| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Evaluating Consumption Risk And Toxicity Index: A Case Study of tridax procumbens.
Ozioma Prince EMMANUEL1*, Uraku Anayo Joseph2, 1Olawale Otitoju1
1Department of Biochemistry Federal University Wukari, Taraba
2Department of Biochemistry Ebonyi State University, Abakaliki
1Department of Biochemistry Federal University Wukari, Taraba
Received Date: October 07, 2021; Accepted Date: October 25, 2021; Published Date: November 02, 2021;
*Corresponding author: Ozioma Prince EMMANUEL, Department of Biochemistry Federal University Wukari, Taraba, Email: emmanueloziomaprince@yahoo.com
Citation: Ozioma PE, Joseph UA, Otitoju O (2021) Evaluating consumption risk and toxicity index: a case study of tridax procumbens. Enviro Sci Poll Res and Mang: ESPRM-111.
DOI: 10.37722/ESPRAM.2021102
Abstract
This study was carried out to determine the level of some heavy metals (minerals), and selected vitamins present in Tridax procumbens leaf extracts and its nutritional compositions as it is recently used in phyto-medicine for the treatment of ailments and also used as source of vegetables for human consumption. All compositions were evaluated using modified standard method of AOAC, 2006. The result revealed myriad amounts (mg/100g) of heavy metals in the order of zinc (8.21 ± 0.01) > manganese (7.02 ± 0.01) > Iron (4.02 ± 0.01) > Nickel (0.62 ± 0.01) > lead (0.43 ± 0.01) > magnesium (0.42 ± 0.01) > cobalt (0.21 ± 0.01). The result for vitamins contents were expressed in mg/100g in the order of vitamin A (15.00 ± 1.41) > vitamin B9 (8.65 ± 0.03) > vitamin C (6.33 ± 0.02). It could be deduced that the nutritional composition of this plant must have led to its therapeutic and conventional use as a vegetable but the presence of some heavy metals could be a major health concern as bioaccumulation might lead to impairment of some vital organs in the body.
Introduction
Plants used as vegetables have been employed in development of nutraceuticals, and their effects have been commendable due to the presence of some phytochemicals, vitamins and minerals. One of such plants includes Tridax procumbens. Tridax procumbens is a species of flowering plant in the Aster family (Compositae), a common weed in West Africa, subregion and other tropical zones of world (Funk et al., 2009). The plant is called with different names; In Igbo it is called Mbuli, in Hausa it is called Harantama, in Yoruba it is called Igbalode, in English it is called coat button. It is a semi prostate, annual, creeper herb. Stem is ascending 30-50cm height, branched, sparsely hairy, rooting at nodes. Leaves are simple, opposite, mainly 3-7cm long, 1 to 4cm wide, irregularly toothed margin, base wedge shaped, shortly petioled, hairy on both surfaces. Flowers are tubular, yellow with hairs, inflorescence capitulum. Tridax has two types of flower: ray florets and disc florets with basal palcentation (Bhalerao and Kelkar, 2012).
 Figure 1: Tridax procumbens
Figure 1: Tridax procumbens
Ikewuchi et al., (2009a and b) researched on the nutritional properties of the leaves of Tridax procumbens, also commonly used as vegetables and for medicinal purposes, because of their myriad of pharmacological properties. These includes but not limited to use as analgesic (Prabhu et al., 2011), anti-anemic (Ikewuchi and Ikewuchi, 2013), anti-arthritic (Petchi et al., 2013), anti-diabetic (Bhagwa et al., 2008; Ikewuchi, 2011; Pareek et al., 2008), antihypertensive (Ikewuchi et al., 2011; Salahdeen et al., 2004), anti-inflammatory, antioxidant (Ravikumar, 2005), antimicrobial ( Yoga et al., 2009), hepatoprotective (Ikewuchi, 2012). The present study reports the heavy metal and selected vitamins composition of the leaves of Tridax procumbens, and in addition discusses the bioaccumulation effect of the detected compounds.
