Publication Information
ISSN: 2641-693X
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Hedinger Syndrome: Report of Two Cases
Marina Santos1*, Andreia Pereira1, Susana Gomes1
1Department of Cardiology, Dr. Nélio Mendonça Hospital, Avenida Luís de Camões, Funchal, Portugal
Received Date: October 05, 2019; Accepted Date: October 15, 2019; Published Date: October 24, 2019
*Corresponding Author: Marina Santos, Dr. Nélio Mendonça Hospital, Avenida Luís de Camões, nº 57 – 9004-514 Funchal, Portugal. Tel: +351291705600; Email: m.raquel.santos1992@gmail.com
Citation: Santos M, Pereira A, Gomes S (2019) Hedinger Syndrome: Report of Two Cases. Int Jr Cardiac Scie and Res: IJCSAR-112.
Abstract
Carcinoid tumors are rare malignant neoplasms that arise from enterochromaffin cells located in the gastrointestinal tract and bronchopulmonary system. These are typically indolent and secrete multiple vasoactive substances, which are responsible for the carcinoid syndrome characterized by flushing, diarrhea and bronchospasm. Carcinoid heart disease (also called Hedinger syndrome) occurs in more than 50% of patients with carcinoid syndrome, and in some cases, it represents the initial manifestation of the disease. The valves and endocardium of the right side are often affected by the disease, but the left side of the heart is usually spared. Cardiac involvement is associated with a poor long-term prognosis and multidisciplinary approach is necessary to control symptoms and progression of the disease. Some cases present good control of symptoms with somastatin analogues; however, evolution into heart failure is expected. Valve replacement is a therapeutic option that can be considered in selected patients. The authors describe the case of two female patients with Hedinger syndrome. The first patient, a 74-year-old female, died one year after the diagnosis, before initiation of somastatin analogues. The second patient, a 57-year-old female, has been medicated with octreotide and chemotherapy with symptomatic control.
Keywords: Carcinoid Tumor; Hedinger Syndrome; Somastatin Analogues; Transthoracic Echocardiography; Valvular Disease
Introduction
Carcinoid tumors are rare neuroendocrine tumors (NET) with a prevalence of 1.2 to 2.1 cases per 100.000 inhabitants arising from enterochromaffin cells, so they can occur in a wide variety of organs, including the gastrointestinal tract, lungs and ovaries [1].
These tumors are typically indolent and secret a variety of mediators, including serotonin; however, its precise pathogenesis remains elusive [1]. Indolent growth determines that 20 to 30% of patients present disseminated disease upon diagnosis [1-3]. Patients are often asymptomatic until the tumors metastasize to the liver, which cannot then inactivate the vasoactive substances. The most common manifestations of carcinoid syndrome are vasomotor symptoms (flushing), hypotension, secretory diarrhea, abdominal cramps, and bronchospasm [4, 5].
Carcinoid tumors are a rare cause of acquired valvular heart disease, although cardiac involvement has been described in up to 60% of patients with carcinoid syndrome [6]; therefore, screening for heart involvement with transthoracic echocardiography is essential with a newly diagnosed carcinoid tumor [7].
Carcinoid heart disease (CHD), also called Hedinger syndrome, may be the initial presentation of the syndrome in up to 20% of patients [2, 8]. Cardiac involvement can vary widely in patients, but generally the left side of the heart is spared because the lungs can inactivate the vasoactive substances [9]. In fact, all heart valves may be affected, but the involvement of the tricuspid valve is most common [10]. Tricuspid leaflets show a specific involvement with thickened leaflets that have reduced mobility and are retracted and the concomitant regurgitation can vary from mild to severe [11]. Right ventricular volume overload can result in right heart failure.
The management of Hedinger syndrome necessitates multidisciplinary control of progressive heart failure, treatment of systemic malignancy and neuroendocrine derangements, and surgical correction of right-sided valvular involvement [5]. Despite the treatment, cardiac involvement is associated with a poor long-term prognosis: the estimated 3-year survival rate of 31% is half that of patients without cardiac involvement [12, 13].
In this article we present two patients, each with a metastatic carcinoid tumor, and whose clinical presentations were complicated by cardiac involvement.
