| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Impact of Detergents on the Nitrification Process of Ammonium Ions in Natural Waters
Sandu Maria1, Tărîţă Anatolie1, Moşanu Elena1*, Dragalina Galina2, Lozan Raisa1
1Institute of Ecology and Geography
2State University of Moldova
Received Date: April 01, 2022; Accepted Date: April 12, 2022; Published Date: April 18, 2022;
*Corresponding author: Moşanu Elena, Institute of Ecology and Geography. Email : emosanu976@gmail.com
Citation: Elena M, Maria S, Anatolie T, Galina D, Raisa L (2022) Impact of Detergents on the Nitrification Process of Ammonium Ions in Natural Waters. Enviro Sci Poll Res and Mang: ESPRM-121.
DOI: 10.37722/ESPRAM.2022201
(http://doi.org/10.37722/ESPRAM.2022201)
Abstract
The evaluation of individual effects of surfactants or other ingredients does not reflect the influence of a detergent on the aquatic environment, being important to estimate the effects of detergent as a whole.
The present study, the impact of detergents on the process of nitrification of ammonium ions in natural waters evaluates the influence of 3 types of detergents on the process of nitrification of NH4 + in water: solid detergents (DS-1 and DS-2) and a liquid detergent (DL). Laboratory modeling evaluated the nitrification process in the water of the Dniester River, in which 2.2-2.5 mg / dm3 NH4 +, 1-10-100 mg / dm3 DS-1 and DS-2 were added and 0.1 -0.5-1.0 ml / dm3 of DL.
The nitrification process (stage I) NH4 + → NO2– in water without detergent (control) took 8 days. At the addition of 1-10 mg/dm3 of DS-1 and DS-2 the process took 9-10 days, and at 100 mg/dm3 – 19-24 days, the concentration of nitrates in stage II (NO2– →NO3–) being 19.8-17.2 mg/dm3 for DS -1 and 14.9-1.68 mg/dm3 for DS-2, respectively. The concentration of 0.1 ml / dm3 DL generated the process at stage I to last 19 days, and in the presence of 0.5-1.0 ml/dm3 DL after 30 days there were still 56-88% of the initial ammonium, finally stage II (NO2–NO3–) ending with only 4.67 – 1.01 mg/dm3 of nitrates.
The study confirms the need to assess the impact of detergents (not only surfactants) and wastewater before being discharged into the emissary.
Keywords: Ammonium Ions; Contamination of Aquatic Ecosystems; Detergents; Nitrate; Nitrite; Nitrification
Introduction
In natural waters are present practically all inorganic nitrogen compounds (NH4+, NH3, NO2–, NO3–), the content of which is conditioned by their amount in existing sources of pollution: wastewater discharged into the environment, the presence of industrial, agricultural, household waste illegally stored, atmospheric deposits, surface runoff and as a result of their biochemical transformation [1].
The surface waters are divided into 5 quality classes according to the concentration ammonium, nitrites and nitrates ions: for nitrates from 1,0 to >11,3 mg N/L; nitrites from 0,01 to >0,3 mg N/L and for ammonium from 0,2 to >3,1 mg N/L [2]. Their presence in water determines the excessive growth of algae causing eutrophication of the aquatic basin [3], and by deteriorating the ecological balance of the aquatic ecosystem; the water quality is also diminished.
The ammonium ions in waters are important for the nitrification process for the biota present in surface waters and specific to the nitrogen cycle. Process is also characteristic during decomposition reactions in wastewater treatment technologies, when nitrogen from organic substances is transformed into toxic inorganic nitrogen compounds, including NH4+.
For exemple, as a result of the discharge of wastewater from Chisinau municipality into the water of Bac river were detected 119 mg/L NH4+, and in Cogalnic district, downstream of Hancesti city – 24,2 mg/L NH4+ [4]. By evaluating the physical-chemical composition of the Bac river water at the discharge into the Dniester river was found the presence of 32,6-33,8 mg/L of ammonium ions [5].
