Publication Information
ISSN: 2641-7049
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
"GALENO" NEUROSURGERY DEVICE FOR BRAIN HERNIATION A multidisciplinary approach to complexity
Claudia Piscitelli*
La Sapienza University of Rome, Faculty of Medicine and Surgery, Anagni (FR) 03012, Italy | (+39)3319023168 | claudiapiscitelli@icloud.com
Received Date: March 14, 2023; Accepted Date: March 24, 2024; Published Date: April 03, 2024
*Corresponding author: Claudia Piscitelli, La Sapienza University of Rome, Faculty of Medicine and Surgery, Anagni (FR) 03012, Italy; Phone: (+39)3319023168; Email: claudiapiscitelli@icloud.com
Citation: Claudia Piscitelli (2024) "GALENO" NEUROSURGERY DEVICE FOR BRAIN HERNIATION, A multidisciplinary approach to complexity. Jr Neuro Psycho and Brain Res: JNPBR-201
DOI: 10.37722/JNPABR.202402
Abstract
Neurological disorders affect billions of people worldwide, making the discovery of effective treatments a major challenge.
Requirements: The design of this device requires neurosurgical, neurophysiological, bioengineering and computer skills.
Introduction
“Galeno” is the theoretical medical device that I designed for patients with severe stroke and herniated brain. It is shaped like a helmet, 3D printed.
The design of this device combines: neurosurgery, neurotechnology, drug delivery and modern knowledge of biomedical engineering and computer science.
The goal is to reduce brain edema and optimize homeostasis.
Patients with brain herniation often have intracranial injuries and concomitant or habitual damage to other organs. The mortality rate can be between 60% and 84.6% in cases of TBI (traumatic brain injury) with herniated brain. More than 27 million people worldwide are affected by TBI each year.
Traumatic brain injuries (TBIs) affect thousands of people in the United States every year.
The economic impact of TBIs, in the United States, was estimated to be $ billion in direct and indirect costs. Traumatic brain injury (TBI) is a devastating neurological disease due to its high morbidity and mortality.
Decompressive craniectomy (DC) has been used for decades to treat TBI, severe hemispheric strokes, etc. However, although DC is an effective treatment for patients with TBI, the risk of mortality and morbidity remains high (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7260895/), as it alone is not sufficient to ensure successful surgery and patient recovery.
“Galeno” medical device was created for the management of brain injury and intracranial hypertension and is placed on the brain via DC to protect, treat and monitor it until cranioplasty.
Objectives
Galeno is placed on the site of patients undergoing DC to monitor and contain swelling, reduce inflammation, detect changes in the parameters of ICP (intracranial brain pressure), blood pressure, brain metabolism, etc.
Galeno can monitor the electrographic and biochemical function of the brain and provide a mechanism to intervene directly through the use of drugs (nanoparticles).
Exploratory: Galeno could theoretically facilitate the surgical procedure, reduce hospital stay, and improve the patient's quality of life.
Safety and tolerability: infection, bleeding and other risks will be monitored.
The intent is to make surgery with “Galeno” the first choice of intervention, facilitating the neurosurgeon's work and the recovery of the patient(s).
Methods/discussion/results
“Galeno” represents a Phase 0 (proof of concept) project for subsequent animal and human testing (Phase I). After animal data on feasibility and safety, approval for Phase I safety/tolerability tests will be required.
Normal intracranial physiological process may be altered following TBI, resulting in refractory intracranial hypertension, decreased cerebral perfusion pressure, and cerebral blood flow disturbances. Malignant intracranial hypertension is the leading cause of death in patients with TBI. Therefore, targeted control and treatment of increased intracranial pressure (ICP) is the key issue in the management of severe traumatic brain injury.
Summary/conclusion
“Galeno” device and helmet has a reticular structure to support the injured brain- ischemic and/or suffering from traumatic brain injury (TBI).
Every year, about 70 million people worldwide and 2.5 million people in Europe suffer from traumatic brain injury (TBI).
