Publication Information
ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Evaluation of Antioxidant and Anti-Obesity Potentials of Four Tunisian Medicinal Plants: Mesembryanthemum edule, Atriplex inflata, Rantherium suaveolens and Arthrophytum scoparium
Feten Zar Kalai1.2*, Samia Oueslati1, Riadh Ksouri1, Hiroko Isoda3.4
1Laboratory of Aromatic and Medicinal Plants, Center of Biotechnology, Technopark of Borj Cedria, BP 901, 2050 Hammam-Lif, Tunisia
2Graduate School of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki 305-8572, Japan
3Alliance for Research on the Mediterranean and North Africa (ARENA), University of Tsukuba, Tsukuba, Japan
4Faculty of Life and Environmental Sciences, University of Tsukuba, 1‑1‑1 Tennodai, Tsukuba, Ibaraki 305‑8572, Japan
Received Date: March 15, 2022; Accepted Date: March 22, 2022; Published Date: March 31, 2022
*Corresponding author: Feten Zar Kalai, Laboratory of Aromatic and Medicinal Plants, Center of Biotechnology, Technopark of Borj Cedria, BP 901,2050 Hammam-Lif, Tunisia / Graduate School of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki 305-8572, Japan. Email: zarfeten@gmail.com
Citation: kalai FZ, Oueslati S, Ksouri R, Isoda Hiroko (2022) Evaluation of Antioxidant and Anti-Obesity Potentials of Four Tunisian Medicinal Plants: Mesembryanthemum edule, Atriplex inflata, Rantherium suaveolens and Arthrophytum scoparium. Adv in Nutri and Food Sci: ANAFS-229.
DOI: 10.37722/ANAFS.2022202
Abstract
This study investigated the antioxidant and anti- adipogenic capacities of four Tunisian Extremophile plants (Mesembryanthemum edule (ME), Atriplex inflate (AI), Rantherium suaveolens (RS) and Arthrophytum scoparium (AS)). These plants are well known for their use in the traditional medicine. Mesembryanthemum edule exhibit an important amount of total phenolic compound (73.10 mg EGA/g DW) followed by Rantherium suaveolens (58.92 mg EGA/g DW). Mesembryanthemum edule presented the highest antioxidant capacity (82.15 mg EGA/g DW) followed by Rantherium suaveolens (32.19 mg EGA/g DW). For the anti-radical activity, RS extract showed considerable radical scavenging activity with an IC50 equal to 8.52 and in important anti-adipogenic activity by the inhibition of 3T3-L1 differentiation. HPLC analysis indicated that RS extract contained transcinnamic acid, isorhamnetin 3-O –glucoside and isoquercetrin as major compounds.
Keywords: Antioxidant; Anti-adipogenic; Rantherium suaveolens; 3T3-L1 cells; HPLC
Introduction
Obesity is fundamentally a problem of energy balance and it can develop when energy intake exceeds energy expenditure, resulting in fat accumulation and excessive adipose tissue mass. Possible mechanisms for preventing or treating obesity involve decreasing triglycerides formation, increasing fatty acid oxidation, or increasing lipolysis [1].Many studies have demonstrated that adipocyte differentiation and fat amount as well as adipocyte dysfunction are strongly associated with the development of obesity, which is a major risk factor for many metabolic disorders and cardio-vascular diseases [2, 3]. One of the treatment/prevention of fat accumulation in adipose tissue is via the control of adipocyte differentiation and adipogenesis using in vitro cell models specific to obesity research. Among the cell line models, 3T3-L1 cells is a cell line derived from mouse widely used in this kind of biological research purpose. Based on in vitro and in vivo experiments, a lot of research studies agree that plants can be used as a treatment for metabolic problems such as obesity [4-6]. Pharmacological strategies are recommended for the treatment of obesity. In fact, the choice of the potent obesity treatment depends on the right diagnosis [7]. There are several integrative and corresponding practices, including dietary strategy, physical activity, behavioral therapy, surgical interventions, medicinal therapies, drug addiction treatments, hypnosis, acupuncture and the use of medicinal plants. These approaches also grant to the selection of species to be considered and the improvement of phytotherapeutic medicines based on ethnopharmacological exploration.
Among these therapies are plant-based medications that may contribute to satiety, increased metabolism and accelerated weight loss [8]. In this context, some plants as halophytes and xerophytes are a rich source of natural active compounds including polyphenols, flavonoids, and anthocyanin [9].