Case One
A 74-year-old female patient presented to general practitioner in February 2019 with anorexia, chronic diarrhea and weight loss (about 5 kg) in the last 3 months. On physical exam there was abdominal pain on right lower quadrant and a solid and heterogeneous mass was palpated. An abdominal ultrasound was requested which showed hepatic nodules compatible with metastasis but was non-diagnostic. Therefore, computed tomography (CT) of the abdomen was performed (Figure 1) and a solid mesenteric mass (4x3cm on axial plan) was highlighted in the right iliac fossa, with central calcification and surrounding desmoplastic reaction, conditioning retraction of the adjacent intestinal loops. Multiple hipervascular liver lesions (the largest with 4x3.5cm) compatible with secondary lesions and multiple adenopathy were also demonstrated. Suspicion for carcinoid syndrome was raised, and consequently, plasma and urine biomarkers were measured. Serum chromogranin A (96.5 µg/dL, normal range <10.2 µg/dL) and urinary 5-HIIA (60.0 mg/24h, normal range 0.7-8.2 mg/24h) excretion levels were severely elevated. In March 2019 the patient was admitted to the hospital to do CT-guided biopsy. The pathological anatomy confirmed the diagnosis of grade 2 neuroendocrine tumor and the patient was referred to oncology and started and appetite stimulant.

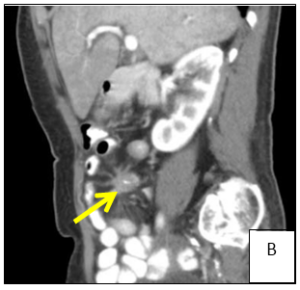
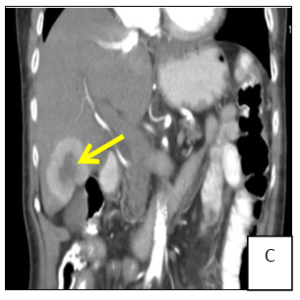
Figure 1: Computer tomography showing a solid mesenteric mass (arrow) in the right iliac fossa, with central calcification and surrounding desmoplastic reaction, conditioning retraction of the adjacent intestinal loops (A/B). Multiple hipervascular liver lesions (the largest with 4x3.5cm, arrow) compatible with secondary lesions and multiple adenopathy (C).
In April 2019 she presented to the emergency room with fatigue and worsening dyspnea [class III, New York Heart Association (NYHA)]. She also complained of leg swelling in the last week. She denied diarrhea. On physical exam, blood pressure was 134/34 mmHg, heart rate was 80 bpm (sinus rhythm), peripheral capillary oxygen saturation was 91% with increased respiratory rate and body mass index was 20 kg/m2. She had no fever. Clinical pathological findings included bibasilar pulmonary crackles, jugular vein distension and edema in both legs. On cardiac auscultation a grade 3/6 pansystolic murmur was heard at the left sternal border. Arterial blood gas analysis showed respiratory alcalemia with mild hypoxemia. The electrocardiogram (ECG) showed left axis deviation and poor R wave progression on precordial leads. Thoracic radiography was compatible with cardiac heart failures. Blood tests showed a mild increase in inflammatory markers and an N-terminal pro-brain natriuretic peptide (NT- proBNP) of 17350 pg/ml. She was admitted to the cardiology unit for clinical stabilization and intravenous (IV) diuretics were started. The patient underwent transthoracic echocardiography (TTE) (Figure 2) which showed mild left ventricle dilation and severe left auricle dilation (49 ml/m2) with no wall motion abnormalities and preserved global systolic function. The right chambers were mildly enlarged with good right ventricular global systolic function [tricuspid annular plane systolic excursion (TAPSE) 23 mm]. Severe aortic regurgitation due to retraction of left coronary cuspid (tricuspid valve) was found. The tricuspid and mitral valve leaflets were visibly thickened, preventing central coaptation and causing moderate tricuspid and mitral regurgitations. Systolic pulmonary artery pressure (SPAP) was found to be 63 mmHg. During hospitalization an oncologist observed the patient but was of the opinion in not initiating analogues of somastatin while she not stabilized. Patient showed a favorable evolution and was discharged after 10 days medicated with furosemide (60mg id). However, the patient died a few days later, even before the start of somastatin analogues.
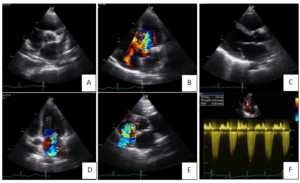
Figure 2: Transthoracic echocardiography. A. Parasternal short axis view showing tricuspid aortic valve with thickened leaflets. B. Apical 4 chamber view shows how retraction of left coronary cuspid of the aortic valve conditions a severe regurgitation. C. Parasternal long axis view showing mitral valve leaflets thickened, with good opening but retraction of posterior leaflet. D. Moderate mitral regurgitation. E. Moderate tricuspid regurgitation. F. Systolic pulmonary artery pressure of 63mmHg.