The device and helmet are 3D printed (with biocompatible polymers: ceramic and hydrogel) and integrated with other components: brain-computer interface, neuromonitoring, sensors, fluorescence, wireless system, subdural and fluid drainage, etc. Each component chosen has, theoretically, suitable properties to facilitate neural regeneration and repair, following damage.
The innovative network structure, equipped with sensors and fluorescence, makes it possible to map and identify any area of the brain, even the anatomically “difficult” ones. It can function as an anatomical and brain activity scanning device to allow the neurosurgeon a complete acquisition of biochemical-anatomical information.
Swelling, inflammation, ICP, blood pressure, metabolism, etc. will be constantly monitored and treated. The drugs (nanoparticles) are delivered directly into the brain by a cross-system capable of precisely identifying where action is needed.
The patent application was filed in Italy in October 2022 and in October 2023 I applied to extend it to the United States as well.
Application in Research
“Galeno” was born with the intent to combine medicine-surgery and technology.
Modern times require innovative solutions that using the support of technology, help doctors and patients. The project also explores the possibility of using “Galeno” in different areas of medical research.
Military
Traumatic brain injury (TBI) is a serious threat to the readiness of soldiers and currently appears to lack approved and effective treatment options.
“Galeno” aims to be created and developed as an innovative solution to manage and counter TBI at the time of injury, reducing morbidity and mortality. Through its sophisticated technology, Its purpose is to provide
a better understanding of brain functioning, appropriate treatment of brain lesions, and visualization and decoding of brain activity.
Space
Human spaceflight is becoming more frequent and longer, inducing widespread changes in brain morphology (ventricular volume, gray matter shifts, CSF changes, etc.).
Studies have shown that upon return from Space, the deficits most commonly found in astronauts are caused by pressure changes in the brain and spinal fluid, thus suggesting the need to find a cure and/or minimize neurological risks for long-duration missions (also with a view to future trips to Mars).
Surgery
“Galeno” is theoretically suitable for use in both routine and emergency surgical procedures. By modifying the choice of drugs, it could also enable treatment of brain tumors currently considered “inoperable”.
Neuroscience
Analyzing the full potential of “Galeno-m.d.”, in the future it could be considered to deepen and improve complex and still little understood neurophysiological knowledge.
The innovative structure of the helmet aims specifically to facilitate the study of the brain, mapping its connections and its biochemical, electrical and metabolic changes.
Military Research
In October 2007, the U.S. Department of Defense (DoD) established a formal definition of traumatic brain injury: “a traumatically induced structural injury and/or physiologic disruption of brain function as a result of (the) external force.”- https://apps.dtic.mil/sti/pdfs/AD1027329.pdf
Traumatic brain injury (TBI) is a serious threat to soldier readiness, and currently there appears to be a lack of approved and effective treatment options.
The intent is to create and develop innovative solutions to manage and counteract head trauma at the point of injury, reducing morbidity and mortality.
In addition to physical damage, head injury can significantly impair mental capacity, including learning ability, decision making, attention span, memory, and emotion. Without these and other abilities, the Military is seriously at risk in all phases of conflict.
Military TBI: the numbers (https://media.defense.gov/2022/Sep/08/2003072142/-1/- 1/1/WRAIR_DISPATCH_BRAIN_HEALTH.PDF).
Soldiers are at greater risk of sustaining repeated concussions that can cause second concussion syndrome, in which the brain swells rapidly, leading to more severe symptoms.
Data (https://media.defense.gov/2022/Sep/08/2003072142/-1/-1/1/WRAIR_DISPATCH_BRAIN_HEALTH.PDF) indicate that more than 96% of TBIs occur in conjunction with extremity trauma, increasing the complexity of brain injury and requiring tailored medical solutions in the context of TBI/polytrauma-associated.
The presence of parenchymal hematomas contributes to edema formation, causing an increase in intracranial pressure (ICP) and a decrease in cerebral perfusion pressure (CPP), fueling endocranial hypertension (EH).
An uncontrolled increase in ICP, due to a mass effect, causes brain herniation with abnormal protrusion of brain tissue, cerebrospinal fluid (CSF) and blood vessels from their usual location. They can occur at any level of the central nervous system (CNS) and are a life-threatening condition.
