Publication Information
ISSN 2691-8803
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Gallic Acid Novel Derivatives Improve Cytotoxicity and Oxidative Stress in Non-Small Cell Carcinoma Cell Lines
Shohreh Jafarinejad1, William H. C. Martin1, Richard D. Bowen1, Zahra Adol1, Mohammad Isreb2, Amie Saidykhan1, Badie Jacob3, Mojgan Najafzadeh1⁕
1School of Chemistry and Biosciences, Faculty of Life Sciences, University of Bradford, Bradford BD7 1DP, UK
2School of Pharmacy, Faculty of Life Sciences, University of Bradford, Bradford BD7 1DP, UK
3Bradford Royal Infirmary, Bradford Teaching Hospitals NHS. Foundation Trust, Duckworth Lane, Bradford, West Yorkshire, BD9 6RJ, UK
Received Date: July 02, 2023; Accepted Date: July 10, 2023; Published Date: Aug 12, 2023;
*Corresponding author: Mojgan Najafzadeh. Assistant professor in Biomedical Sciences, The University of Bradford, Richmond Road, Bradford, West Yorkshire, BD7 1DP, Tel: + 441274 232323, Fax: + 441274 309 742. Email: m.najafzadeh1@bradford.ac.uk
Citation: Jafarinejad S, Martin W H C, Bowen R D, Adol Z, Isreb M, et al. (2023) Gallic Acid Novel Derivatives Improve Cytotoxicity and Oxidative Stress in Non-Small Cell Carcinoma Cell Lines. Adv Pub Health Com Trop Med: APCTM-182.
10.37722/APHCTM.2023401
Abstract
Gallic acid (GA) is a natural and potent antioxidant which stimulates cancer cell apoptosis. However, its therapeutic use is limited due to poor oral permeability. In order to increase GA’s antioxidant capacity and oral permeability, we synthesised a series of GA analogues: 4-methoxybenzenesulfonamide (MBS), 3,4-dimethoxybenzenesulfonamide (DMBS), 3,4-dimethoxy-N-methylbenzenesulfonamide (DMBS-Me), N-ethyl-3,4-dimethoxybenzenesulfonamide (DMBS-Et) and 3,4,5-trimethoxybenzenesulfonamide (TMBS).
In these compounds, we replaced hydroxyl groups with various numbers of methoxy groups to increase hydrophobicity and oral permeability in comparison to GA. We also replaced the carboxylic group with a sulfonyl group to increase the compounds’ molecular polarity and antioxidative activities. The cell counting kit-8 (CCK-8) assay was used to detect the effect of each compound on cell proliferation and apoptosis in peripheral blood mononuclear cells (PBMCs) from healthy individuals and in non-small cell lung carcinoma A549 cells. In addition, the comet assay was used to assess the genotoxic potential of these compounds in A549 cells. Tested concentrations of GA and TMBS were not cytotoxic in PBMCs from healthy donors but drastically reduced the survival of carcinoma cells. Furthermore, in comparison to GA, TMBS was more cytotoxic in A549 cells, suggesting that TMBS demonstrates therapeutic potential in cancer. In PBMCs from healthy donors as well as A549 cells, 30 µM DMBS, DMBS-Me and DMBS-Et showed reduced cell survival rates (p<0. 0001) in a dose-dependent manner compared to untreated cells. In A549 cells, 30 µM DMBS, DMBS-Me and DMBS-Et caused greater DNA damage than other compounds in comparison to untreated cells. Overall, the results demonstrate that TMBS, a novel sulfonamide derivate of GA, demonstrates therapeutic potential in cancer.
Introduction
Naturally occurring chemicals present in plants are promising options to improve the efficiency of treatments in cancer patients with improved treatment outcomes and reduced adverse reactions. Numerous studies have demonstrated that natural polyphenols can be used for the prevention and treatment of cancer (Zhou et al., 2016). Polyphenols possess a common chemical structure comprising at least one aromatic ring attached to at least one hydroxyl group (Pandey et al.,2009). Polyphenols are secondary metabolites of plants, the complexity of which increases from simple phenolic acids to highly polymerised tannins (Pandey et al.,2009). Based on their chemical structures, natural polyphenols are classified into subclasses including flavonoids, phenolic acids, lignans, stilbenes and other polyphenols. The anticancer activity of polyphenols is attributed to their potent antioxidant and anti-inflammatory properties, and their regulation of molecular targets and signalling pathways that serve as drivers for carcinogenesis including proliferation, migration, differentiation, angiogenesis, cell survival and immune responses (Pandey et al.,2009).