Based on the above considerations, this study presents an exploration of some extremophile plants (Mesembryanthemum edule, Atriplex inflata, Rantherium suaveolens and Arthrophytum scoparium) known for their richness in phenolic compounds, by evaluating antioxidant capacity and their potential therapeutic properties in the prevention or treatment of obesity.
Materials and Methods
Sampling and Plant extraction
Mesembryanthemum edule sample was collected from the coastal region “Soliman” (Nabeul, Tunisia) with sub-humid climate. Atriplex inflata was collected from salt flat (sebkha) of Kairouan city with semi-arid climate. Rantherium suaveolens and Arthrophytum scoparium samples were collected from the south region of Tunisia (Gabes) with a Saharan climate. The plant material was identified at the Centre of Biotechnology of Borj Cedria by Professsor Smaoui. The collected samples were rinsed with distilled water, kept in laboratory temperature; oven dried at 60°C and then ground finely using a ball mill type “Dangoumeau”. Afer that, 10 g of dry powder was extracted with 100 ml of ethanol/water (70%) solution for 30 min under a magnetic stirring. All extracts were filtered using a Whatman filter paper (No. 4). Then the extracts were stored at 4°C until analysis.
Determination of total phenolic contents
Total polyphenols were assayed by the Folin-Ciocalteu reagent and gallic acid as standard according [10]. Absorbance was checked at 760 nm. Contents were expressed as mg gallic acid equivalent per gram of dry weight (mg GAE/g DW) through the calibration curve with gallic acid, ranging from 0 to 500 µg/ml. All samples were analysed in triplicate.
Determination of flavonoid contents
Total flavonoid content of all extracts was estimated using a colorimetric assay developed by [11]. An aliquot (250 µl) of the samples was mixed with 75 µl NaNO2 solution (5%; w/v) for 6 min, before adding 150 µl AlCl36H2O (10%; w/v). After 5 min at room temperature, 500 µl of NaOH (1 M) was added. The final volume was adjusted to 2.5 ml with H2O and scrupulously mixed. Absorbance of the mixture was determined at 510 nm and converted to flavonoid concentration from a catechin standard curve and expressed as mg catechin equivalents/g of dry weight (mg CE/g DW).
Determination of condensed tannin contents
The condensed tannins content was determined according to the method [12]. Fifty ml of diluted extract were mixed with 3 ml vanillin (4%; w/v). Then, 1.5 ml 2M hydrochloric acid was added to the mixture. After incubation for 15min at room temperature, absorbance was measured at 500 nm. Results were expressed as mg catechin equivalents/g of dry weight (mg CE/g DW).
Evaluation of antioxidant activities
DPPH radical scavenging assay
Radical-scavenging activity was determined according [13]. Briefly, extract at different concentrations were added to 0.2 mM of 2,2-diphenyl-1-picrylhydrazyl (DPPH) methanolic solution then the absorbance was measured at 517 nm. DPPH radical scavenging activity was expressed as IC50, defined as the concentration of the extract generating 50% inhibition. All samples were analyzed in triplicate.
Evaluation of total antioxidant activity (TAA)
Total antioxidant ability of all extracts was based on the reduction of Mo (VI) to Mo (V) and subsequent formation of a green phosphate/Mo5+ complex at acid pH according a protocol described previously [14]. An aliquot (100 µl) of diluted extracts was combined with 1ml of reagent solution (0.6N sulfuric acid, 28mM sodium phosphate and 4mM ammonium molybdate). The tubes were incubated in a thermal block at 95°C for 90 min. Then, the mixtures were cooled to room temperature and the absorbance of each solution was measured at 695 nm against blank in a UV–vis spectrophotometer. Antioxidant capacity was expressed as mg gallic acid equivalent per gram dry weight (mg GAE/g DW).
Iron Reducing Power
This antioxidant activity was focused on the reduction of the trivalent iron produced by the FeCl3: Iron (III) chloride anhydrous [15]. The intensity of the appearing blue-green colour was measured at 700 nm. The EC50 value (µg/ml) for the reducing power is the extract concentration at which the absorbance was 0.5 and ascorbic acid was used as a positive control. All samples were analysed in triplicate.
