Publication Information
ISSN 2692-1529
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Evaluation of ME-PRO® and EnviroMeal Combinations as Partial and Complete Fish Meal Replacements in Diets for Juvenile Atlantic Salmon (Salmo salar) Reared in A Recirculating Aquaculture System
Luke Fredrickson1, Breanna Modica2, Daniel Adams2, Carrie Kuball2, Liz Koutsos2, Brandon White1*, Sergio F Nates1
1Prairie AquaTech, LLC DBA Houdek, Brookings, SD, USA
2EnviroFlight LLC, Maysville, KY, USA
Received Date: April 02, 2023; Accepted Date: April 10, 2023; Published Date: April 17, 2023;
*Corresponding author: Brandon White, Houdek, 705 32nd Ave S, Brookings, SD 57006, USA. Tel +1(605)692-1266; Email: Brandon@prairieaquatech.com
Citation: Fredrickson L, Modica B, Adams D, Kuball C, Koutsos L, et al. (2023) Evaluation of ME-PRO® and EnviroMeal Combinations as Partial and Complete Fish Meal Replacements in Diets for Juvenile Atlantic Salmon (Salmo salar) Reared in A Recirculating Aquaculture System. Jr Aqua Mar Bio Eco: JAMBE-121.
DOI: 10.37722/JAMBE.2023201
Abstract
A feeding trial was conducted in a recirculation aquaculture system (RAS) to test the growth of juvenile Atlantic salmon (Salmo salar). A standard salmon diet (Control) was used as the template for two experimental diets that contained a combination of Black Soldier Fly (Hermetia illucens) larvae (BSFL) defatted meal (EnviroMeal) and fermented soy protein (ME-PRO®). The two ingredients were combined to replace fish meal by 50 or 100% (MEP/Meal 50 and MEP/Meal 100) in the experimental diets. At the 12-week trial conclusion, all growth parameters were similar among the three treatments. The diet containing no fish meal, MEP/Meal 100, demonstrated slightly higher numeric growth performance compared to the Control and MEP/Meal 50 treatment. Similarly, a histological analysis of distal intestines and organosomatic indices showed no significant difference among treatments. All intestinal samples were healthy in appearance showing no apparent enteritis. The results of this study show a combination of fermented soy and BSFL meal may be a viable option in sustainable diets for commercial Atlantic salmon production.
Introduction
Atlantic salmon aquaculture has changed dramatically over the last twenty years [1]. Due to social pressures and sustainability demands, feed producers have reduced their use of fish meal in formulations. This reduction in fish meal use could have been correlated with decreases in fish growth, but the industry continues to see fish grown to market size faster than previous years. Feed companies continue to use all ingredients at their disposal to formulate feeds that can drive the industry forward. Feeds are not only evaluated on growth performance and digestibility, but also on the sustainability of their ingredients [2].
The industry 0068as shown an increasing interest in the use of insects as feed ingredients in sustainable aquafeeds [3, 4]. Black Soldier Fly Larvae (BSFL) and fermented soy protein are great contributors to the sustainability story. BSFL can be grown on food by-products or other lower quality feed ingredients which they convert into high quality protein and lipid sources [5].
In addition, supplementing salmon feeds with “functional” ingredients represents the largest opportunity towards improvement and optimization of recirculation aquaculture systems. Physical characteristics of such ingredients include high viscosity, which can improve fecal material stability, low dust and small particle size, both of which can improve water quality. Some functional ingredients, such as fermented co-products, also offer significant amounts of short-chain peptides and free amino acids that confer excellent attractability and palatability properties [6]. The presence of biologically-active factors can increase gut micro-biota, reduce intestinal-inflammation, and boost metabolic processes which lead to improved animal health [7, 8].
In this article, we report on a recent study that was carried out to evaluate the combination of a fermented soybean meal, ME-PRO®, and a Black Soldier Fly larvae defatted meal, EnviroMeal, as partial and complete fish meal replacements in diets for juvenile Atlantic salmon (Salmo salar). We evaluated its effects on fish growth, feed utilization, and health.