Tridax procumbens has been adduced to have many phytochemical, mineral, and vitamins constituents of, which has resulted it its use in phyto-medicine and consumption as vegetables in villages like Abakaliki, Ebonyi State, Nigeria, likewise other African countries. However, limited concern has been placed on its potential to bio-accumulate some heavy metals such lead, nickel, cobalt which might be present in an insignificant quantity.
Specific Objectives:
- To determine the level of minerals present in Tridax procumbens.
- To determine level of some specific vitamins (A, B9 and C) in Tridax procumbens.
Materials and Methods
All materials, chemical and reagent used are of analytical standard.
Collection of plant material
Fresh leaf of Tridax procumbens was collected at the back of Biochemistry Laboratory, Ebonyi State University, Abakaliki and was authenticated by Professor S.S.C Onyekwu of Taxonomy unit of the Department of Applied Biology, Ebonyi State University.
Preparation of Plant Material
The bulk sample was sorted to remove dried and infected plants, the rest were washed in running tap water to eliminate dirt. It was dehulled manually, spread on a Laboratory tray and shade dried for 2 weeks at room temperature. The dried sample was homogenized into powdered form using manual grinder. The ground sample was sieved through 1mm test sieve to obtain a powdered processed sample used for analysis.
Method of Mineral Determination
Minerals were estimated by the use of an Atomic Absorption Spectrophotometer using a modified standard method of AOAC (AOAC, 2006). The sample solutions in the sample bottles were analyzed for the concentration of the individual elements. Each element has specific cathode discharge lamp and this lamp was used to determine a particular element. Discharge lamp emits radiation at a wavelength specific for each element being assayed. This specificity can be obtained only from a pure sample of the element that is excited electrically to produce an arc spectrum on that element.
Determination of content Retinol (Vitamin A)
This was done using spectrophotometric using a modified standard method of AOAC (AOAC, 2006). 5g of sample was dissolved into a 30mls of absolute alcohol (ethanol), and 3mls of 5% of potassium hydroxide was dissolved into it. The mixture was boiled under reflux for 30mins and was cooled rapidly with running water and filtered. 30mls of distilled water was cooled rapidly with running water and filtered. 30mls of distilled water was added and also the mixture was transferred into a separating funnel. Three portions of 50mls of ether were used to wash the mixture, the layer as discarded and the upper layer was washed with 50mls of distilled water. The extract was evaporated to dryness and dissolved in 10mls of isoprophyl alcohol and its absorbance was measured at 320nm.
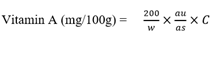
Where,
au = absorbance of test sample
as = absorbance of standard solution
C = concentration of test sample
W = weight of sample
Determination of Folic Acid (vitamin B9)
This was determined spectrophotometrically using a modified standard method of AOAC (AOAC, 2006). Homogenized leaves powder (500 mg) was weighed into 100 ml volumetric flask. It was dissolved with 50 ml, 3% of disodium hydrogen orthor-phosphate and shaked for 20 minutes, and make up to mark with the same solution, and then filter.
The standard folic acid powder (28 mg) was weighed and treated as above. The filtrate (40 ml) was taken from the test and 3ml of the filtrate was taken from the standard and each into separate 100 ml volumetric flask and was made up to mark with 3% disodium hydrogen orthorphosphate. Five milliliters of both standard and test each was added into 50 ml volumetric flask and was treated for colour development as follows;
Two millilitres of KMnO4 (0.4%) was added and allow to stand for one minute, two milliliters of sodium nitrate (2%) was added. Two millilitres of 5M HCl was also added and both standard tests were shaken. Two millilitres of sulphuric acid (5%), 2 ml of sodium edadate/EDTA (5%) was added, shake and allow standing for 10 minutes. Two millilitres of Azodye (0.1%w/v) was also added and allowed to stand for 10 minutes and absorbance read at 550 nm.
Potency = AT/AS × WS/WT × 3/100 ×100/40 × Average fill weight.
AT = Absorbance of test.
AS = Absorbance of standard.
WS = Weight of standard.
WT = Weight of test.
Average fill weight = 480 mg.