Analysis of phenolic compounds by analytical RPHPLC/ UV
During this experiment, extracts used previously was kept. Before injection into the HPLC system, extract was passed through a 0.45 μm nylon filter. The separation of selected phenolic compounds was carried out using HPLC system (consisting of a vacuum degasser, an autosampler, and a binary pump with a maximum pressure of 600 bar; Agilent 1260, Agilent technologies, Germany) equipped with a reversed phase C18 analytical column of 4.6 x100 mm and 3.5μm particle size (Zorbax Eclipse XDB C18). Column temperature was maintained at 25°C. The injected sample volume was 2 μl and the flow-rate of mobile phase was 0.4 ml/min. Mobile phase B was milli-Q water consisted of 0.1% formic acid and mobile phase A was methanol. The optimized chromatographic condition was revealed as follows: 10% A, 90% B (0 min); 20% A, 80% B (5 min); 30% A, 70% B (10 min); 50% A, 50% B (15 min); 70% A, 30% B (20 min); 90% A, 10% B (25 min); 50% A, 50% B (30 min); 10% A,90% B (35 min). UV-vis absorption spectra were recorded online during the HPLC analysis. The DAD detector was set to a scanning range of 200-400 nm. Peak identification was obtained comparing the retention time and the UV-vis spectra of all extracts phenolics chromatograms with those of available standards [16]. Quantification was performed by reporting the measured integration area in the calibration equation of the corresponding standard.
Cell Culture
Murine 3T3-L1 preadipocytes (Riken Tsukuba japan) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin (5000 μg/ml)-streptomycin (5000 IU/ml) in 75-cm2 tissue culture flasks. Medium was changed every 3 days and cell passage was carried out at 80% confluence at one on two ratio using 0.25% trypsin (1 mM EDTA). 3T3-L1 cells were cultured in a humidified incubator at 37°C and 5% CO2.
Pre-adipocytes differentiation and Oil-Red-O staining procedures
3T3-L1 pre-adipocytes were seeded into 96-well plates at 1.0 x104 cells/well, and cultured for additional two days until full confluence. Two days later (Day0), cells were incubated with a differentiation cocktail (MDI) containing 1/10 insulin solution, 1/10 dexamethasone solution and 1/10 3-isobutyl-1-methylxanthine solution in standard culture medium for 3 days followed by additional 48h with standard culture medium containing insulin alone. The differentiation-maintenance medium was changed every 2 days. To investigate the effect of extracts on adipogenesis in 3T3-L1, at different doses; (25, 50, 100, 200 and 400μg/ml) were added to the differentiation-induction and differentiation-maintenance media. The same procedure was conducted to investigate the effect of the determined phenolic compounds of NR; Luteolin-7-O-glucoside (5, 25, 50, 100 and 200 μM), Isorhamnetin (5, 25, 50, 100 and 200μM) and Isorhamnetin-3-O-rutinoside (5, 25, 50, 100 and 200μM). The staining procedure was conducted according to the adipogenesis assay kit (Cayman chemical company). The absorbance was read at 490 nm with a 96-well plate reader. The lipid droplet content was reported as percentage of control cells.
Cell proliferation assay (MTT assay)
Cell proliferation was investigated by MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay. 3T3-L1 cells were seeded in 96-well plates at 1×105cells/ml. After incubation for 7 days (adipocytes), leaf and stem extract samples diluted in medium were added at final concentrations of 25, 50, 100, 200, 400 μg/ml. The same procedure was conducted to investigate the effect of the determined phenolic compounds of NR; Luteolin-7-O-glucoside (5, 25, 50, 100 and 200 μM), Isorhamnetin (5, 25, 50, 100 and 200μM) and Isorhamnetin-3-O-rutinoside (5, 25, 50, 100 and 200 μM). MTT was added after treatment for 7 days, and the resulting formazan was completely dissolved by 100 μl of 10% sodium dodecyl sulfate (SDS) for 24h. The absorbance was determined at 570 nm in a multi-detection microplate reader (Power-scan HT, Dainippon Pharmaceutical, NJ, USA). Absorbance caused by the ability of the sample to reduce MTT or by its color, was corrected using plates as blanks, prepared in the same conditions in the absence of cells.
Results and Discussion
Total phenolic, flavonoid and condensed tannin contents
Phenolic contents depend on several factors such as species. In fact, total polyphenol, flavonoid and tannin content are listed in (Table 1). Results showed a significant difference between species. Mesembryanthemum edule exhibit an important amount of total phenolic compound (73.10± 0.72 mg EGA/g DW) followed by Rantherium suaveolens (58.92± 1.32 mg EGA/g DW). Arthrophytum scoparium and Atriplex inflata present the lowest TPC with 6.80± 0.09 mg EGA/g DW and 5.32± 0.07 mg EGA/g DW, respectively.
