Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Establishment of an Efficient Paraffin Embedding Method for 3-Dimension Cultured Oral Cell Organoids
Bao-Feng Wang, MD, PhD1*; Ying-Ying Wang, BS1; Yun-Lan Yi, BS2
1Department of Otolaryngology-Head and Neck Surgery, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, P.R. China
2Department of Otolaryngology-Head and Neck Surgery, Jinzhou medical College, Jinzhou, China
Received Date: April 08, 2024; Accepted Date: April 10, 2024; Published Date: April 11, 2024;
*Corresponding author: Bao-Feng Wang, Department of Otolaryngology-Head and Neck Surgery, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, P.R. China. E-Mail: wangbaofeng2013@aliyun.com
Citation: Wang BF, Wang YY, Yi YL (2024) Establishment of an Efficient Paraffin Embedding Method for 3-Dimension Cultured Oral Cell Organoids. Annals of Otorhinolaryngology - Head and Neck: AOHNS-112.
DOI: 10.37722/AOHNS.2023201
Abstract
Objective: This study was to develop a streamlined and efficient paraffin embedding technique method specifically tailored for 3-dimensional (3D) cell culture models.
Methods: Oral epithelial cells (OECs) were utilized to culture 3D cell organoids, and subsequently, paraffin embeddings were prepared. Hematoxylin and eosin (HE) and immunofluorescence (IF) stains were carried out to assess the morphology and protein expression of the OECs organoids.
Results: Both HE and IF stains provided accurate detection of the morphological structure and protein expression within the 3D OECs organoids.
Conclusion: The implementation of this paraffin embedding technique represented a substantial advancement in the potential of 3D cell culture models, thereby cultivating broader utility across multiple domains. This progress paved the way for its adoption in diverse domains such as disease model researches, translational medical studies, and the realm of pharmaceutical innovations.
Keywords: 3D culture; organoids; paraffin embedding.
Introduction
In the realms of medical and pharmacology researches, experimental animals have played pivotal roles. Nevertheless, with the growing emphasis on animal welfare and the widespread acceptance of the reduction, replacement, refinement (3R) principles, alternative techniques to replace experimental animals are gaining recognition1. In recent years, the advancement of 3-dimensional (3D) cell model technologies, such as organ-on-a-chip, organoids and spheroids, have facilitated the shift towards alternatives to using experimental animals [1, 2].
The investigation of tissue morphology and histopathology in organoids often entails employing frozen or paraffin sections in combination with histochemical and immunofluorescence staining. While frozen sections offer the advantages of conveniences and rapid preparations, they do have some drawbacks including limited preservation times, potential detachment from slides, and the formation of ice crystals during sections, all of which can impact antigen localizations [3]. On the other hand, paraffin sections offer several benefits, including exceptional tissue structure preservations, secure adhesion to slides, accurate antigen localizations and long- term storage capabilities. However, due to the complexities of the culture systems, small sizes of organoids, creating pathological sections using traditional paraffin embedding methods can be challenging. These methods involve pre- embedding in substances like agar, agarose, or egg white, resulting in increased complexity and prolonged processing time, along with issues related to insufficient wax infiltration due to the influence of agarose concentration and melting state, difficulties in sectioning, and gelation problems during agar addition due to temperature, as well as shrinkage during dehydration and excessive dehydration time resulting from high agarose water content [4, 5]. These limitations have, to some extent, hindered the application of paraffin sectioning techniques in 3D cell model research. To address this, our study has taken into account the unique characteristics of 3D cell models and improved the classical paraffin embedding to establish an efficient paraffin sectioning method suitable for 3D cell culture models. This development provides a convenient pathological platform for exploring the morphologies, functionalities and applications of 3D cell culture models.
Methods
3D organoids culture
Oral epithelial cells (OECs) were first plated in several wells of two 6-well culture plates pre-coated with collagen (Vitrogen; Collagen Biomaterials, Palo Alto, Calif) for expansion respectively. After confluence, OECs were trypsinized with 0.25% trypsin and plated in 0.4-µm pore membrane inserts of two 12-well transwell plates (Costar, Corning, NY). OECs were grown in Oral Kertinocyte Medium (OKM, ScienCell, USA) until they reached confluence. Then the cells were cultured under air-liquid interface (ALI) conditions. Apical medium was discarded, and the basal medium was replaced with ALI medium containing OKM with
1.5 mM calcium chloride (Sigma-Aldrich, WI, USA) and 50 µg/mL vitamin C (Sigma-Aldrich) for OECs to induce cell differentiation. The ALI mediums were changed every other day for 2 weeks. After 15 days, the organoids prepared for paraffin embedding, making paraffin sections.
Paraffin embedding and addition of eosin dye
The cultured 3D cell organoids added an appropriate volume of 10% neutral formalin for fixation in the transwells. Once fixation was completed, removed the formalin. To dehydrate the organoids, performed the following sequential steps: immersed the organoids in 75% ethanol for 5 minutes, followed by 85% ethanol for 5 minutes, and then 95% ethanol for 5 minutes. Consecutively immersed the organoids in the absolute ethanol twice, with each immersion lasting for 10 min. Performed the rest consecutive steps of transparention by immersion into an appropriate amount of xylene-free wax remover alternative twice, each for 10 minutes. Due to the organoids’ white color, which makes them difficult to distinguish from the concretionary paraffin, eosin dye can be added to the paraffin during wax dip for 30 min. This labeling with red color will help to distinguish the organoids from the paraffin during further processing.