Nitrification is characterized by high oxygen consumption (for oxidation 1 mg/L of NH4+ consumes about 3,6 mg/L O2), and an insufficient supply of oxygen can lead to the accumulation in water only of nitrites, which leads to creating exceptional situations [6].
Previously has been studied the influence of phenol, petroleum products, heavy metals, pesticides, surfactants, etc., on the process of ammonium nitrification in surface waters [7-11], because these substances are used in domestic activity and in various fields of the economy.
The following paper includes the results obtained by laboratory modeling of the impact of detergent that contained the different type of surfactants, in the biochemical process of ammonium ions nitrification in natural water.
According to European Committee of Surfactants and their Organic Intermediates reports, half of the detergent consumption is used in household applications, and the other half in industries (cosmetics, metal processing, papermaking, leather, etc.). In 2008, it was estimated that more than 4,2 million tonnes of detergents and 1,2 million tonnes of balms were used annually in Eastern Europe, up from 2006 [12], the annual global production of surface active agents being about 13 million tons, and 65% are anionic surfactants, the second and third places in the global production correspond to non-ionic and cationic compounds, respectively [13].
Materials and Methods
The conversion of ammonium ions or ammonia into nitrite and respectively in nitrate (oxidation, nitrification) is prods by Nitrosomonas and Nitrobacter aerobic organisms, so the result of study will demonstrate the influence of detergents on the nitrification bacterium.
The influence of detergents on the process of biochemical oxidation of ammonium ions was assessed by determining for 30 days the concentration of NH4+, NO2– and in final NO3– ions and pH, using national standards [14-16] and NO3– ion by the method in the presence of NO2– [17].
In the present study is evaluating the influence of 3 detergents on the ammonia nitrification process in water: solid detergent DS-1 (5-15% anionic active agents, <5% nonionic active agents, zeolites, enzymes, optical bleaches, fragrance, benzyl benzoate, butylphenyl methylpropional, citronellol, geraniol), DS-2 (5-15% anionic active agents, <5% soap, sodium silicate, 15-30% sodium carbonate (Na2CO3), optical bleaches, aromatize) and liquid detergent DL (5-15% anionic and cationic active agents, <5% phosphonates, soap, enzymes, benzisothiazolinone, methylisothiazolinone, perfume, alpha-isomethyl ion, citronellol, geraniol).
The nitrification process was evaluated in the water from river Dniester, Palanca village, Stefan Voda district, Republic of Moldova, in which were added of 2,2 – 2,5 mg/dm3 NH4+ (permissible in urban and industrial wastewater at discharges – 2 mg/L [18]).
The initial concentration of NH4+ in water was of 0,11 mg/L and 2,7 mg/L NO3–. The comparison sample includes natural water with added 2,36 mg/L of NH4+. In the models with detergent in experiments was added: 2,2-2,5 mg/dm3 NH4+, by 1-10-100 mg/dm3 of DS-1 and DS-2 and by 0,1-0,5-1,0 ml/dm3 of DL, using information as a detergent for a wash is about 1 g/L for DS-1, 0,4 g/L for DS-2 and 1,1 ml/L for DL.
In the study were used chemicals of “pure for analysis” or “chemically pure” quality. The modeling was performed at a temperature of 20-22°C and pH of 7,65 in conditions presented by Grunditz C., etc. (2001): optimum temperature is 35°C for Nitrosomonas and 38°C for Nitrobacter; optimum pH is 8.1 for Nitrosomonas and 7,9 for Nitrobacter [19].
Results and Discussions
The European Union adopted in 2004 Regulation 648/2004 on detergents [20], which includes the notion of detergent: ‘Detergent’ means any substance or mixture containing soaps and/or other surfactants intended for washing and cleaning processes. Detergents may be in any form (liquid, powder, paste, bar, cake, moulded piece, shape, etc.) and marketed for or used in household, or institutional or industrial purposes.