One such polyphenol with anti-tumour potential is gallic acid (GA), which is also known as 3,4,5-trihydroxybenzoic acid and has the chemical formula C6H2(OH)3COOH (Kahkeshani et al., 2019). GA is a simple planar compound of low molecular weight (170.12 g/mol) ( Badhani et al., 2015) that chemically contains a benzene ring linked to three hydroxyl groups and a carboxylic acid group ( Sroka et al., 2003). GA is one of the most abundant phenolic acids in the plant kingdom and is present in tea leaves, gallnuts, walnuts, sumac, witch hazel, blueberries, and numerous other sources (Kahkeshani et al., 2019). GA exhibits anticancer, anti-inflammatory, antimalarial and antiviral properties. In addition, GA has antimicrobial effects against Bacillus subtilis, Escherichia coli and Staphylococcus aureus (Subramanian et al., 2015). Furthermore, GA has potent antioxidant activities relating to radical scavenging activity, metal chelation and redox properties, as well as being an efficient apoptosis inducing agent (Badhani et al., 2015).
Mono- and dimethyl ether sulfonamides MBS and DMBS have been extensively studied in medicinal chemistry and have been used as synthetic intermediates in drug discovery (Moffatt and Lerch, 2002; Daly et al., 2002). However, three GA derivatives were synthesised, namely mono-, di- and trimethoxy sulfonamides (MBS, DMBS and TMBS). Here, the hydroxyl groups were replaced with 1, 2 or 3 methoxy groups which are stronger electron-donating groups, with a view to increasing hydrophobicity and oral permeability in comparison to GA. However, this reduces the antioxidant capacity of GA derivatives (Subramanian et al., 2015). Therefore, the carboxylic acid of GA derivatives was replaced with a sulfonamide moiety (figure 1), a stronger electron withdrawing group (Rehman et al., 2017) with a view to increasing the molecular polarity and antioxidative activities of the GA derivatives (Zhongbing, 2006). Since sulfonamide derivatives are powerful antioxidants (Rehman et al., 2017; Muhammad-Ali et. al., 2019), the sulfonamide moiety mitigates the loss of antioxidative activity which the loss of phenolic groups would otherwise cause. Thereafter, the CCK-8 assay was used to assess the cytotoxicity of GA derivatives in PBMCs and non-small cell lung carcinoma A549 cells. Additionally, the compounds were tested for their genotoxicity in A549 cells using the comet assay.
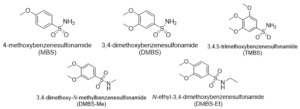
Figure 1: Target compounds.
Methodology
Materials and Methods
General information
Chemicals were purchased from Sigma-Aldrich and were used without further purification. All reactions were carried out in a fume cupboard and monitored by analytical thin layer chromatography (TLC) under ultraviolet (UV) light. 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were obtained on a Bruker 400/100 MHz NMR spectrometer using chloroform-D (CDCl3) as a solvent. Infrared spectroscopy (IR) spectra were obtained using a Bruker FTIR spectrophotometer.
Synthesis of gallic acid derivatives
The synthetic sequence of the target compounds began with the use of commercially available anilines, which followed the well-established sequence of conversion to diazonium salt. Next, anilines underwent sulfonation to sulfonyl chloride by the action of chlorosulfonic acid. Afterwards, primary sulfonamide was obtained from sulfonyl chloride via treatment with ammonium hydroxide.