Materials & Methods
Atlantic salmon eggs were obtained from Benchmark Genetics (Iceland). Eggs were hatched at Houdek (Brookings, SD) and fed a control diet until they reached an average weight of approximately seven grams. The juvenile Atlantic salmon were graded to decrease the overall size variation between individuals. After grading, the average fish weight was 7.7 grams. Fifteen fish were stocked into each tank. Initial tank biomass (Day 0) for the fifteen tanks averaged 116 grams per tank, resulting in tank densities of 1.02 kg m-3. The low density at the start of the trial ensured the fish had adequate space for optimal growth rates throughout the trial.
Prior to the start of the trial, all tank biomasses had less than 10% variation (± 5% of the mean). The recirculating aquaculture system (RAS) that was utilized for this trial consisted of fifteen, 40-gallon (151 L) tanks. Each treatment was randomly assigned five replicate tanks. The RAS was equipped with mechanical and biological filtration, UV sterilizers, pure oxygen injection, and chiller. The freshwater temperature was maintained at 14 degrees Celsius, and all water parameters were maintained at optimum levels for culture.
The fish were not given an acclimation period to transition onto their new feeds. Tanks were randomly assigned treatments and fish were switched from their holding feed to their respective diets. Fish were fed by hand to satiation three times per day for 12 weeks. Satiation was determined when a small amount of feed remained in each tank. Uneaten feed was collected, and pellets weighed and subtracted from the total daily feeding. Incremental tank biomass weights were recorded at 3, 6, 9, and 12 weeks. During the incremental weigh points, feed containers were weighed to determine feed conversion ratios (FCR). Tank biomass calculations were used to determine relative growth (RG), specific growth rate (SGR), and average weight per fish.
Test Diets and Feed Regime
All diets were formulated to meet the nutritional requirements of juvenile Atlantic salmon using a commercial feed formulation software. The trial design included two experimental formulations with one positive control diet (Control). The Control contained 21% menhaden fish meal and was void of soy protein and BSFL ingredients (Table 1). The remaining experimental diets (MEP/Meal 50 and MEP/Meal 100) replaced 50 or 100% of fish meal with combinations of ME-PRO® and EnviroMeal. The blended ratio of ME-PRO/EnviroMeal in the two experimental diets was 60/40. This ratio was determined by closely matching the proximate composition of the menhaden fish meal (63.3% crude protein, 8.6% crude fat) that was used in this study. By targeting the fish meal proximate composition, the “experimental combination” was a close 1-to-1 replacement with fish meal. This allowed the inclusion levels of nearly all other ingredients in the formulations to remain constant across the three diets.
Feeds were manufactured at Houdek (Brookings, SD) using identical commercial extrusion methods. Dry ingredients were ground using a Fitzpatrick hammer mill (Waterloo, ON) equipped with a 0.65 mm screen prior to blending. Complete ingredient mixes were transferred to a ribbon mixer (Patterson Equipment, Toronto, ON) and blended to obtain homogeneity. The homogenous feedstuff was extruded using an Extru-Tech E325 single-screw extruder (Sabetha, KS) equipped with a 1.6 mm die to produce 2.0 mm diameter floating pellets. Extruded pellets were dried with a conveyor oven drier, sifted with a screener (Rotex Inc., Cincinnati, OH), and then lipid coated using a Phlauer vacuum coater (A&J Mixing, Oakville, ON). The diets were bagged and stored at room temperature until use.
Prior to feed manufacturing, samples of primary protein ingredients were sent to a third-party laboratory (Midwest Laboratories, Omaha, NE) for proximate analysis. The reported values were entered into the feed formulation software and the experimental feed formulations were adjusted to ensure diet calculations were isonitrogenous and isolipidic.
Table 1: Diet formulation (%) and calculated proximate composition of diets reported on an as-fed basis.