Space research
Long and prolonged periods in Space result in far-reaching problems with possible permanent effects on some organs of the human body, particularly the brain, with changes in its volume and deformation of the pituitary gland.
Astronauts who have stayed on the Space Station have reported vision damage as a result of prolonged exposure to microgravity; evaluations upon return to Earth have revealed optic nerve swelling, retinal hemorrhage and other ocular structural changes. According to scientists, these changes can be attributed to increased intracranial pressure during spaceflight.
According to a new study published (in JAMA Neurology) being in Space for a long time can cause brain damage. Being in space has well-documented negative effects on the body, but what happens to the brain is less studied.
Five Russian cosmonauts aboard the International Space Station for about five and a half months were examined. The researchers found elevated blood levels of five different biomarkers associated with brain damage on Earth: NfL (neurofilament light chain), GFAP (glial fibrillary acidic protein) and amyloid proteins were significantly increased after their return from Space.
Human spaceflight is becoming more frequent and longer, drawing more attention to changes in brain morphology (ventricular volume, distribution, CSF, etc.), suggesting that brain injury may be a previously unknown risk for humans in long-duration spaceflight, and there is a need to minimize neurological risks for long-duration missions and/or planning a trip to Mars.
Despite continuous improvements in emergency care (stroke units, trauma centres, etc.), it’s still difficult to identify/treat brain injury and intracranial hypertension.
The lack of understanding of their consequences also hinders the reintegration of patients into society, underlining the importance of research in this area.
Therefore, it is necessary to monitor brain tissue and implement neuroprotective strategies in a timely manner.
The passion for neurosurgery, research, innovation and technological progress led me to write “Galeno”, the theoretical medical device I designed for patients with severe stroke and brain hernia. Brain herniation, also known as acquired intracranial herniation, refers to the displacement of brain tissue from its normal position, into an adjacent space due to mass effect.
It is a life-threatening condition that requires early diagnosis.
The goal is to provide a specific device that can guide the neurosurgeon with precision and at the same time able to monitor and protect the damaged brain, leading to a reduction in surgery time and cost with an improvement in the patient's quality of life.
Analysis of the patient's brain structure and simultaneous acquisition of biochemical/physiological information would allow the surgeon to reduce the margin of error. An "instant-by-instant" analysis of what is happening inside the brain, in which even the smallest change is detected in real time, would provide a better understanding of what needs to be done for successful surgery and prevent future damage.
Neurological disorders affect billions of people worldwide, making the discovery of effective treatments a major challenge.
The normal intracranial physiological process can be altered as a result of severe traumatic brain injury, resulting in refractory intracranial hypertension, decreased cerebral perfusion pressure, and cerebral blood flow disorders. Malignant intracranial hypertension is the leading cause of death in patients with TBI. Therefore, targeted control and treatment of increased intracranial pressure (ICP) is the key point in the management of severe traumatic brain injury.
“Galeno" has the shape of a helmet, 3D printed. The goal is to contain swelling, reduce edema and optimize brain homeostasis. The intent is to make surgery with “Galeno” the first choice of intervention, facilitating the neurosurgeon's work and the recovery of the patient(s).
The design of this device combines: neurosurgery, neurotechnology, direct drug delivery into the brain, and modern knowledge of biomedical engineering and computer science.
Patients with brain herniation often have intracranial injuries and concomitant or habitual damage to other organs. The mortality rate can be between 60% and 84.6% in cases of TBI (traumatic brain injury) with herniated brain. More than 27 million people worldwide are affected by TBI each year. Traumatic brain injuries (TBIs) affect thousands of people in the United States every year.
The economic impact of TBIs, in the United States, was estimated in billions of dollars divided between direct and indirect costs.
Traumatic brain injury (TBI) is a devastating neurological disease due to its high morbidity and mortality.
Decompressive craniectomy (DC) has been used for decades to treat TBI, severe hemispheric strokes, etc. However, although DC is an effective treatment for patients with TBI, the risk of mortality and morbidity remains high (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7260895/), as it alone is not sufficient to ensure successful surgery and patient recovery.