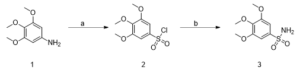
Scheme 1: Synthesis of a gallic acid derivative (3) Reagents and conditions: (a) 3,4,5-trimethoxyaniline, acetic acid, 12M HCl, NaNO2, CuCl2, SO2, H2O, -5 (3h) to room temp, 12 h (RT), 60%; (b) 25% NH4OH solution, THF, 70°C, 30 min, 50%.
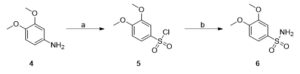
Scheme 2: Synthesis of a gallic acid derivative (6) Reagents and conditions: (a) 3,4-dimethoxyaniline, acetic acid, 12M HCl, NaNO2, CuCl2, SO2, H2O, -5 (3h) to room temp, 12 h, 60%; (b) 25% NH4OH solution, THF, 70°C, 30 min, 70%.
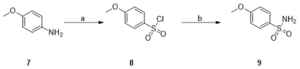
Scheme 3: Synthesis of a gallic acid derivative (9) Reagents and conditions: (a) 3,4-dimethoxyaniline, acetic acid, 12M HCl, NaNO2, CuCl2, SO2, H2O, -5 (3h) to room temp, 12 h, 60%; (b) 25% NH4OH solution, THF, 70°C, 30 min, 80%.
General procedures for the synthesis of precursor compounds 2, 5, and 8 (a)
Compound 2, 3,4,5-trimethoxybenzenesulfonyl chloride was synthesised based on the procedure described in Binisti et al., 1997 with slight modification. A solution of 3,4,5-trimethoxyaniline (3.0 g, 0.02 mol) in acetic acid (16 ml) and 12M HCl (27 ml) at -10 ◦C was slowly treated with a solution of NaNO2 (1g, 0.01 mol) in 4 ml water. The mixture was stirred at -10◦C for 30 minutes. After 30 minutes, the solution of diazonium salt was slowly added to a cold (-5◦C) solution of acetic acid (7 ml) saturated with copper (II) chloride. Following this, sulfurous acid was added then stirred at -5◦C for 3 hours. Then the solution was stirred at room temperature for 24 hours and poured over ice. The solid precipitation was filtered and dried to obtain 3,4,5-trimethoxybenzenesulfonyl chloride (compound 2). Compounds 5 and 8 were synthesised in a similar manner, adjusting for the molar concentration of reagents based on the molecular weight of starting compounds.
General procedures for the synthesis of TMBS, DMBS and MBS (b)
Compound 2 was used in the next step of the synthetic route based on the procedure described in Burri et al. 1993. Compound TMBS was prepared as follows: a solution of 3,4,5-trimethoxybenzenesulfonyl chloride, 2, (3.0 g, 0.01 mol) in tetrahydrofuran was cooled in an ice bath, then 15 ml of 25% NH4OH was added dropwise. The reaction mixture was stirred vigorously in a flask placed in an oil bath at 70◦C for 30 minutes. Next, thin layer chromatography (TLC) confirmed the completion of the reaction, and the tetrahydrofuran was removed by distillation. The residue was extracted with ethyl acetate. The crude was chromatographed (1:2 EtOAc-petroleum ether) to obtain the brown product TMBS. MBS and DMBS were synthesised in a similar manner, adjusting for the molar concentration of reagents based on the molecular weight of starting compounds.
3,4-dimethoxy-N-methylbenzenesulphonamide, 3,4-(CH3O)2C6H3SO2NHCH3
A solution of 3,4-dimethoxybenzenesulphonyl chloride (1.25 g, 5.28 mmol) in warm tetrahydrofuran (20 mL) was prepared, cooled to 0 °C, and added dropwise during 20 minutes to a magnetically stirred ethanolic solution of methylamine (33%, w/v, 10 mL, corresponding to 159 mmol of methylamine) whilst maintaining the temperature between -5 and 0 °C). After 10 minutes, TLC showed no residual starting material but only a single new spot at much lower rf, corresponding to the sulphonamide product (together with a little baseline material).