So, the detergents used in everyday life are complex mixtures of various compounds. These components can interact antagonistically, additively or synergistically, increasing or diminishing the toxicity of the detergent in the aquatic environment. Thus, evaluating the individual effects of surfactants or other ingredients does not reflect either the actual or net influence of a detergent on the aquatic environment, it is thus necessary to estimate the effects of detergent formulations as a whole [21, 22].
In the Regulation (EC) Nr. 648/2004 [20] is mention: (7) As confirmed by the Commission White Paper on the strategy for a future Chemical Policy, appropriate measures concerning detergents should ensure a high level of environmental protection, especially of the aquatic environment, so it is important to study the influence of detergents on NH4+ ion nitrification.
The results of nitrification process evaluated in the water from river Dniester are presented in figures 1-6.
The NH4+→NO2– step in water without detergent (0) lasted 8 days. At the addition of 1-10 mg/dm3 of DS-1 the process took 9 days and at 100 mg/dm3 the duration of the step was 19 (figure 1). The NO2–→ NO3– step in water without detergent lasted 13 days (0). When adding 1-10 mg/dm3 of DS-1 the process took 13 days and in the presence of 100 mg/dm3 the stage duration was 26 days for DS-1 (figure 2).
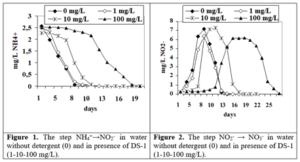
The first stage of the process of nitrification NH4+→NO2– in water at the addition of 1-10 mg/dm3 of DS-2 the process took 10 days and at 100 mg/dm3 the duration of the step was 24 days (figure 3). The NO2–→ NO3– step in water when adding 1-10 mg/dm3 of DS-2 the process took 17 days and in the presence of 100 mg/dm3 the stage duration was more than 30 days for DS -2 (figure 4).
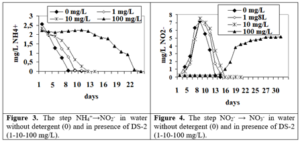
It was shown that at the addition of 0,1 ml/dm3 DL the step lasted 19 days, and in the presence of 0,5-1,0 ml/dm3 after 30 days there were still 56-88% of ammonium ions left (figure 5). DL has a specific influence in the second stage of nitrification (NO2–→ NO3–). At the addition of 0,1 ml/dm3 DL the step lasted 20 days. In the presence of 0,5-1,0 ml/dm3 DL after 30 days in solutions were only 0,63-1,38 mg/dm3 NO2– (figure 6).
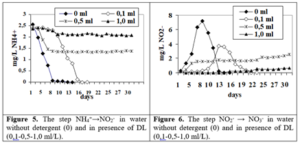
Finally nitrates formed after nitrification in the presence of DS-1 and DS -2 is of 14,6-19,8 mg/dm3, and in water without the addition of detergents – 21,4 mg/dm3 (table 1).
DL has a specific influence on the nitrification process. At the addition of 0,1 ml/dm3 DL in final was 14,6 mg/dm3 of nitrates, in the presence of 0,5-1,0 ml/dm3 DL in final being only 4,67-1,01 mg/dm3 of nitrates (table 1).
| Water river Nistru | NH4+, mg/L | Detergent | NO3–, mg/L | |
| Named | Additioned | |||
| 0,11 | 2,7 | |||
| 1 | 2,56 | 0 | 21,4 | |
| 2 | 2,47 | DS-1 | 1 mg/L | 19,8 |
| 3 | 2,26 | 10 mg/L | 18,4 | |
| 4 | 2,51 | 100 mg/L | 17,2 | |
| 5 | 2,23 | DS-2 | 1 mg/L | 14,9 |
| 6 | 2,33 | 10 mg/L | 17,8 | |
| 7 | 2,38 | 100 mg/L | 1,68 | |
| 8 | 2,40 | DL | 0,1 ml/L | 14,6 |
| 9 | 2,46 | 0,5 ml/L | 4,67 | |
| 10 | 2,37 | 1,0 ml/L | 1,01 | |
Currently the world production of detergent amounts to 10 millions of tons per years [23]. The detergents used are complex mixtures of various compounds that can interact antagonistically, additively or synergistically, increasing or diminishing its toxicity on aquatic biota [21, 22].