The same tendency was observed for total flavonoid content which exhibit that Mesembryanthemum edule and Rantherium suaveolens contain the highest amount of these metabolites, respectively with 45.00± 0.82 mg EC/g DW and 21.75± 1.11 mg EC/g DW.
R.suaveolens extract was characterized by considerable amount of condensed tannin with 38.82 ± 2.13 mg EC/g DW. While the lowest quantities were detected in Arthrophytum scoparium and Atriplex inflata. In fact, Halophytes can be an interesting source of bioactive compounds, as they grow in conditions extremely hard with continuous stress, which can stimulate their metabolism to produce bioactive molecules [17]. These changes could be attributed to the effect of species, organs or environmental, genetic and technical factors [18]. All studied plants had high TPC compared to values of some known medicinal plants such as Dactyloctenium aegyptium, Withania somnifera and Zygophyllum simplex which exhibit 3.31 mg EGA/g DW, 4.79 mg EGA/g DW and 5.21 mg EGA/g DW, respectively [17].
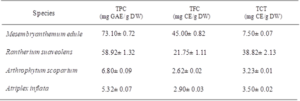
Table 1: Total phenolic, flavonoid and condensed tannin contents from six plant ethanolic extracts. Values are the means of three replicates and standard deviation. Values with different superscripts (a, b) are significantly different at P<0.05.
Antioxidant potentialities of Mesembryanthemum edule, Atriplex inflata, Rantherium suaveolens and Arthrophytum scoparium
Extracts of four medicinal halophytes have been studied for their antioxidant capacity using three complementary tests: total antioxidant activity, DPPH and reducing power (Table 2). Several researches have proven the potent role of phenolics as major antioxidant agents in plants, particularly within tolerant species14. Mesembryanthemum edule presented the highest antioxidant capacity (82.15± 2.21 mg EGA/g DW) followed by Rantherium suaveolens (32.19± 1.23 mg EGA/g DW). This activity was correlated with their richness in phenolic compounds. However, Atriplex inflata exhibited the lowest activity with 10.28± 0.5 mg EGA/g DW. The assessment of the total antioxidant activity showed that the total antioxidant ability was species dependent [18].
Concerning antiradical activity, the results of DPPH test indicated that Rantherium suaveolens extract showed considerable radical scavenging activity with an IC50 equal to 8.52±0.56 µg/ml. Furthermore, Rantherium suaveolens extract exhibited higher antioxidant activity than BHT (IC50 of 11.5µg/ml). Besides, Mesembryanthemum edule was considered as a potent antioxidant (IC50 = 21.36 ± 2 µg/ml). On the other hand, our results were better than those found by [19] who reported that flower extract of Rhanterium sueaveolens exhibit an antiradical capacity with an IC50 equal to 333 µg/ml. In addition, our results were efficient than those found by [20] which reported that antiradical activity of halophytes such as Achillea collina, Aster tripolium subsp. pannonicus and Suaeda maritima were (106.65 mg/ml), (117.73 mg/ml) and (132.61 mg/), respectively.
The assessment of antioxidant activity using FRAP test showed that Mesembryanthemum edule exhibited a potent reducing capacity with a lower EC50 of 110 µg/ml. EC50 values of all tested extracts were ranged from 110 to 2150 μg/ml and arranged in the following decreasing efficiency order: Mesembryanthemum edule (110 μg/mL) > Rhanterium sueaveolens (180 ±5.10 µg/ml) > Arthrophytum scoparium (1020 ±15.10 μg/mL) > Atriplex inflata (2150 ±17.21 μg/mL). These results suggested that Mesembryanthemum edue is considered as a source of antioxidant reductants. In the same context [21] demonstrate that roots extract of M. edule present an EC50 equal to 217 μg/mL. In fact, it is evident that halophytes enriched in phenolics present an important antioxidant activity [22]. Besides, due to the diversity of antioxidants it will be interesting to use various antioxidant tests to elucidate the mechanism of action of different compounds [23]. Previous researches indicated the presence of a clear relationship between antioxidant potentialities of plant extracts and total phenolic contents [24]. Besides, it is necessary to use a wide range of antioxidant tests in order to assess the antioxidant ability of plant extracts known by the diversity of compounds, where the mechanism of action differs depending on the species [25, 26]. Furthermore, compound can act independently or in synergetic with other compounds [27].