The reaction mixture was poured into water (150 mL), acidified with dilute hydrochloric acid (2M), and the sulphonamide was extracted with dichloromethane (6 x 40 mL). The combined organic extracts were washed with aqueous sodium carbonate solution (10% w/v, 20 mL), dried (MgSO4), filtered and rotary evaporated to constant mass, with final removal of residual solvent at rotary pump pressure to give a white solid (0.81 g, 66%). Careful recrystallisation from ethanol/water gave N-methyl-3,4-dimethoxybenzenesulphonamide as white crystals (0.67 g, 55%).
N-Ethyl-3,4-dimethoxybenzenesulphonamide, 3,4-(CH3O)2C6H3SO2NHC2H5.

A solution of 3,4-dimethoxybenzenesulphonyl chloride (1.25 g, 5.28 mmol) in warm tetrahydrofuran (20 mL) was prepared, cooled to 0 °C and added dropwise during 20 minutes to a magnetically stirred aqueous solution of ethylamine (25%, w/v, 20 mL, corresponding to 111 mmol of ethylamine) whilst maintaining the temperature between -5 and 0 °C). After 10 minutes, TLC showed no residual starting material but only a single new spot at much lower rf, corresponding to the sulphonamide (together with a little baseline material).
The reaction mixture was poured into water (150 mL), acidified with dilute hydrochloric acid (2M), and the sulphonamide was extracted with dichloromethane (6 x 40 mL). The combined organic extracts were washed with aqueous sodium carbonate solution (10% w/v, 20 mL), dried (MgSO4), filtered and rotary evaporated to constant mass, with final removal of residual solvent at rotary pump pressure to give a white solid (0.87 g, 67%). Careful recrystallisation from ethanol/water gave N-ethyl-3,4-dimethoxybenzenesulphonamide as white crystals (0.69 g, 53%).
3,4,5-trimethoxybenzenesulfonamide (TMBS)
Brown solid (0.5 g, 40%); mp: 193-196°C; IR: Vmax (ATR) 3358 (N-H asymmetric), 3262 (N-H symmetric), 2944 (C-H aromatic), 2839 (C-H aromatic), 1589 (C=C aromatic), 1500 (C=C aromatic), 1464 (C=C aromatic), 1408 (S=O), 1310 (S=O), 1232 (S=O), 1072 (S=O); 1H NMR (400 MHz, CDCl3): δ 7.09 (1H,S), 4.69 (2H, S, N-H2), 3.84 (6H, S), 3.78 (3H, S); 13C NMR (100 MHz, CDCl3): 205.2, 153.3, 103.6, 59.7, 55.8, 29.1; MS: 247.0503.
3,4-dimethoxybenzenesulfonamide (DMBS)
White solid (2.0 g, 75%); mp: 136-138°C; IR: Vmax (ATR) 3321 (N-H asymmetric), 3231 (N-H symmetric), 3119 (C-H aromatic), 2841 (C-H aromatic), 1584 (C=C aromatic), 1509 (C=C aromatic), 1462 (C=C aromatic), 1264 (S=O), 1235 (S=O), 1135 (S=O), 1094 (S=O) ; 1H NMR (400 MHz, CDCl3): δ 7.88 (1H, d, J=2.2 Hz), 7.43 (1H, d, J=2.2 Hz), 6.95 (1H, d, J=8.52 Hz), 4.85 (2H, S, N-H), 3.96 (6H, d, J=4.68 Hz); 13C NMR (100 MHz, CDCl3): 152.8, 148.7, 133.6, 119.9, 110.6, 108.8, 56.4 ppm; MS: (217 + H).
4-methoxybenzenesulfonamide (MBS)
White solid (1.0 g, 70%); mp: 111-113°C; IR: Vmax (ATR) 3346 (N-H asymmetric), 3257 (N-H symmetric), 2947 (C-H aromatic), 2842 (C-H aromatic), 1597 (C=C aromatic), 1578 (C=C aromatic), 1546 (C=C aromatic), 1324 (S=O), 1301 (S=O), 1150 (S=O), 1021 (S=O); 1H NMR (400 MHz, CDCl3): δ 7.88 (2H, d, J= 8.8 Hz), 7.00 (2H, d, J= 8.9), 4.87 (2H, S, N-H2), 3.89 (3H, S); 13C NMR (100 MHz, CDCl3): 163.0, 133.5, 128.5, 114.1, 55.6 ppm; MS: (187 + H).