The contamination of aquatic ecosystems is increasing at an alarming rate as a consequence of the discharge of untreated sewage of urban and industrial origin into coastal zones, rivers, streams and lakes. Among the contaminants found in these sewage effluents are organic pollutants such as detergents, which can cause toxicity problems for the aquatic biota found in the receiving water bodies [24].
So, is important to study the influence of used detergents on the systems of wastewaters treatment and were wastewater is discharged in the natural waters.
Conclusions
- In the presence of solid detergents: DS-1 (5-15% anionic active agents, <5% non-ionic active agents, zeolites, enzymes, optical bleaches, perfume, benzyl benzoate, butiphenyl methylpropional, citronellol, geraniol); DS-2 (5-15% anionic active agents, <5% soap, sodium silicate, 15-30% sodium carbonate (Na2CO3), optical bleaches, aromatized) the content of nitrates ,formed after nitrification is 14.6-19.8 mg/dm3, and in water without the addition of detergents (control sample) is 21.4 mg/dm3.
- DL liquid detergent (5-15% anionic and cationic active agents, <5% phosphonates, soap, enzymes, benzisothiazolinone, methylisothiazolinone, perfume, alpha-isomethyl ion, citronellol, geraniol) has a specific influence on the nitrification process. The concentration of 0.1 ml/dm3 DL in water finally generates an amount of 14.6 mg/dm3 of nitrates, and 0.5-1.0 ml/dm3 only 4.67-1.01 mg/dm3 of nitrates.
- The study confirms the need to assess the impact of detergents (not only surfactants) and wastewater before being discharged into the emissary.
References
- Belingher Mihaela-Liliana, Chimerel Mircea-Eleodor, (2011) Sursele de azot şi bazele procesului de nitrificare-denitrificare. Analele Universităţii “Constantin Brâncuşi” din Târgu Jiu, Seria Inginerie, nr. 2:196-203. ISSN 1842-4856.
- Hotărârea Guvernului nr. 890 din 12.11.2013 pentru aprobarea Regulamentului cu privire la cerinţele de calitate a mediului pentru apele de suprafaţă. MO din 22.11.2013, Nr. 262-267, art. Nr.: 1006.
- Dumitru Mihail, Simota Cătălin, Cioroianu Traian, (2015) Codul bunelor practici agricole pentru protecţia apelor împotriva poluării cu nitraţi din surse agricole. Bucureşti, eKarioka, 164. ISBN 978-606-94088-0-3.
- Lozan Raisa, Tărîţă Anatol, Sandu Maria, et al. (2015) Starea Geoecologică a apelor de suprafata si subterane in bazinul hidrografic al Marii Negre (in limitele Republicii Moldova). Ch, 326. ISBN 978-9975-9611-2-7.
- Tărîţă Anatol, Sandu Maria, Moşanu Elena, Cozari Tudor, Lozan Raisa, (2020) Evaluarea componenţei fizico-chimice şi indicii de calitate a apelor conexe ariilor naturale protejate de stat din raionul Anenii Noi. “Instruire prin cercetare pentru o societate prosperă”, conferinţă ştiinţifico-practică, 21-22 martie 2020. 1: Biologie. Chişinău: S. n, 143-154. ISBN 978-9975-76-306-6.
- Garrido J, W van Benthum M, van Loosdrecht, J Heijnen, (1997) Influence of dissolved oxygen concentration on nitrite accumulation in a biofilm airlift suspension reactor. Biotechnol. Bioeng, nr. 53, 168-178.
- Sandu Maria (2005) Toxicity of pesticides in the presence of heavy metals on biochemical oxidation of ammonia ions. Environmentaly sound management (ESM) practices on cleaning up obsolete stockpiles of pesticides for Central European and EECCA Countries, Sofia, 179-181. ISBN-10: 954-9467-11-2; ISBN-13:978-954-9467-11-6.
