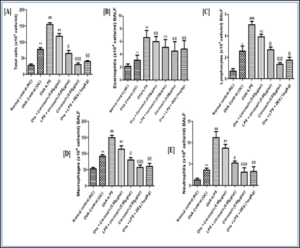
Figure 5.2: Effects of curcumin treatment on Differential leukocyte count.
The values are provided as mean standard error (n=6). The significance of the values was evaluated using a one-way ANOVA followed by Tukey's multiple comparison test. ###p < 0.001 significant, versus normal control (NC); @p < 0.05 significant versus Ovalbumin; **p < 0.01, ***p < 0.001 significant, versus Ova+LPS and ns is non-significant versus OVA+LPS.
Effect of weight variation on the rats by orally administration of curcumin
Ovalbumin induced asthma was evaluated in wistar rat by orally administration of estimated dose of curcumin, and then blood sample was collected through retro orbital plexus and then rats were sacrificed to evaluate the difference of weight variations.
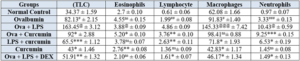
Table 5.3: Effects of curcumin treatment on weight variations.
The values are provided as mean standard error (n=6). The significance of the values was evaluated usinga one-way ANOVA followed by Tukey's multiple comparison test. ###p < 0.001 significant, versus normal control (NC); @p < 0.05 significant versus Ovalbumin; **p < 0.01, ***p < 0.001 significant, versus Ova+LPS and ns is non-significant versus OVA+LPS.

Conclusion
According to results curcumin has shown therapeutic potential in the treatment of bacterial asthma management in ovalbumin asthma animal model. This compound can be a best treatment option for airways inflammation. Curcumin increase the level GSH, GPx and Catalase in comparison to standard Dexamethasone and curcumin decrease the leukocytes level as well as decrease the serum Eosinophils, Lymphocyte, Macrophages and Neutrophils level as compared the disease control groups. So that it is concluded the curcumin is effective against airway inflammation. As well as curcumin also improve the activity of oxidative stress markers so we can say curcumin reduces the oxidative stress level and the drug also increases the body mass and body weight against the disease.
References
- Al-Kinani, M. F. H. and S. G. Sayyah (2016). "Evaluate of antioxidant enzymes superoxide dismutase, glutathione peroxidase and catalase levels in asthma patients." J Nat Sci Res 6:42-48.
- Barnes, P. and J. Drazen (2002). Pathophysiology of asthma. Asthma and COPD, Elsevier, Amsterdam.
- Baxi, S. N. and W. Phipatanakul (2010). "The role of allergen exposure and avoidance in asthma." Adolescent medicine: state of the art reviews 21:57.
- Brannan J D, M D Lougheed (2012). "Airway hyperresponsiveness in asthma: mechanisms, clinical significance, and treatment." Frontiers in physiology 3:460.
- Cellat, M., M. Kuzu, C. T. İşler, M. Etyemez, N. Dikmen, A. Uyar, İ. Gökçek, E. Türk and M. Güvenç (2021). "Tyrosol improves ovalbumin (OVA)-induced asthma in rat model through prevention of airway inflammation." Naunyn-Schmiedeberg's Archives of Pharmacology 394:2061-2075.
- Dias Araujo Mazzari, A. (2017). In vitro effects of selected medicinal plants shortlisted for clinical use in the Brazilian public health system in CYP3A4 mRNA gene expression, glutathione levels and P-glycoprotein activity and their implications for herb-drug interactions, UCL (University College London).
- Johnson, C. C. and D. R. Ownby (2017). "The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases." Translational Research 179:60-70.
- Karadogan, B., S. Beyaz, A. Gelincik, S. Buyukozturk and N. Arda (2022). "Evaluation of oxidative stress biomarkers and antioxidant parameters in allergic asthma patients with different level of asthma control." Journal of Asthma 59:663-672.
- Kearney, S. C., M. Dziekiewicz and W. Feleszko (2015). "Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma." Annals of Allergy, Asthma & Immunology 114:364-369.
- Kodama, Y., Y. Kishimoto, Y. Muramatsu, J. Tatebe, Y. Yamamoto, N. Hirota, Y. Itoigawa, R. Atsuta, K. Koike and T. Sato (2017). "Antioxidant nutrients in plasma of Japanese patients with chronic obstructive pulmonary disease, asthma‐COPD overlap syndrome and bronchial asthma." The Clinical Respiratory Journal 11:915-924.
- Kraft, M. (2000). "The role of bacterial infections in asthma." Clinics in chest medicine 21:301-313.
- Lambrecht, B. N. and H. Hammad (2015). "The immunology of asthma." Nature immunology 16:45-56.
- Moorman, J. E., L. J. Akinbami, C. M. Bailey, H. S. Zahran, M. E. King, C. A. Johnson and X. Liu (2012). "National surveillance of asthma: United States, 2001-2010." Vital & health statistics. Series 3, Analytical and epidemiological studies (35):1-58.
- Moselhy, H. F., R. G. Reid, S. Yousef and S. P. Boyle (2013). "A specific, accurate, and sensitive measure of total plasma malondialdehyde by HPLC [S]." Journal of lipid research 54:852-858.
- Postma, D. S. (2007). "Gender differences in asthma development and progression." Gender medicine 4: S133-S146.
- Schatz, M., C. Sorkness, J. Li, P. Marcus, J. Murray and R. Nathan (2007). "Asthma Control Test: reliability, asthma." Eur Respir J 30:452-456.
- Sharpe, R. A., N. Bearman, C. R. Thornton, K. Husk and N. J. Osborne (2015). "Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors." Journal of Allergy and Clinical Immunology 135:110-122.
- Tabatabaei, A., M. Babaee, N. Moradi, M. Nabavi, S. Arshi and S. Fallah (2020). "Serum concentration of selenium and GPX enzyme activity in iranian children with asthma." Modern Care Journal 17(2).
- Vural, H. and K. Uzun (2000). "Serum and red blood cell antioxidant status in patients with bronchial asthma." Canadian respiratory journal 7:476-480.
- Yunis J J (1969). "Biochemical methods in red cell genetics."