Case Two
A 57-year-old female patient with no relevant personal history presented to emergency department in September 2018 with abdominal pain and dyspnea (NYHA class III). On physical examination a solid mass at right upper quadrant was palpated. She underwent and thoracic/ abdominal/ pelvic CT (Figure 3) which showed an enlarged liver with hypertrophy of the left lobe and multiple hipervascular lesions. She was admitted at surgery unit to further study. The patient underwent an upper and lower digestive endoscopy which showed no significant lesions. Elevation of serum chromogranin A (50.3/dL, normal range <10.2 µg/dL) and urinary 5-HIIA (65.6 mh/24h, normal range 0.7- 8.2 mh/24h) raised the suspicion of NET. Pelvic ultrasound showed an ill-defined lesion adjacent to the right ovary and CEA was elevated. Liver laparoscopic biopsy confirmed the diagnosis of a well differentiated NET of unknown origin (digestive system? ovary?). Positron emission tomography only demonstrated multiple sites of abnormal uptake in the liver. After multidisciplinary approach, lesions were considered unresectable and the patient was discharged and referred to oncology. Analgesics were started.

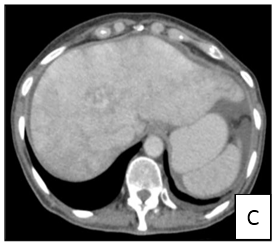

Figure 3: Computer tomography showing enlarged liver with hypertrophy of the left lobe and multiple hipervascular lesions in multiple projections (1A, B and C).
Two months later she was admitted to the emergency room with complains of dyspnea (class III NYHA), orthopnea and paroxysmal nocturnal dyspnea. She also complained of hot flashes, diarrhea and abdominal swelling. On physical exam, bool pressure was 110/62mmHg and heart rate was 131bpm (arrhythmic). Clinical pathological findings included erythema on the face, jugular vein distension, bibasilar reduction of pulmonary vesicular sounds, systolic murmur in tricuspid focus and abdominal swelling with signs of ascites. The ECG showed atrial flutter. Thoracic radiography exhibited bilateral pleural effusion. Blood tests showed cholestasis and a NT-proBNP of 3674pg/ml. She was admitted to the hospital to start IV medication. TTE (Figure 4) showed mild left auricle dilation (42ml/m2) with no wall motion abnormalities and preserved global systolic function. The right chambers were enlarged (right auricle 54.6ml/m2), but there was a good right ventricular global systolic function. Aortic and mitral valves leaflets were thickened conditioning moderate regurgitations. The tricuspid valve was semi-opened and fixed with thickened leaflets, causing a severe tricuspid regurgitation. Systolic pulmonary artery pressure (SPAP) was found to be 76mmHg. Patient was discharged after 6 days clinically better. She was medicated with furosemide (40mg id), rivaroxaban (20mg id) and carvedilol (6.25mg bid).
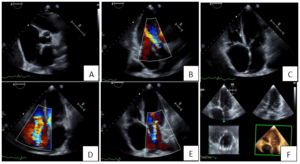
Figure 4: Transthoracic echocardiography. A. Short axis view showing tricuspid aortic valve with thickened leaflets. B. Apical 3 chamber view shows a moderate aortic regurgitation. C. Apical 4 chamber view shows semi-opening and fixation of tricuspid valve leaflets. D. Moderate tricuspid regurgitation. E. Moderate mitral regurgitation. F. 3D imaging of mitral valve leaflets.
In January 2019, on a routine consultation, despite slight symptomatic improvement, the patient still had dyspnea (class II NYHA) and diarrhea. On physical examination there was still a reduction of pulmonary vesicular sounds and cardiac frequency was 80bpm. For those reasons’ digoxin (0.125mg id) and spironolactone (25mg id) was prescribed. Also, with the aim to improve vasoactive symptoms, the patient started octreotide. During the follow-up the patient referred improvement of diarrhea complains and dyspnea.