“Galeno” medical device was created for the management of brain injury and intracranial hypertension and is placed on the brain via DC to protect, treat and monitor it until cranioplasty.
The aim is to provide optimal and precise control of the conditions in which the patient’s brain is involved, giving the possibility to intervene in any area/zone.
Starting from the main notions of neurophysiology and the mechanisms of cerebral plasticity I imagined and designed this helmet for cerebral herniation that has the shape of a lattice, articulated in horizontal and vertical lines and, like a kind of fishing net, “traps” and protects the brain, without sending it into fatigue.
Anatomically, brain hernias can occur anywhere in the central nervous system (CNS); the affected areas can be different and not always easily accessible and this is the reason why the helmet must also be able to cover the entire surface of the brain.
“Galeno” theoretically monitors and contains swelling, reduces inflammation, detects changes in the parameters of ICP (cerebral intracranial pressure), blood pressure, brain metabolism, etc.
All this is made possible thanks to the physical-mechanical specialties of the materials with which the helmet is made. The combination of materials makes it possible to create a medical device that can mimic the physiological environment of neural tissue without damaging nerve structures.
Through the sensors, placed in the interface between the device and the brain parenchyma, there is continuous monitoring of the parameters of cerebral perfusion pressure, temperature, cerebral blood flow, partial pressure of O2, etc.
"Galeno" can (theoretically) monitor the electrographic and biochemical function of the brain and provide a mechanism to intervene directly through the use of drugs (nanoparticles).
Understanding the functioning of the brain is essential to governing brain processes with the goal of managing pathological dysfunctions.
Neural networks in the brain should meet two requirements that could also be regarded as basic organizing principles: functional segregation and integration, enabling both rapid information extraction and the generation of coherent brain states.
Traumatic brain injury (TBI) has been classified as hyperconnectivity syndrome, because the reaction that is triggered, in response to a lower efficiency of neuronal networks, is an increased level of connectivity and this is why I theorized that the brain-computer interface (BCI) can be an important tool to assess the level of damage, supporting diagnosis and therapy.
“Galeno” uses a bi-directional interface that combines the direct reception of information, resulting from brain activity, with a return line that allows the exchange of information between the device and the brain.
Research results confirm that decompressive craniectomy (DC) is efficient in the treatment of patients with severe cranial-brain lesions accompanied by malignant intracranial hypertension and cerebral edema but not sufficient in the overall management of pathology; Galeno was (theoretically) designed to facilitate the surgical procedure but also to reduce hospital stay and improve the patient's quality of life.
Before proceeding to tell you what “Galeno” is, forme, at least for me, it is good to point out that there is no prototype of this device yet, It is in “phase 0” (proof of concept) but the written project is complete.
I hope that at the end of this meeting you will be intrigued and eager to deepen with me its complexity and its potential.
The helmet is made by 3D printing, whose external part (outer part) is (made) in bioceramics, while the inner part, in contact with the brain parenchyma, is made in hydrogel.
Using bioceramics and hydrogels aims to provide the structural support the brain needs and create a first condition suitable for the nerve tissue, helping it to counteract the pathological event and all that it causes.
The helmet, precisely because it is in close contact with the brain parenchyma, must be made of polymers that are biocompatible with brain tissue, using a 3D printer and equipped with certain essential features: it must be elastic and compressible while at the same time capable of transferring electrical and chemical signals.
For example: hydrogels have high water absorption capacity (able to swell and de-swell reversibly in water), can retain large volumes of fluid in a swollen state and can be designed to possess mechanical and physiological properties similar to those of the brain.
Cell growth benefits from their exceptional physical and chemical properties, providing the ideal 3D microarchitecture for neural regeneration.
Hydrogels are optimal candidates as carriers in drug delivery systems, transporting them to target sites and releasing them at a controlled rate.
“Galeno” is a single device that is placed at the site of patients undergoing decompressive craniectomy (DC) in which sensors, located in the interface between the device and the brain parenchyma, allow continuous monitoring of the parameters of cerebral intracranial pressure, cerebral blood flow, blood pressure, cerebral metabolism, etc.