Ingredients
Control
MEP/Meal 50
MEP/Meal 100
ME-PRO®
0
6.3
12.6
EnviroMeal
0
4.2
8.4
Fish Meal (Menhaden)
21
10.5
0
Land Animal Protein (LAP)
12.5
12.5
12.5
Marine Protein Meal
3
3
3
Plant Protein Meals
25
25
25
Wheat Flour
18.9
18.0
17.7
Vit/Min Premix
2
2
2
Stay C 35%
0.3
0.3
0.3
L-Lysine
1
1
1
DL-Methionine
0
0.1
0.2
L-Threonine
0.15
0.15
0.15
L-Taurine
0
0.05
0.1
Monodical Phosphate 21%
0.3
0.75
1
Choline Chloride 60%
0.3
0.3
0.3
Astaxanthin, 10%
0.01
0.01
0.01
Soy Lecithin
0.5
0.75
0.75
Fish/Canola Oil
15
15
15
Dry Matter
92.8
93.4
93.9
Crude Protein
45.0
45.0
45.1
Crude Lipid
20.3
20.1
19.8
Crude Fiber
0.8
1.5
2.1
Ash
7.6
6.3
4.9
Necropsy & Histology
A subset of ten fish per treatment (n=30) were randomly selected at the 9-week sampling point for necropsy and histological analysis. Fish were euthanized with an overdose of tricaine methanesulfonate (MS222) no more than 24 hours after last feeding. Blood samples were collected via heparinized capillaries from the caudal vein. Hematocrit values were measured after spinning samples for five minutes in a hematocrit centrifuge. Liver, viscera, and visceral fat were removed and weighed immediately (live wet weight) to calculate hepatosomatic (HSI), viscerosomatic (VSI), and visceral fat indices (VFI). Distal intestine tissue aliquots were fixed in 10% phosphate buffered formalin for histological analysis. Three fixed distal intestine samples were cut to smaller sizes, dehydrated in ethanol and Paraclear (Scientific Device Laboratory, IL, USA) and embedded in paraffin according to standard histological techniques. Sections were cut and affixed to slides. A total of 2 to 4 sections per sample were mounted per slide and stained with hematoxylin and eosin (H&E). H&E stained slides were examined using light microscopy and representative fields of view were digitally photographed. Samples were analyzed for enteritis with emphasis on enterocyte supranuclear vacuolization (SNV) and the width of lamina propria (LP) and submucosa. Fusion of mucosal folds were noted if present. Qualitative analysis of enteritis severity was scored as none, low, medium, and high.
Calculations & Statistics
All data was subjected to ANOVA and pairwise comparisons by Tukey’s test for significance (p<0.05, n=5 tanks, ± SD).
- Feed Conversion Ratio (FCR)
= feed weight consumed/weight gained
- Relative Growth (RG, %) = ((final average weight of fish-initial average weight)/initial average weight)*100
- Specific Growth Rate (SGR) = ((ln(final average weight)-ln(initial average weight))/feeding days)*100
- Fulton’s Condition Factor (K)
= [(weight(g)/length(mm)^3)]*100000
- Hepatic Somatic Index (HSI) = liver weight (g)/body weight (g) * 100
- Visceral Somatic Index (VSI) = viscera weight (fat removed)/body weight * 100
- Visceral Fat Index (VFI) = visceral fat (g)/body weight* 100
- HMT = hematocrit values (% red blood cells in blood)
Results & Discussion
The juvenile Atlantic salmon grew to an average tank density of 2.5 kg m-3 by the 9-week point. It was at this point that ten fish per treatment were randomly selected for necropsy. All fish tripled their weight during the feeding trial. Relative growth (RG) did not significantly differ among treatments upon conclusion of the trial (Table 2). Fish fed MEP/Meal 50 had the lowest growth at the final weigh point in the trial (Table 3). Final average weights of fish fed MEP/Meal 100 were numerically higher than the Control. FCR was lowest for the MEP/Meal 100 followed by Control and MEP/Meal 50 (Table 4).
Table 2: Relative growth (RG, %) based on the average initial weight per fish (n=5, mean ± SD).
Treatment
Relative Growth ± S.D.