Anti-adipogenic activities of Mesembryanthemum edule, Atriplex inflata, Rantherium suaveolens and Arthrophytum scoparium extracts
Adipogenesis assay was performed to investigate the effect of halophyte samples on the adipocyte differentiation and on the lipid droplets accumulation in 3T3-L1 cells using Oil red O staining. Differentiated 3T3-L1 cells were treated every two days with different halophyte extracts at various concentration and with 25 μM isorhamnetin (as positive control), for 7 days. Based on Oil-Red-O content quantification, results showed that not all these samples are effective in reducing the lipid content within cells. Only some high concentrations of certain plant extracts were shown to inhibit lipid droplets accumulation within 3T3- L1 cells. For example, Cells treated with RS and ME at 400 μg/ml were less differentiated than other treated cells showing similar results as isorhamnetin (about 50%), so the inhibition of cell differentiation and adipogenesis of these cells (Figure 1). The important anti-adipogenic effect could be correlated to the potential antioxidant capacities of ME and RS in comparaison with other plants. In other hand, cell viability of 3T3-L1 cells was cheked using MT assay and results showed that ethanolic extracts of halophyte medicinal plants did not significantly affect the cell viability except for RS which reduced the cell proliferation especially for the high dose as shown in (Figure 2).
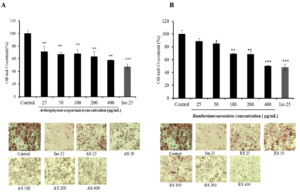
Figure 1: Effects of plant extracts: AS (A), RS (B), ME (C) and AI (D) using different concentrations (25, 50, 100, 200 and 400 μg/mL), on lipid droplet content in 3T3-L1 cells. Fat droplets in adipocytes differentiated for 7days with or without sample extracts and isorhamnetin at 25 μM dose (the positive control) treatments were stained with oil Red-O dye and examined used a light microscope. Relative Oil-Red-O absorbance was measured at 490nm. Lipid droplet accumulation in treated cells was expressed as a percentage of control (untreated cells). Bars represent mean ± SD, n=3, * p<0.05, ** p<0.01.

Figure 2. Effects of plant extracts: AS (A), RS (B), ME (C) and AI (D) using different concentrations (25, 50, 100, 200 and 400 μg/mL) and isorhamnetin at 25 μM dose (Iso 25), on 3T3-L1pre-adipocytes viability after cell differentiation (7 days’ treatment). Bars represent mean ± SD, n=3, * p<0.05, ** p<0.01
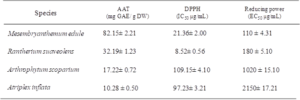
Table 2: Antioxidant activities of the four halophyte plants: Total antioxidant activity, DPPH radical scavenging activities and ferric reducing power. Values are the means of three replicates and standard deviation. Values with different superscripts (a, b) are significantly different at P<0.05.
Identification of phenolic compounds in potent species by RPHPLC/ UV
In this section only extracts with the best potentialities (Rhanterium suaveolens) have been selected for phenolic identification. RP-HPLC coupled with a UV-Vis multi wavelength detector was employed to separate and to quantify phenolic compounds. A total of 8 phenolics were detected in this extract (Figure 3). These compounds have been identified according to their retention time and the spectral characteristics of their peaks compared to those of standards, as well as by spiking the sample with standards. Phytochemical investigation allowed us to depict trans-cinnamic acid as major phenolic acid with a high proportion (7.321 mg/g R), followed by isorhamnetin 3-O -glucoside and Isoquercetrin with the proportions of the order of 5.40 and 3.29 mg/g R, respectively. All these natural compounds especially the ones containing phenolic hydroxyl group are well-known for their several health benefits due to inherent strong free radical scavenging properties. In fact, cinnamic acid and its derivates are being studied as potential antioxidants due to the multi-functional activities they exhibit [28]. Besides, isorhamnetin has an efficient protective consequence against oxidative stress in human cells, and the mechanism of action is principally related to the activation of PI3K/Akt signal transduction pathway. Then, isorhamnetin can reinforce the cellular antioxidant resistance capacity by activation of the Nrf2/ HO-1 and ERK pathways, thus preventing C2C12 cells from H2O2 induced cytotoxicity [29]. In addition, cinnamic acid treatment considerably decreased oleic acid which induce lipid accumulation in HepG2 cells and significantly reduced the triglycerides content in a dose-dependent manner [30]. In the same context, these authors demonstrate that cinnamic acid down-regulated genes such as branched-chain ketoacid dehydrogenase kinase (BDK) and up-regulated branched-chain ketoacid dehydrogenase phosphatase (PPM1K), which led to a decreased BDK: PPM1K ratio. This may also indicate its inhibitory effect on the transcription factor carbohydrate-responsive element-binding protein ChREBP, as well as reflecting cinnamic acid therapeutic effects against obesity. Moreover, isorhamnetin can be implicated in prevention of obesity with inhibition of fat formation and the promotion of mitochondrial biogenesis, reduction of body weight, improvement of insulin resistance, and liver fat degeneration in 3T3-L1 preadipocytes and female C57/BL6 mice and increase of insulin secretion and energy consumption in mice fed with a high-fat diet [31, 32].