3,4-dimethoxy-N-methylbenzenesulphonamide, 3,4-(CH3O)2C6H3SO2NHCH3 (DMBS-Me)
White crystals (0.67 g, 55%); mp: 123-125°C; IR: Vmax (ATR) 3075 (N-H asymmetric), 32293 (N-H symmetric), 2984 (C-H aromatic), 2951 (C-H aromatic), 2042 (C=C aromatic), 1470 (C=C aromatic), 1454 (C=C aromatic), 1324 (S=O), 1233 (S=O), 1154 (S=O), 1143 (S=O); 1H NMR (400 MHz, CDCl3): δ 7.37 (1H, d, J=2.2 Hz), 7.41 (1H, d, J=2.2 Hz), 6.88 (1H, d, J=8.49 Hz), 4.50 (2H, S, N-H), 3.85 (6H, d, J=8.61 Hz); 2.56 (3H, d, J=5.43 Hz); 13C NMR (100 MHz, CDCl3): 152.6, 149.2, 130.4, 121.3, 110.5, 109.7, 56.3, 56.2, 29.4 ppm; MS: (232 + H).
N-Ethyl-3,4-dimethoxybenzenesulphonamide, 3,4-(CH3O)2C6H3SO2NHC2H5 (DMBS-Et)
White crystals (0.69 g, 53%); mp: 104-106°C; IR: Vmax (ATR) 3294 (N-H asymmetric), 2949 (N-H symmetric), 3010 (C-H aromatic), 2948 (C-H aromatic), 1892 (C=C aromatic), 1587 (C=C aromatic), 1439 (C=C aromatic), 1255 (S=O), 1232 (S=O), 1154 (S=O), 1142 (S=O) ; 1H NMR (400 MHz, CDCl3): δ 7.57 (1H, d, J=2.2 Hz), 7.41 (1H, d, J=2.2 Hz), 6.88 (1H, d, J=8.49 Hz), 4.80 (2H, S, N-H), 3.91 (6H, d, J=8.61 Hz); 2.92 (2H, m); 1.2 (3H, t, J=7.2); 13C NMR (100 MHz, CDCl3): 152.4, 149.1, 131.6, 121.3, 121.1, 110.5, 109.6, 56.3, 56.2, 38.3, 14.98 ppm; MS: (246 + H).
Results
The CCK-8 assay was performed to assess the cell survival of PBMCs from healthy donors (figure 2) and non-small cell lung carcinoma A549 cells (figure 3) following treatment at 1 h, 24 h and 48 h. The treatments used were various doses of GA (20, 30 and 40 µM), DMBS (30 µM), DMBS-Me (25, 30, 35 µM), DMBS-Et (25, 30, 35 µM) and TMBS (30, 40, 60 µM). The results of the CCK-8 assay on PBMCs from healthy donors demonstrated that at all times, all concentrations of GA increased cell survival compared to the negative control (NC) (p<0. 0001), whereas all concentrations of TMBS showed similar cell survival rates to the negative control (NC). Meanwhile, DMBS, DMBS-Me and DMBS-Et showed reduced cell survival rates compared to the NC significantly after 24, and 48 hours of treatment (p<0. 0001)(figure 2).


Figure 2: The cell survival of PBMCs from healthy donors at 1 h, 24 h and 48 h following treatment with GA (20, 30, 40 µM), TMBS (30, 40, 60 µM), DMBS (30 µM), DMBS-Me (25, 30, 35 µM) or DMBS-Et (25, 30, 35 µM). NC (negative control, cells without treatment); PC (positive control, 80 µM H2O2); GA (gallic acid); TBMS (3,4,5-trimethoxybenzenesulfonamide); DMBS(3,4-dimethoxybenzenesulfonamide); DMBS-Me(3,4-dimethoxy-N-methylbenzenesulphonamide); DMBS-Et(N-Ethyl-3,4-dimethoxybenzenesulphonamide); ns = non-significant. The number of asterisks denotes the degree of significance between results: ** = p < 0.0013; *** = p < 0.0008; **** p<0. 0001. Errors bars represent the standard error of the mean (SEM).