Their functioning is very inspired by the chemoceptors present in our body and represent, for the neurosurgeon, real navigators strategically distributed for the entire extension of the helmet.
The use of these brain sensors must be incorporated with wireless microelectrodes (MEAs) that placed on the side of the brain parenchyma are able to penetrate it. (Obviously, the substrate of the microelectrode must be flexible so as not to cause trauma, inflammation, etc. to the brain parenchyma.)
Polylactic-co-glycolic acid could be used as a biodegradable polymer, able to move like a “second skin” on the brain tissue, without causing trauma, in response to changes in the pressure of the fluid surrounding the organ.
The sensors in the helmet should be located on the entire surface of the helmet, and taking into account the human anatomy, the following arrangement can be estimated:
2 front pole
2 parietal pole
2 occipital pole
2 time pole
2 lateral groove (one sn and one dx)
1 central groove
about 11 sensors distributed over the entire area.
The chip that makes up the sensors is equipped with electronic components that, detecting the chemical of interest, produce an electrical signal that can be detected and analyzed outside the body.
The lighting system (fluorescence NICE: near infrared fluorescent coatings of medical devices) represents the illuminated road that maps the brain and signals, thanks to the work done by sensors, the pathway and the precise point of the entire organ where there is edema and it is therefore necessary to intervene through the release of drugs that serve to counteract and treat the ongoing pathological phenomenon.
To develop a fluorescent biosensor suitable for investigating the health/activity status of nerve tissue, a pair of FRET (fluorescence resonance energy transfer) of FP (fluorescent proteins) or cpFP is combined (fluorescent proteins of the cyanine family) with a respective binding protein, called the detection domain.
In the case of FRET-based helmet biosensors using Cy5.5 as a donor on a filament and Cy7.5 as an acceptor on the complementary filament, it is possible to detect and characterize the NF-kappaB p50 transcription factor.
The drugs (mannitol and corticosteroids or glutathione) pass (biotransporters, nanoparticles) inside tubular structures, arranged vertically and horizontally and extending over the entire surface of the helmet, giving it the particular network structure.
The point at which these horizontal and vertical structures meet is where the release of drugs takes place.
The combination of silk and electroconductive polymers has been shown to have good potential for peripheral nerve regeneration, indicating extreme compatibility.
In addition, silk/CNT composite scaffolds (carbon nanotubes, CNTs) have shown excellent results in bioengineering of neural tissue, promoting neural differentiation and acting as a supporting matrix for nerve tissue regeneration.
Silk fibroin (SF) hydrogels have shear-thinning behavior-Guziewicz et al., 2011- (they always need a finite amount of time to bring back the required rearrangements in the microstructural elements that produce the shear-thinning) that provides injection capability and can be used as a carrier for a drug to provide bioavailability and sustainable release at the site.
The combination of silk and electrically conductive polymers has shown good potential for peripheral nerve regeneration, thus indicating extreme compatibility.
In addition, silk/CNT composite scaffolds have shown excellent results in bioengineering neural tissue, promoting neural differentiation and serving as a supporting matrix for nerve tissue regeneration.
In light of these data and because these structures must penetrate the brain parenchyma to enable the release of pharmacological substances, it is, in my opinion, imperative that the material chosen to make the channels possess characteristics that will not damage the nerve structure.
Within tubular structures, pharmacological substances travel to their point of release in the brain.
In view of the drug treatment currently available to counter edema formation, I hypothesized that the substance released along the vertical stretch "|" could be mannitol, in non-continuous solution. Whereas, in the horizontal tract intersecting the vertical "_" tract would be the release of corticosteroids or glutathione.
The possible choice of using these drugs is due to their pharmacokinetic characteristics, which can reduce edema and counteract inflammation.
The biggest obstacle to drug delivery directly into the brain is crossing the blood-brain barrier (BBB).
The human brain is considered one of the most challenging therapeutic areas for drug delivery because of its physiological-protective barriers: blood-brain barrier and blood-cerebrospinal barrier. (Abbot et al. 2010).