Determination of Vitamin C (Ascorbic Acid)
The method employed by Hussian et al., (2006) was used. 1g of every ground sample was weighed in a very 25 ml conical flask. Then 10ml of the ethanedioic acid (0.05 M)-EDTA (0.02 M) solution was added and also the mixture allowed standing for twenty-four h, to supply the desired time interval. After 24 h, the samples were filtered through 0.45 μm Whatman paper No.1. Then 2.5 ml of every sample was transferred to a separate 25 ml volumetric brown flask, after which 2.5 ml of the acid (0.05 M)-EDTA (0.02 M) solution was added. Subsequently, meta-phosphoric acid was added separately with ethanoic acid (0.5 ml), acid (5% v/v) solution (1 ml) and ammonium molybdate solution (2 ml) in each volumetric brown flask and therefore the volume was made up to 25 ml with water. The absorbance was measured at 760 nm in a very visible Spectrophotometer.
Statistical Analysis
Statistical analysis was done using statistical program for social science (SPSS) 22.0 (SPSS, Inc. Chicago, Illinois, USA). The results of replicate measurements were presented as means ± standard deviation.
Results
Results of Mineral content of Tridax procumbens leaf extract.
The result of mineral content of Tridax procumbens showed significant high levels of minerals such as, zinc, manganese, iron and moderate levels of cobalt, nickel, lead, magnesium lower than one (1) mg/100g,
Minerals
Values(mg/100g)
Magnesium
0.42 ± 0.01
Iron
4.02 ± 0.01
Zinc
8.21 ± 0.01
Manganese
7.02 ± 0.01
Cobalt
0.21 ± 0.01
Nickel
0.62 ± 0.01
Lead
0.43 ± 0.01
Values are expressed in mean ± standard deviation of triplet determination
Results of Vitamin content of Tridax procumbens leaf extract.
The result of vitamin content of Tridax procumbens leaf extract indicated high levels of Vitamins A, B9 and C as shown in table 2 below.
Vitamins
Values (mg/100g)
A
15.00 ± 1.41
B9
8.65 ± 0.01
C
6.33 ± 0.02
Values are expressed in mean ± standard deviation of triplet determinate
Discussion
Tridax procumbens leaf extract showed high level of vitamin A as shown in table 1.This high level of vitamin A might be responsible for the use of the leaf as vegetables and neutraceticals as they as pose antioxidant property, and maintenance of good vision property. The level of vitamin C showed (6.33 ±0.02mg/100g) found to be present in tridax procumbens leaf extract is lower than (10.63 ± 0.00mg/100g) found in the same leaf (Catherine and Jude in 2009). It also contains high level of vitamin B9 (8.65 ± 0.03mg/100g) which is higher than (0.60 ± 0.06), found in Seige leaf extract, (Odunfa, 2007), which aid in body metabolism and wound healing.
The levels of Zinc, Manganese, Iron were not significantly different from the result reported by Ikewuchi and Ikewuchi (2009) on same plant. Nevertheless, Punsiri in (2015), reported a lesser concentration of Zinc (2.7 ± 3.3) in same plant in a work conducted in Thailand. The differences in concentration could be as a result of changes in geographical locations and plant growth conditions. Zinc and Iron helps in boosting the Immune system boosting, bone formation and other properties. The concentration of magnesium is much lesser compared to the report of Ikewuchi and Ikewuchi (2009) whose magnesium concentration was (8.86 ± 2.22) on same plant. Punsiri in (2015) reported concentrations of lead (0.23 ± 0.22 %) and Nickel (0.26 ± 0.25%) which was higher than the result of this present research. It has been reported that metals such as Iron, Lead, Mercury, and Nickel have the ability to produce reactive oxygen species; thus, results in lipid peroxidation, DNA alteration, and hampered calcium homeostasis (Stohs and Bagchi, 1994; Otitoju and Onwurah, 2005). Therefore, consumption of Tridax procumbens leaf extract as medicine or vegetable because of its pharmacological properties might require some level of assessment in order to save the exposed or vulnerable population seeking for treatment to their ailments.
References
- O.A.C. (Association of Official Analytical Chemist), 2006. Official Methods of Analysis of the AOAC. 18th Edition, Horwitiz, Washington D.C, USA.
- Bhagwat DA, Suresh KG, Rahul AS (2008) Anti-Diabetic Activity of Leaf Extract of Tridax procumbens. International Journal of Green Pharmacy, 2:126-128.