Figure 3 shows the results of the CCK-8 assay on A549 cells, which demonstrate that at 24 and 48 h of treatment, all concentrations of GA and TMBS drastically reduced cell survival compared to the NC (p<0.0001). Additionally, at 1 h, all TMBS concentrations exhibited increased cytotoxicity compared to GA, and at 24 and 48 h of treatment, TMBS exhibited increased cytotoxicity compared to all compounds. All concentrations of DMBS-Me and DMBS-Et exhibited increased cytotoxicity compared to GA at 1 h of treatment, but after 24 h and 48 h their cytotoxicity reduced or became similar to that of GA at specific concentrations. Moreover, the highest concentrations of DMBS-Me and DMBS-Et were the most cytotoxic treatments against A549 cells, hence their inhibition of cell proliferation appeared to be dose-dependent.
The half-maximal inhibitory concentration (IC50) of treatments were calculated based on the results of the CCK-8 assay on A549 cells. 30 µM GA, DMBS, DMBS-Me and DMBS-Et and 40 µM TMBS were selected as the optimal concentrations for treatment of A549 cells at 24 hours, and these concentrations were also used to treat cells in the comet assay.
Figure 3: The cell survival of A549 cells at, 1 h, 24 h and 48 h following treatment with GA (20, 30, 40 µM), TMBS (30, 40, 60 µM), DMBS (30 µM), DMBS-Me (25, 30, 35 µM) or DMBS-Et (25, 30, 35 µM). NC (negative control, cells without treatment); PC (positive control, 80 µM H2O2); GA (gallic acid); TBMS (3,4,5-trimethoxybenzenesulfonamide); DMBS(3,4-dimethoxybenzenesulfonamide); DMBS-Me(3,4-dimethoxy-N-methylbenzenesulphonamide); DMBS-Et(N-Ethyl-3,4-dimethoxybenzenesulphonamide); ns = non-significant. The number of asterisks denotes the degree of significance between results: (* = p <0.05; ** = p < 0.0013; *** = p < 0.0008; **** p<0. 0001). Errors bars represent the standard error of the mean (SEM).
Figure 4 shows the results of the comet assay which was performed to assess the genotoxicity of treatments in A549 cells as measured by OTM and % tail DNA. The treatments were 30 µM GA, 40 µM TMBS, 30 µM DMBS-Me, and 30 µM DMBS-Et, in addition to all these treatments co-supplemented with 80 µM H2O2. There was no significant difference in DNA damage between untreated cells and cells treated with 30 µM GA or 40 µM TMBS. However, treatment with 30 µM DMBS, DMBS-Me and DMBS-Et caused significant damage in cells compared to the NC.
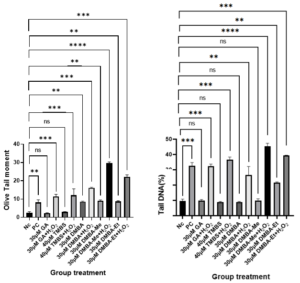
Figure 4: Effect of GA and its derivatives on A549 cells as measured using OTM in the comet assay. NC (negative control without treatment); PC (positive control 80 µM H2O2); H2O2 (hydrogen peroxide); GA (gallic acid); TMBS (3,4,5-trimethoxybenzenesulfonamide); DMBS(3,4-dimethoxybenzenesulfonamide); DMBS-Me(3,4-dimethoxy-N-methylbenzenesulphonamide); DMBS-Et(N-Ethyl-3,4-dimethoxybenzenesulphonamide); ns stands for non-significant. The number of asterisks denote the degree of significance between results: (* = p <0.05; ** = p <0.01; *** = p<0.001; ns = non-significant). Errors bars represent the standard error of the mean (SEM).