Week 3
Week 6
Week 9
Week 12
Control
26 ± 504
89 ± 11.89
159 ± 17.88
223 ± 25.63
MEP/Meal 50
25 ± 2.74
82 ± 8.15
145 ± 13.16
201 ± 19.28
MEP/Meal 100
23 ± 4.20
86 ± 11.68
163 ± 15.73
230 ± 34.31
Treatment
Week 0
Week 3
Week 6
Week 9
Week 12
Control
7.72 ± 0.2
9.74 ± 0.5
14.60 ± 1.2
19.99 ± 1.7
24.92 ± 2.3
MEP/Meal 50
7.71 ± 0.2
9.62 ± 0.2
14.01 ± 0.6
18.88 ± 1.0
23.24 ± 1.8
MEP/Meal 100
7.77 ± 0.2
9.59 ± 0.5
14.44 ± 1.2
20.40 ± 1.4
25.64 ± 2.8
Treatment
FCR
SGR
Control
1.01 ± 0.16
1.39 ± 0.10
MEP/Meal 50
1.10 ± 0.11
1.31 ± 0.08
MEP/Meal 100
0.92 ± 0.17
1.42 ± 0.13
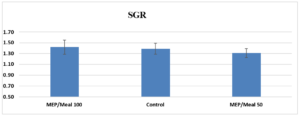
Figure 1: Total specific growth rate (SGR) for each treatment for the final weigh point in the trial. Error bars show standard deviation.
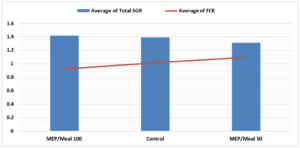
Figure 2: SGR (blue bars) and FCR (red line) for final weigh point in the trial (n=5). A greater overlap of values is indicative of better feed utilization.
Histology
All distal intestine samples showed similar structure. There were no differences in width of the lamina propria (LP) or supranuclear layer (SL) among treatments. The control diet displayed normal villa within the SL and no inflammation of the LP (Figure 3). Distal intestine samples did not show a significant difference among treatments and the experimental treatments with the supranuclear layer (SL) and lamina propria (LP) displayed normal intestinal morphology. (Figures 4, 5). All samples were healthy in appearance and given an enteritis score of none.
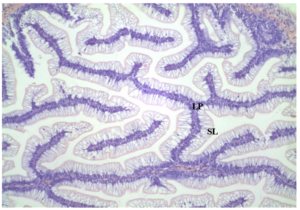
Figure 3: Distal intestine samples (10x magnification) from Control. No inflammation of lamina propria (LP) and no reductions in supranuclear layer (SL).
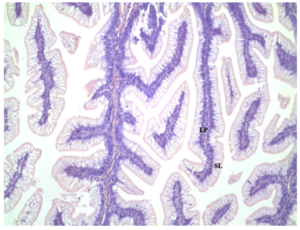
Figure 4: Distal intestine samples (10x magnification) from MEP/Meal 50. No inflammation of lamina propria (LP) and no reductions in supranuclear layer (SL).
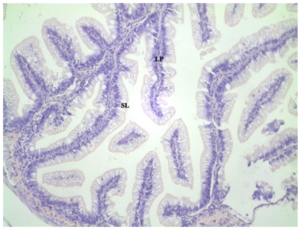
Figure 5: Distal intestine samples (10x magnification) from MEP/Meal 100. No inflammation of lamina propria (LP) and no reductions in supranuclear layer (SL).
Fish were left off feed for 18 hours prior to necropsy. Lengths and weights of the individual fish were recorded to calculate Fulton’s Condition Factor (K). All treatments showed values that represented good overall body shape and size. The liver, viscera, and visceral fat were all weighed to determine organosomatic values (HSI, VSI, VFI) (Table 5).
Table 5: Histology results at the 9-week sampling point (n=10).