The blood-brain barrier, composed of endothelial cells, astrocytes, and pericytes, is the main factor limiting drug transport to the brain: about 98 percent of small molecules and nearly 100 percent of drugs cannot reach the brain parenchyma (Pardridge 2003).
Nanoparticle (NP) biopolymers are a powerful carrier for the delivery of drugs, peptides, proteins, and nucleic acids to the central nervous system, and because they can protect the drug from biological and chemical degradation, they implement its enormous potential thereby increasing its bioavailability. In addition, nanoparticle (NP) biopolymers are able to increase the therapeutic effect of the drug transported and absorption by the brain, also decreasing the side effects of typical drug delivery to this region.
NPs are small molecules ranging in size from 1 to 1000 nm. As the size of NPs decreases, studies conducted have shown an increasing ability to permeate the BBB (no permeability above 200 nm). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9032478/
“Galeno” was also designed as a surgical acquisition tool, for this reason I theorized (within it) a bi-directional interface (neural interface or BCI) able to detect, record and process the electrographic function of the brain.
The entire organ is scanned and the wireless system allows the immediate transfer of data to an external monitor, so as to make them easily accessible and viewable.
The drainage system that surrounds the helmet, perimeters its entire circumference, functioning as a collection and outflow system, equipped with a suction valve with the intent of eliminating what “weighs down” the brain.
The helmet is surrounded by an outflow system (a roughly tubular-shaped structure that perimeters the helmet), equipped with suction and pressure pumps that allow the removal (literally the "throwing out" of the brain) of anything that is in excess and thus aggravating the patient's pathological picture.
The outflow system represented by a silicone drainage catheter perimeter the helmet and penetrates into the surgical site at the intracranial level, in correspondence of the cerebral ventricles, when the device is applied after DC.
The drainage concerns the edematous fluid (transudate) coming from the blood vessels, composed mainly of plasma, that compressing the blood capillaries and the walls of the skull causes a blockage in the supply of oxygen and nutrients slowly leading to necrosis of brain tissue and cephalorachidian fluid (liquor – CSF) that could remain confined in the ventricles of the brain leading to an excessive accumulation of the same inside the brain.
To the bellows container is connected a graduated emptying bag, from 0 to 600 ml, into which liquid can be drain and measured. On the bottom of the bags you have a tap for emptying.
These are referred to as positive displacement piston (or plunger) pumps in which the volume change is obtained by the alternating flow of a piston in a cylinder and appropriate check valves that force the liquid to flow in only one direction preventing the reflux during the return stroke of the piston.
In this case the pump has a double effect because the piston performs work both on the outward and return.
This type of pump offers great design flexibility, variety of sizes and piston resistances that, combined with the adjustable displacement characteristics, integrated into the pump head, allow for extensive design flexibility.
Sensors can be included to monitor speed, temperature and pressure/vacuum.
The combination of these features results in a long-lasting fluid intake system.
In the suction action the piston causes a depression that causes the lifting of the liquid along a tube and its expulsion from the pump at ambient pressure. With the pressing action, the fluid enters the cylinder at room pressure and the pump is raised by pressure. In this case the cylinder is at the same level or below the liquid to be lifted.
The outflow system, comparable to a surgical drainage, has a unidirectional circuit and the drained fluid does not return back bacause the emptying bag is equipped with an anti-flow valve. Such a system is necessary to avoid excessive drainage potentially dangerous for the patient.
The pump has a central cavity in which the fluid is sucked and expelled by the movement of the piston.
This movement has a direct influence on performance, although it is limited by the power of the drive motor (which can be internal or external to the pump) and the number of revolutions the pump can start.
Flow and pressure are affected by the size of the pump, a small area of the pump cavity will correspond to a higher pressure and lower flow rate; conversely, a larger area of the cavity will correspond to an increase in flow rate and a decrease in pressure.
The pumping action provides precision and repeatability for long periods of operation.
The pump, with medium viscous fluids, also benefits from good control and constant flow rates, while with variable viscosity fluids it maintains a good flow rate and can withstand medium to high pressures.