Discussion
NETs are heterogeneous neoplasms arising from neuroendocrine precursor cells of different tissues. Approximately 90% of all carcinoid tumors are in the gastrointestinal system and other less common sites include the bronchus and gonads [13]. Carcinoid tumors can secrete a large amount of vasoactive substances (serotonin, histamine, tachykinins and prostaglandins), which are largely inactivated by the liver. Carcinoid tumors usually grow slowly, over years, commonly causing few or no symptoms until they are large or have metastasized to the liver and, less frequently, to other sites (e.g., the lungs or bone) [14]. In the setting of liver metastasis vasoactive substances produce systemic effects leading to characteristic symptoms [13].
Patients with carcinoid syndrome have a propensity to develop plaque-like deposits consisting of smooth muscle, myofibroblasts, connective tissue and an overlying epithelial layer on heart valves. Retraction and fixation of valves ensues, leading to severe deformity and resultant regurgitation and stenotic lesions [2, 13]. Usually only carcinoid tumors that invade the liver result in pathological changes to the heart. The vasoactive substances will cause structural changes in the endocardium of the right heart chambers [9], in approximately 90% of the cases [14]. Left-sided disease can be encountered in up to one-third of cases in the presence of patent foramen ovale with right-to-left shunt, bronchial carcinoid, and high circulating levels of vasoactive substances overwhelming the hepatic and pulmonary degradative capacity [2].
CHD typically involves the tricuspid and pulmonary valves, causing right heart failure. Involvement of the aortic and mitral valves is rarely reported [9, 15]. Tricuspid valve with severe regurgitation appears in 90% of the cases; pulmonary valve with stenosis in 53%, and regurgitation in 81% [16]. The tricuspid valve leaflets and subvalvular structures are often thickened, shortened and retracted, leading to incomplete coaptation and moderate to severe tricuspid regurgitation.
The diagnosis of Hedinger syndrome depends upon strong clinical suspicion, biomarker levels, cardiac imaging and possibly the use of cardiac nuclear medicine [14]. In the presence of CHD, increased urinary 5-HIAA levels and chromogranin a are useful in evaluating cardiac involvement and disease progression. NT-proBNP has both diagnostic and prognostic significance [17]. The combination of NT-proBNP and chromogranin A has been shown to determine survival probability, as patients with elevated levels of both parameters have only a survival probability of 16% compared with 81% in patients with normal levels of both parameters [18]. Transthoracic Echocardiography is the imaging modality of choice in assessing the presence and degree of cardiac involvement [19]. The European neuroendocrine tumor society (ENTS) recommends TTE screening for patients diagnosed with carcinoid syndrome, annually for patients with no cardiac involvement and semiannually for those with it [20].
The main predictor of clinical outcome in patients with carcinoid syndrome is the extent of cardiac involvement. The therapeutic approach to the heart disease is complex, and careful evaluation with complementary imaging tests is critical for proper recognition of the disease patterns [21]. In those with carcinoid syndrome treatment tends to be palliative as metastatic spread as already occurred. Management of patients with CHD can be divided into the treatment of right heart failure, pharmacotherapy to reduce the secretion of tumor products, and surgical/ interventional treatment of valvar pathology [22].
To alleviate symptoms and to prevent development of carcinoid syndrome, long-acting somastatin analogues are used as a first-line treatment [22]. Octreotide is an eight amino acid peptide that, by binding to somastatin receptors, has the direct effect of reducing the vasoactive peptides that provoke the carcinoid syndrome [23]. Although some cases present good control of symptoms with the use of somastatin analogs, evolution into heart failure is expected. Diuretics together with fluid and salt restriction are only temporarily effective for symptom control [22]. In advanced right ventricular failure, these therapies become ineffective. In patients with carcinoid heart, valve surgery is the only effective therapy. In suitable candidates, valve surgery is the only definitive treatment for severe right heart failure, as the carcinoid syndrome may produce pronounced morphological defects of the right sided valves. Patients with carcinoid heart disease usually die because of severe tricuspid regurgitation rather than carcinomatosis [22].
Conclusion
CHD is a frequent occurrence in patients with carcinoid syndrome, accounting for substantial morbidity and mortality. Its pathophysiology remains unclear; however chronic exposure to circulating vasoactive substances seems one of the most important contributing factors.
Patients with carcinoid syndrome and high suspicion of Hedinger syndrome, according to clinical features or raised NT-proBNP and/or 5-HIAA and/or chromogranin A, should perform a transthoracic echocardiography. Transthoracic echocardiography remains the gold standard for diagnosis and follow-up of CHD.