Treatment
HSI
VSI
VFI
HMT
K
Control
1.57
7.15
2.21
42.00
1.22
MEP/Meal 50
1.46
6.66
2.57
42.00
1.22
MEP/Meal 100
1.35
6.51
2.20
41.00
1.22
Conclusions
The purpose of this trial was to evaluate the combination of fermented soy protein (ME-PRO®) and defatted BSFL meal (EnviroMeal) in salmon diets with reduced or no fish meal. The Control diet, having the highest fish meal inclusion, was defined as the positive control in this trial and therefore performed expectedly well. The combinations of ingredients that were used to replace fish meal at 50 and 100% may provide the foundation for how insect meal and fermented soy protein can be formulated into diets in all fish species to reduce the dependence on fish meal. The feeds all produced growth rates that were satisfactory for pre-smolt salmon production.
Formulation trends indicate that salmon feed producers will continue to use at least a minimal amount of fish meal in diets [9, 10], except in specialty markets where consumers are driving the demand for fish meal free feeds. The results of this trial indicate that a combination of EnviroMeal and ME-PRO® may be able to reduce the dependence on fish meal in diets for juvenile Atlantic salmon, without significantly impacting growth performance. The use of BSFL defatted meal coupled with fermented soy protein will help the aquafeed industry push towards its goal of continuously improving sustainability.
References
- Thorstad, Eva B., D. Bliss, C. Breau, K. Damon, L. E. Sundt-Hansen, E.M.C. Hatfield, G. Horsburgh, H. Hansen, N.O. Maoiléidigh, T. Sheehan and S.G. Sutton (2021). Atlantic salmon in a rapidly changing environment—Facing the challenges of reduced marine survival and climate change. Aquatic Conservation: Marine and Freshwater Ecosystems, 31:2654-2665.
- Zlaugitne, B., J. Pubule and D. Blumberga (2022). Advantages and disadvantages of using more sustainable ingredients in fish feed. Heliyon, 8(9): e10527.
- Kannan, M., D.K. Rajan, T. Muralisankar, A.R. Ganesan, P. Sathishkumar and R. Nagarajan (2022). Use of black soldier fly (Hermetia illucens) larvae meal in aquafeeds for a sustainable aquaculture industry: A review of past and future needs. Aquaculture, 553:738095.
- Radhakrishnan, G., M.S. Silva, E-J Lock, I. Belghit and A.J. P. Philip (2022). Assessing amino acid solubility of black soldier fly larvae meal in Atlantic salmon (Salmo salar) in vivoand in vitro. Frontiers in Physiology, 13:1028992.
- Shahida, A.S., B. Ristow, T. Rahayu, N.S. Putra, N.W. Yuwono, K. Nisa, B. Mategeko, S. Smetana, M. Saki, A. Nawaz and A. Nagdalian (2022). Black soldier fly larvae (BSFL) and their affinity for organic waste processing. Waste Management, 140: 1-13.
- White, B., L. Fredrickson, O. Araujo, N. Araujo, S.F. Nates (2020). The Role of the Microbial Enhanced Protein ME-PRO® in Recirculation Aquaculture Systems (RAS) Using Precision Feeding. Journal of Aquaculture, Marine Biology and Ecology, 1:1-9.
- Li, Y., K. Gajardo, A. Jaramillo-Torres, T. M. Kortner, and Å. Krogdahl (2021). Consistent changes in the intestinal microbiota of Atlantic salmon fed insect meal diets. Animal Microbiome 4:1-15.
- Brezas A, Hardy RA (2020). Improved performance of rainbow trout selected strain is associated with protein digestion rates and synchronization of aminoacid absorption. Scientific Reports 10: 4678.
- Foroutani, M.B., C.C. Parrish, J. Wells, R.G. Taylor, M.L. Rise and F. Shahidi (2018). Minimizing marine ingredients in diets of farmed Atlantic salmon (Salmo salar): Effects on growth performance and muscle lipid and fatty acid composition. Plos One.
- Kousoulaki, K., L. Sveen, F. Niren and A. Espmark (2022). Atlantic Salmon (Salmo salar) Performance Fed Low Trophic Ingredients in a Fish Meal and Fish Oil Free Diet. Frontiers in Physiology, 13:884740.