Figure 3: RP-HPLC chromatograms of Rhanterium suaveolens extract. Signal was monitored at 254 nm. The peak numbers correspond to: 1: Catechol; 2: Catechin; 3: Chlorogenic acid; 4: Trans 3-Hydroxycinnamic acid; 5: Isoquercetrin; 6: Kaempferol 3-O-rutinoside; 7: Isorhamentin- 3-O-glucoside; 8: Transcinnamic acid.

Table 3: Phenolic compounds identified and quantified by analytical RP-HPLC/UV from ethanolic extract of Rhanterium suaveolens. Values are the means of three replicates and standard deviation. Values with different superscripts (a, b) are significantly different at P<0.05.
Conclusion
Taken together all the results obtained in our findings, halophytes and xerophytes originated from Tunisia could be a potential source of natural products and bioactive molecules which could be used as ingredients in the formulation of food supplements and pharmaceutical drugs for the treatments and prevention of obesity and its linked disorders.
Acknowledgments: This work was supported by the Japanese JICA/JST Science and Technology Research Partnership for Sustainable Development Project and the Tunisian Ministry of Higher Education and Scientific Research (LR19CBBC06).
Disclosure Statement: The authors declare that they have no conflict of interest.
References
- Mir SA, Shah MA, Ganai SA, Ahmad T, Gani M (2019) Understanding the role of active components from plant sources in obesity management. Saudi Soc. Agric. Sci 18 : 168-176.
- Lee J, Jung E, Lee J, Kim S, Huh S, et al. (2009) Isorhamnetin represses adipogenesis in 3T3-L1 cells. Obesity 17: 226-32.
- Longo M, Zatterale F, Naderi J, Parillo L, Formisano P, et al. (2019) Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci 20: 1-23.
- Patra S, Nithya S, Srinithya B (2015) Review of Medicinal Plants for Anti-Obesity Activity. Biomed 6: 1-22.
- Chang E, Hafner H, Varghese M, Clemente J, islam M, et al. (2019) Programming effects of maternal and gestational obesity on offspring metabolism and metabolic inflammation. Sci Rep 9:16027.
- Sim WS, Choi S, Cho BY, Choi SH, han H, et al. Anti-Obesity Effect of Extract from Nelumbo Nucifera L., Morus Alba L., and Raphanus Sativus Mixture in 3T3-L1 Adipocytes and C57BL/6J Obese Mice. Foods 8: 1-18.
- Hardy LL, Mihrshahi S, Gale J, Nguyen B, Baur LA, et al. (2015) Translational research: are community-based child obesity treatment programs scalable?. BMC Public Health 15: 652.
- Luciano FJ, Eduardo BA (2017) Medicinal plants for the treatment of obesity: Ethnopharmacological approach and chemical and biological studies. Am. J. Transl. Res 9: 2050–2064.
- Tungmunnithum D, Thongboonyou A, Pholboon A (2018) Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects : An Overview. Medicines 5: 93.
- Dewanto V, Wu X, Adom KK (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50 :3010-3014.
- Falleh H, Ksouri R, Lucchessi ME, Abdelly C (2012) Ultrasoundassisted extraction: effect of extraction time and solvent power on the levels of polyphenols and antioxidant activity of Mesembryanthemum edule Aizoaceae shoots. Trop. J. Pharm. Res 11: 243-249.