The standard treatments to alleviate symptoms related to the syndrome and prevent the development and/or progression of CHD are the somatostatin analogues. The patient with CHD should be managed by a specialized multidisciplinary team, within a setting of a specialized NET center. Management should be individualized for each patient, and a holistic approach should be considered.
Competing interests: The authors declare that they have no competing interests.
References
- Modlin IM, Sandor A (1997) An analysis of 8305 cases of carcinoid tumors. Cancer 79: 813-829.
- Bhattacharyya S, Davar J, Dreyfus G, Caplin ME (2007) Carcinoid heart disease. Circulation 116: 2860-2865.
- Schnirer II, Yao JC, Ajani JA (2003) Carcinoid: a comprehensive review. Acta Oncol 42: 672-692.
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD (2005) Current status of gastrointestinal carcinoids. Gastroenterology 128: 1717-1751.
- Grozinsky-Glasberg S, Grossman AB, Gross DJ. (2015) Carcinoid heart disease: from pathophysiology to treatment - ‘something in the way it moves’. Neuroendocrinology 101: 263- 273.
- Liu J, Backx PH (2014) Patch-clamp technique in ESC-derived cardiomyocytes. Methods Mol Biol 1181: 203-214.
- Poulin H, Bruhova I, Timour Q, Theriault O, Beaulieu JM, et al. (2014) Fluoxetine blocks Nav1.5 channels via a mechanism similar to that of class 1 antiarrhythmics. Mol Pharmacol 86: 378-389.
- Westberg G, Wängberg B, Ahlman H, Bergh CH, Beckman-Suurküla M, et al. (2001) Prediction of prognosis by echocardiography in patients with midgut carcinoid syndrome. Br J Surg 88: 865-872.
- Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, et al. (1993) Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation 87: 1188-1196.
- Dobson R, Burgess MI, Pritchard DM, Cuthbertson DJ (2014) The clinical presentation and management of carcinoid heart disease. Int J Cardiol 173: 29-32.
- Engelsman AF, van Duijvendijk P, Groenemeijer BE, van der Zaa E, Spronk PE, et al. (2012) Tricuspid valve regurgitation as a presenting symptom of metastasized carcinoid tumor. Case Rep Gastroenterol 6: 643-649.
- Moller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J, et al. (2005) Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation 112: 3320-3327.
- Fox DJ, Khattar RS (2004) Carcinoid heart disease: presentation, diagnosis, and management. Heart 90: 1224- 1228.
- Davar J, Connolly HM, Caplin ME, Pavel M, Zacks J et al. (2017) Diagnosing and managing carcinoid heart disease in patients with neuroendocrine tumors: an expert statement. J Am CollCardiol 69:1288-1304.
- Connolly HM, Schaff HV, Mullany CJ, Rubin J, Abel MD, et al. (2001) Surgical management of left-sided carcinoid heart disease. Circulation. 104: I36-140.
- Moerman VM, Dewilde D, Hermans K. (2012) Carcinoid heart disease: typical findings on echocardiography and cardiac magnetic resonance. Acta Cardiol 67: 245-248.
- Bhattacharyya S, Toumpanakis C, Caplin ME, Davar J (2008) Usefulness of N-terminal pro-brain natriuretic peptide as a biomarker of the presence of carcinoid heart disease. Am J Cardiol 102: 938-942.
- Korse CM, Taal BG, de Groot CA, Bakker RH, Bonfrer JM (2009) Chromogranin- A and N-terminal pro-brain natriuretic peptide: an excellent pair of biomarkers for diagnostics in patients with neuroendocrine tumor. J Clin Oncol 27: 4293-4299.
- Bernheim AM, Connolly JM, Hobday TJ, Abel MD, Pellikka PA (2007) Carcinoid heart disease. Prog Cardiovasc Dis 49: 439-451.
- Arnold R, Chen YJ, Costa F, Falconi M, Gross D, et al. (2009) ENETS consensus guidelines for the standards of care in neuroendocrine tumors: follow-up and documentation. Neuroendocrinology 90: 227-233.
- Gustafsson BI, Hauso O, Drozdov I, Kidd M, Modlin IM (2008) Carcinoid heart disease. Int J Cardiol 129: 318-324.
- Simona Grozinsky-Glasberg ABG, Gross DJ (2015) Carcinoid heart disease: from pathophysiology to treatment– ‘something in the way it moves’. Neuroendocrinology 101: 263-273.
- Matthew HK, Robert JM (1999) Carcinoid tumours. N Engl J Med 340: 858-868.