- Sandu M, Lozan R, Ropot V, Munteanu V, Rusu V Rolul (1995) fenolului în procesele de autoepurare ale apelor de suprafaţă. Conf. “Protecţia mediului – parte a restructurării economiei româneşti”, Bucureşti, 21-23 septembrie, 272-277.
- Spătaru P, Sandu Maria, Dragalina Galina, Arapu Tatiana, Lozan Raisa, (2003) Influenţa produselor petroliere asupra procesului de oxidare biochimică a ionilor de amoniu. Buletinul AŞM. Seria de ştiinţe biologice, chimice şi agricole. Chişinău, nr. 2(291), 128-130.
- Spătaru P, Sandu M, Tărîţă A, Lupaşcu T, Negru M, et al. (2011) Ţurcan S. Impactul substanţelor tensioactive asupra oxidării biochimice a ionilor de amoniu în apele naturale. Buletinul Academiei de Ştiinţe a Moldovei, Seria Ştiinţele Vieţii. Chişinău, 2 (311), 178-184. ISSN 1857-064X.
- Sandu Maria. Impactul agenţilor activi de suprafaţă asupra mediului şi ajustarea legislaţiei naţionale la condiţiile UE. Buletinul AŞM. Ştiinţele vieţii. Nr. 2(332) 2017, p. 173-179. ISSN 1857-064X.
- Ivankovic T, Hrenovic J, (2010) Surfactants in the Environment. Arh. Hig. Rada Toksicol, no. 61:95-110.
- Olkowska E, Ruman M, Kowalska A, Polkowska Ż, (2013) Determination of surfactants in environmental samples. Part II. Anionic compounds. Ecological Chemistry and Engineering S. vol. 20, no. 2, 331-342.
- SM SR EN 26777:2006. Calitatea apei. Determinarea conţinutului de nitriţi. Metoda prin spectrometrie de absorbţie moleculară.
- SM SR ISO 10523:2011. Calitatea apei. Determinarea pH-lui.
- SM SR ISO 7150-1:2005. Calitatea apei. Determinarea conţinutului de amoniu. Partea 1: Metoda spectrometrică manuală.
- Sandu Maria, Lupascu Tudor, Tarita Anatol, (2014) Method for nitrate determination in water in the presence of nitrite. Chemistry Journal of Moldova. General, Industrial and Ecological Chemistry. Chişinău, 9:8-13. ISSN 1857-1727.
- Hotărârea Guvernului nr. 950 din 25.11.2013 pentru aprobarea Regulamentului privind cerinţele de colectare, epurare şi deversare a apelor uzate în sistemul de canalizare şi/sau în emisaruri de apă pentru localităţile urbane şi rurale. MO nr. 284-289 din 06.12.2013, art. nr. 1061.
- Grunditz C, Dalhammar G, (2001) Development of nitrification inhibition assays using pure cultures of Nitrosomonas and Nitrobacter. Water Res, 35:433-40.
- Regulation (EC) No 648/2004 of the European Parliament and of the Council of 31.03.2004 on detergents.
- Markina ZV, Aizdaicher NA (2007) Influence of laundry detergents on the abundance dynamics and physiological state of the benthic microalga Attheya surensis(Bacillariophyta) in laboratory culture. Russ J Mar Biol 33:391-398.
- Azizullah A, Richter P, Jamil M, Häder DP (2012) Chronic toxicity of a laundry detergent to the freshwater flagellate Euglena gracilis. Ecotoxicology 21:1957-1964.
- Info Mine Research Group (2012) Synthetic detergents and cleaning products in the CIS and Baltic Countries: production, market and forecast. Info Mine Market Research Group.
- Uc-Peraza RG, Delgado-Blas VH (2012) Determinación de la concentración letal media (CL50) de cuatro detergentes domésticos biodegradables en Laeonereis culveri(Webster, 1879) (Polychaeta, Annelida). Rev. Int. Contam. Ambient 28:137-144.