The limits of the pressing pump are due to the problems of containing the high pressure from by the pump chamber, seals and valves.
A possible solution to overcome their limitation could be to adopt piston pumps without valves. The immediate advantage is that there are fewer moving parts that wear out or break, thus improving both durability and chemical compatibility.
The technology is based on the movement of a “split” dovenest piston that serves to block the entrance and exit while moving back and forth in the chamber.
The arrangement of these pumps is, in my opinion, as follows:
1 front
1 rear
2 lateral.
In view of the anatomy of the brain it will be:
1 pump located at the front pole
1 pump located at the occipital pole
2 pumps located at temporal lobes
To conclude, the helmet in addition to all the features listed above and with all the necessary appendices: sensors, channels, outflow system, etc. that allow the containment/ compression of the brain structure, It must also function as a kind of topographic map that can instantly detect what the brain needs to limit its damage.
All this is made possible, according to my idea, thanks to a finely regulated and integrated work of structures that operate in series and in parallel.
The advantage of using this surgical device can be found first of all in the control that it offers to the neurosurgeon, in the operating phase, instant by instant, of all the possible variables and to intervene promptly, with less invasiveness and therefore less risks.
“Galeno” (device and helmet) is suitable in its use for both routine and emergency procedures performed.
In case of cancer, ischemic stroke, diffuse edema, etc.
References:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7260895/
- https://link.springer.com/article/10.1007/s00415-019-09541-4
- https://apps.dtic.mil/sti/pdfs/AD1027329.pdf
- JAMA Neurology
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9454297/
- https://pubmed.ncbi.nlm.nih.gov/29421552/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6786932/
- https://www.mdpi.com/1424-8220/23/13/6001
- https://pubmed.ncbi.nlm.nih.gov/32871471/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9032478/
- https://pubmed.ncbi.nlm.nih.gov/35011559
Supplement Figures Supporting Topic:
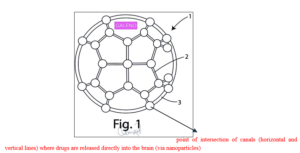
Surgical device (Fig. 1) for cerebral herniation applied to the brain after decompressive craniectomy. The device (the helmet) has along the perimeter an outflow system comprising at least one drainage catheter and means of connection to an aspiration system, said outflow system being positioned along the perimeter of said structure and being adapted to convey waste liquids or blood outside.
Surgical device characterised in that said plurality of sensors comprises fluorescent biosensors, said sensors being coated with an ultra-bright and stable biocompatible fluorescent coating, for illuminating one or more areas of said surgical device (1), in particular corresponding to areas of the brain wherein said sensors determine the presence of an edema.
The sensors comprise at least one transducer of a neural interface, said transducer being adapted to register the impulses of a neuron.
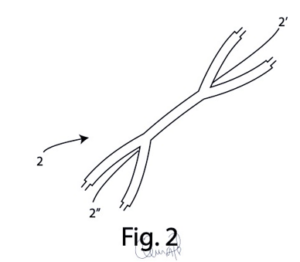
Tubular canals including an inlet extremity (2’) of one or more drugs and an outlet extremity (2”) for the release of said one or more drugs into the cerebral tissue.
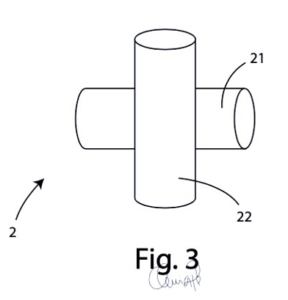
Detail of canals
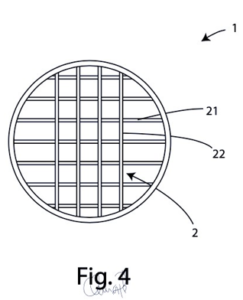
A plurality of tubular canals (Fig. 4) divided into horizontal and vertical lines covering the entire surface of the helmet.
Surgical device characterised in that said structure comprises a first and second level of tubular administration canals, said first level comprising a first plurality of canals (21) parallel to each other and said second level comprising a second plurality of canals (22) parallel to each other and perpendicular to said first plurality of canals (21).