- Oueslati S, Ben mansour R, Medini F, Ksouri R, Megdiche W (2020) Effect of the extraction method on the content of phenolic compounds and the antioxidant and antibacterial potential of Mentha spicata L. JNS 77: 4503-4509.
- Hatano T, Kagawa H, Yasuhara T (1998)Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects." Chem pharm bull 36: 2090-2097.
- Ben Mansour R, Wasli H, Serairi-Beji R, Soumaya B, et al. (2020) In vivo gastroprotective effect and biological potentialities of six Tunisian medicinal plants using multivariate data treatment, Plant Biosystems 1-12.
- Oyaizu M (1986) (Studies on Products of Browning Reactions: Antioxidative Activities of Product of Browning Reaction Prepared from Glucosamine. Japan J Nutr 44: 307-315.
- Guyot S, Doco T, Souquet JM, et al. (1998) Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a french cider apple variety (Malus). J Agri Food Chem 46: 1698-1705.
- Qasim M, Abideen Z, Adnan MY, et al. (2017) Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas, Afr. J. Bot110: 240-250.
- Bourgou S, Ezzine Y, Ben Mansour R, et al. (2020) Preliminary phytochemical analysis, antioxidant, anti-inflammatory and anticancer activities of two Tunisian Ephedra species: Ephedra alata and Ephedra fragilis. Afr. J 135: 1-8.
- Hitana M, Dupas C, Oulahal N, et al. (2019) Assessment of antioxidant activities of an endemic species from Tunisia: Rhanterium sueaveolens Desf related to its phenolic composition. Agr. Biot 22: 101355.
- Stanković MS, Petrović M, Godjevac D, et al. (2015) Screening inland halophytes from the central Balkan for their antioxidant activity in relation to total phenolic compounds and flavonoids: Are there any prospective medicinal plants. Arid Environ 120: 26-32.
- Falleh H, Ksouri R, Medini F, et al. (2011) Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind Crops Prod 34: 1066-1071.
- Boulaaba M, Medini F, Hajlaoui H, et al. (2019) Biological activities and phytochemical analysis of phenolic extracts from Salsola kali L. Role of endogenous factors in the selection of the best plant extracts. Afr. J. Bot 123:193-199.
- Moukette BM, Pieme CA, Njimou JR, et al. (2015) In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non –timber forest product used as spice Monodora myristica. based compl. Alter. Med 48: 1-17.
- Gali L, Bedjou F (2019) Antioxidant and anticholinesterase effects of the ethanol extract, ethanol extract fractions and total alkaloids from the cultivated Ruta chalepensis. Afr. J. Bot 25:163-69.
- Bellik Y, Benabdesselam F, Ayad A, et al. (2013) Antioxidant activity of the essential oil and oleoresin of Zingiber officinale Roscoe as affected by chemical environment. J. Food Prop 16: 1304-1313.
- Zengin G, Menghini L, Di Sotto A, et al. (2018) Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa essential oil: a multidisciplinary study. Molec 23: 1-26.
- Oueslati S, Ellili A, Legault J, et al. (2015) Phenolic content, antioxidant and anti-inflammatory activities of Tunisian Diplotaxis simplex (Brassicaceae). Nat Prod Res 29: 1-3.
- Oladimeji OH, Promise OO, Anthony PC (2021) Acetylation of Cinnamic Acid and Evaluation of Antioxidant Activity of the Resultant Derivative. Biomed J Sci & Tech Res 39 : 31085-31088.
- Choi YH (2016) The cytoprotective effect of isorhamnetin against oxidative stress is mediated by the upregulation of the Nrf2-dependant HO-1 expression C2C12 myoblasts through scavenging reactive oxygen species and ERK inactivation. Physio. Biophys 35: 45-53.
- Wu Y, Wang M, Yang T, et al. (2021) Cinnamic Acid Ameliorates Nonalcoholic Fatty Liver Disease by Suppressing Hepatic Lipogenesis and Promoting Fatty Acid Oxidation. Bas. Complement. Alterna Med 9561613: 1-13.
- Lee MS, Kim Y (2018) Effects of isorhamnetin on adipocyte mitochondrial biogenesis and AMPK activation. Molecules 23: 1853.
- Zhang Y, Gu M, Cai W, et al. (2016) Dietary component isorhamnetin is a PPARγ antagonist and ameliorates metabolic disorders induced by diet or leptin deficiency. Sci Rep 6: 19288.