Discussion
The anticancer activity of mono-, di- and trimethoxybenzenesulphonamides appears to reflect several factors, including the number and position of the methoxy substituents. Methoxy groups contribute to the cytotoxicity of GA derivatives in cancer cell lines (Dhingra et al., 2021). In general, the anticancer activity rises as the number of methoxy groups increases. Thus, 4-methoxybenzenesulphonamides have only a little activity; the dimethoxy homologues are more active, and the trimethoxy species have the greatest activity. This trend follows that found in substituted benzoic acids. The antioxidant activity of TMBS is greater than that of GA which is mainly related to its O-demethylation by CYP2D6 (Dhingra et al., 2021).
GA is a natural product with numerous properties, including being a plant metabolite, an astringent, an antioxidant, a human xenobiotic metabolite, an apoptosis inducer and a geroprotector. Eudesmic acid (3,4,5-trimethoxybenzoic acid) is a plant metabolite, a human xenobiotic metabolite and a human urinary metabolite. Both are substantially more active than their lower homologues with only two or one hydroxyl or methoxy groups.
The position of the methoxy substituents also influences the activity of the sulphonamides. In most cases (perhaps all), the 3,4-dimethoxybenzenesulphonamides have a higher activity than the corresponding 2,5-dimethoxy analogues. This trend also is consistent with the observed activity of gallic and eudesmic acids, in which there are hydroxyl or methoxy groups in the 3 and 4 positions.
Similarly, replacing one of the methoxy substituents with a methyl group reduces the anticancer activity. Thus, the first three members of the 2-methoxy-5-methylbenzenesulphonamide series, 2-CH3O-5-CH3C6H3SO2NHR (R = H, CH3 or C2H5) are less active than their dimethoxybenzenesulphonamide counterparts. This point is consistent with the interpretation that demethylation of the methoxy groups in vivo leads to formation of the more active hydroxyl analogues. When only one methoxy group is present, it is possible to form only one hydroxyl substituent, thus explaining the reduced activity of the 2-methoxy-5-methylbenzenesulphonamides compared to the 2,5-dimethoxybenzenesulphonamides.
The solubility of the methoxybenzenesulphonamides may also be relevant. Hydrophobic moieties of GA derivatives e.g., methoxy groups (Mei et al., 2008) increase the compounds’ affinity for cell membranes and permeability (Saeki et al., 2000). Whereas the higher N-alkyl derivatives, containing a larger group, are almost insoluble in water, the lower members of the series, especially the parent sulfonamides, (CH3O)nC6H5-nSO2NH2 (n = 1-3), are sufficiently soluble in water permit recrystallisation from aqueous media containing little or no ethanol cosolvent. This higher solubility in water may facilitate transport in vivo. It may also account, at least in part, for the greater activity of the dimethoxy- and trimethoxybenzenesulfonamides compared to eudesmic acid, which is only sparingly soluble (~ 2.5 g per L), and gallic acid, which is slightly more soluble (10-15 g per L) in water at neutral pH. Alternatively, the sulfonamides may simply be less prone to oxidation and other modifications in vivo than their carboxylic acid analogues.
The methylation of hydroxy groups significantly increased the oral permeability of TMBS compared to GA in human intestinal epithelial cells (Alhyari et al., 2022). N-alkyl derivatives, DMBS-Me and DMBS-Et, might be more hydrophobic than their parent compound that help the compound pass through the cell membrane and reach to the active site of the target cells.
Many studies have shown that GA and its derivatives exhibit antitumour activity in cancer cells (Locatelli et al., 2013). Locatelli and co-workers investigated the antitumour activity of esters of GA against different types of cancer cell lines such as leukaemia, lung cancer and breast cancer in vitro and in vivo assays (Locatelli et al., 2013). It was demonstrated that alkyl gallates with 8-14 carbon atoms in the ester chain exhibits greater anticancer activity than alkyl gallates with fewer than 8 carbon atoms, as well as GA to an even greater extent (Locatelli et al., 2013). The anticancer effects were related to the amphipathic feature of alkyl ester derivatives, of which the hydrophobic moiety shows an affinity for cell membranes and increases permeability (Locatelli et al., 2013).