- Ikewuchi CC, Ikewuchi JC (2009) Comparative Study of the Vitamin Composition of Some Common Nigerian Medicinal Plants. Pacific Journal of Science and Technology, 10:367-371.
- Ikewuchi JC (2011) Alteration of Plasma Biochemical, Haematological and Ocular Oxidative Indices of Alloxan Induced Diabetic Rats by Aqueous Extract of Tridax procumbens Linn (Asteraceae). Experimental and Clinical Science Journal, 11:291-308.
- Ikewuchi JC (2012) An Aqueous Extract of the Leaves of Tridax procumbens Linn (Asteraceae) Protected against Carbon Tetrachloride Induced Liver Injury in Wistar Rats. Pacific Journal of Science and Technology, 13:519-527.
- Ikewuchi JC, Ikewuchi CC (2009) Comparative Study of the Mineral Elements Composition of Some Common Nigerian Medicinal Plants. Pacific Journal of Science and Technology, 10:362-366.
- Ikewuchi JC, Ikewuchi CC (2013) Moderation of Haematological Indices, Plasma Electrolytes and Markers of C.C. Ikewuchi et al. 1001 Hepato-Renal Function in Sub-Chronic Salt-Loaded Rats by an Aqueous Leaf Extract of Tridax procumbens Linn (Asteraceae). Pacific Journal of Science and Technology, 14:362-369.
- Ikewuchi JC, Onyeike EN, Uwakwe AA, Ikewuchi CC (2011) Effect of Aqueous Extract of the Leaves of Tridax procumbens Linn on Blood Pressure Components and Pulse Rates of Sub Chronic Salt-Loaded Rats. Pacific Journal of Science and Technology, 12:381-389.
- Ikewuchi JC, Ikewuchi CC, Igboh NM (2009) Chemical Profile of Tridax procumbens Pakistan Journal of Nutrition, 8:548-550.
- Otitoju O, Onwurah INE (2005) Superoxide dismutase (SOD) activity and serum calcium level in Rats exposed to a locally produced insect powder “Rambo”. Animal Research Journal, 2:261-266.
- Pareek H, Sharma S, Khajja BS, Jain K, Jain GC (2008) Evaluation of Hypoglycemic and Anti-Hyperglycemic Potential of Tridax procumbens (Linn.). BMC Complementary and Alternative Medicine, 9:48.
- Petchi RR, Vijaya C, Parasuraman S (2013) Anti-Arthritic Activity of Ethanolic Extract of Tridax procumbens (Linn.) in Sprague Dawley Rats. Pharmacognosy Research, 5:113-117.
- Prabhu VV, Nalini G, Chidambaranathan N, Kisan NS (2011) Evaluation of Anti-Inflammatory and Analgesic Activity of Tridax procumbens Linn against Formalin, Acetic Acid and CFA Induced Pain Models. International Journal of Pharmacy and Pharmacological Science, 3:126-130.
- Punsiri D (2015) Examination of some heavy metal pollution in Roadside plants: Using X-Ray Spectroscopy. University of Łódź Chair of Modelling Teaching Processes. 1-172.
- Ravikumar V, Shivashangari KS, Devaki T (2005) Hepatoprotective Activity of Tridax procumbens against DGalactosamine/Lipopolysaccharide-Induced Hepatitis in Rats. Journal of Ethnopharmacology, 101:55-60.
- Salahdeen HM, Yemitan OK, Alada ARA (2004) Effect of Aqueous Leaf Extract of Tridax procumbens on Blood Pressure and Heart Rate in Rats. African Journal Biomedical Research, 7:27-29.
- Stohs SJ, Bagchi D (1994) Oxidative mechanisms in the toxicity of metal ions. Free Radical in Biological Medicine, 18:321-336.
- Yoga Latha Jr. L, Darah I, Sasidharan S, Jain K (2009) Antimicrobial Activity of Emilia sonchifolia DC., Tridax procumbens L. and Vernonia cinerea L. of Asteracea Family: Potential as Food Preservatives. Malaysian Journal of Nutrition, 15:223-231.