The results presented in Figure 2 indicate that the tested concentrations of GA and TMBS did not have any cytotoxic effects on PBMCs from healthy donors after 1 hour, 24 hours, and 48 hours. However, Figure 3 reveals that GA significantly reduced the survival of carcinoma cells after 24 hours and 48 hours, and TMBS was also found to be cytotoxic at all timelines. Furthermore, the cytotoxicity of TMBS was found to be greater than that of GA at all concentrations tested. These findings suggest that TMBS could be a promising therapeutic agent for treating non-small cell lung cancer by effectively reducing the viability of cancer cells without causing significant harm to healthy cells.
Although Figure 4 shows that DMBS, DMBS-Me, and DMBS-Et caused significant DNA damage in A549 cells compared to the NC, Figures 2 and 3 demonstrate that these compounds also reduced cell survival rates in both carcinoma and healthy cells when compared to the NC (p<0. 0001). Therefore, these compounds may not be suitable for use in cancer treatments.
Conclusion
At selected concentrations, TMBS may demonstrate therapeutic potential in cancer since it is cytotoxic to carcinoma cells but not to healthy cells. In the future, we will synthesize GA analogues by adding the methyl and ethyl group to the TMBS compound. Next, the biological activity of the novel compounds will be compared with GA and TMBS.
References
- Alhyari D H, Sheldrake H, Kantamneni S, Isreb M, Soumehsaraei S J, et al. (2022) Physicochemical and biopharmaceutical characterization of new sulfonamide derivatives of gallic acid. British Journal of Pharmacy
- Badhani B, Sharma N, Kakkar R (2015) Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Advances 5:27540-27557.
- Daly W H, Holle H J, Fouad F M (2002) Lewis acid catalyzed addition of isocyanates to sulfonamides. Journal of Organic Chemistry 39:1600-1603.
- Dhingra N, Kar A, Sharma R (2021) Gallic acid derivatives with antibreast cancer and antioxidant action: synthesis and pharmacological assessment. Acta Pharmaceutica Hungarica 91:53-66.
- Kahkeshani N, F Farzaei, M Fotouhi, S S Alavi, R Bahramsoltani, et al. (2019) Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iranian Journal of Basic Medical Sciences 22:225-237.
- Locatelli C, F B Filippin-Monteiro, T. B. Creczynski-Pasa (2013) Alkyl esters of gallic acid as anticancer agents: a review. European Journal of Medicinal Chemistry 60:233-239.
- Mei B C, Susumu K, Medintz I L, Delehanty J B (2008) Modular poly (ethylene glycol) ligands for biocompatible semiconductor and gold nanocrystals with extended pH and ionic stability. Journal of Materials Chemistry 18:4949-4958.
- Moffatt J G, Lerch U (1971) Carbodiimide-sulfoxide reactions. XII. Reactions of sulfonamides. Journal of Organic Chemistry 36:3686-3691.
- Muhammad‑Ali M A, Salman H H, Jasim E (2019) Antioxidant activity of some newly prepared symmetrically azo dyes derived from sulfa drugs. Asian Journal of Pharmaceutical and Clinical Research 12:479-483.
- Pandey K B, Rizvi S I (2009) Plant polyphenols as dietary antioxidants in human health and disease 2:270-278.
- Rehman H, Qadir A, Ali Z, Nazir S, Zahra A, et al. (2017). Synthesis and characterization of novel sulfonamides derivatives and their antimicrobial, antioxidant and cytotoxicity evaluation. Bulletin of the Chemical Society of Ethiopia 31:491.
- Saeki K, Yuo A, Isemura M, Abe I, Seki T, et al. (2000). Apoptosis-inducing activity of lipid derivatives of gallic acid. Biological and Pharmaceutical Bulletin 23:1391-1394.
- Sroka Z W C (2003). Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids 41:753-758.
- Subramanian A P, John A A, Vellayappan M V, Balaji A, Jaganathan S K, et al. (2015) Gallic acid: prospects and molecular mechanisms of its anticancer activity 45.
- Zhou Y, Zheng J, Li Y, Xu D P, Li S, et al. (2016) Natural Polyphenols for Prevention and Treatment of Cancer 8:515.
- Zhongbing L, Nie G, Belton P S, Tang H, Zhao B (2006) Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochemistry International 48:263-274.