Dropped a wax about 50 μL into the bottom of metal embedding molds, then cut the membranes from the inserts by scalpels, vertical putted the membranes into waxes, then carefully filled molds with liquid paraffin. Upon completion, carefully removed the molds and allowed them to rest at room temperature for approximately 30 minutes, facilitating the solidification of the liquid paraffin enveloping the organoids.
Setting the slicing thickness to 5μm, moved the sliced paraffin ribbons in warm water and carefully flattened them. Using anti-stripping slides, gently lifted the ribbons from the water. Subsequently, subjected the sliced sections to a 62°C heating platform for 1 hour and then stored them at room temperature until the staining process.
Prior to performing stainings, the dewaxing and rehydration steps were carried out. This involved immersing the sections in xylene for a duration of 10 min in room temperature, with the process being repeated twice. Subsequently, the sections were submerged in 100% ethanol for two cycles of 10 min each, followed by sequential immersion in 95%, 85%, and 75% ethanol, each for 5 min.
Hematoxylin and eosin (HE) and immunofluorescence (IF) stainings
After dewaxing and rehydration of the sections, routine HE stainings were performed: sections were rinsed in deionized water for 5min, immersed in hematoxylin stain for 5min, rinsed in tap water for 10min, subjected to hydrochloric acid- ethanol differentiation for 3 seconds, counterstained with lithium carbonate dilute solution for 30 seconds, soaked in tap water for 10 min, stained with eosin solution for 10 min. Subsequently, the sections were dehydrated as up.
For IF staining, the sections were immersed in phosphate- buffered saline (PBS) with a pH of 7.2-7.4 and subsequently washed. The Tris/ethylene diamine tetraacetic acid antigen retrieval solution (EDTA, Boster, Wuhan, China) was heated to 100°C using a microwave. The sections were immersed in the boiling Tris/EDTA retrieval solution and subjected to medium power heating for 7 min, with this process being repeated 4 times. After heating, the containers were taken out and allowed to cool at room temperature for approximately 45-60 minutes. Following that, the sections were incubated in 1×PBS for 5 min, followed by three rounds of PBS washing. Subsequently, the sections were incubated with 5% bovine serum albumin (BSA) for 30 min at room temperature.
The primary antibody, specifically MMP-1 (Abcam, Cambridge, UK) and diluted in 1×PBS containing 1% BSA, was applied by adding 25-30 μL of the primary antibody solution to the sections. The samples were then placed in a humidified chamber and incubated overnight at 4°C. After the primary antibody was removed, the samples underwent three washes with PBS. Subsequently, 25-30 μL of goat-anti-rabbit secondary antibody solution, diluted in 1×PBS, was applied to the sections. The samples were incubated at room temperature in darkness for 1 hour within a humidified chamber.
Following this, 10-20 μL of a mounting medium containing 4, 6-diamidino-2- phenylindole (DAPI, Boster) was added to the samples, and the samples were incubated for 10 minutes. Excess mounting medium was removed through PBS washing. The resultant images were captured using a fluorescence microscope.
Results
HE staining of the OECs organoid
The OECs organoid prepared using this method exhibit intact structures with clear cell boundaries. HE staining reveals distinct chromatin and nuclei, with well-preserved cell morphology (figure 1).

Figure 1: HE staining of OECs organoids with clear visualization of both the cytoplasm and the nuclei.
IF staining of the OECs organoid
The immunofluorescently labeled MMP-1 primarily expresses in the cytoplasm, exhibiting green fluorescence, while the cell nuclei are stained blue (figure 2).
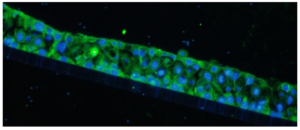
Figure 2: IF staining of OECs organoids with clear visualization of both MMP-1 expression in cytoplasm with green fluorescence and DAPI in nuclei with blue fluorescence.
Discussion
The method introduced in this study is straightforward and eliminates the need for pre-embedding with agarose or egg albumin, and involves optimized steps for fixation, dehydration, clearing, and paraffin embedding tailored to the sample characteristics. The method offers strong operability and practicality, producing intact slices with clear cell boundaries. The resulting sections are suitable for HE and IF staining, all yielding excellent results.
Compared to common tissue samples in pathological sections, 3D cell organ-like structures are tiny, nearly colorless, and composed of relatively simple cell components.
These characteristics make sample collection, retention, and preservation challenging, thereby increasing the difficulty of embedding and sectioning. In this study, a water- soluble eosin stain was used for sample marking before dehydration, overcoming these challenges. The stained cell organoids appeared red and were clearly visible in the paraffin blocks, facilitating subsequent sectioning and slide preparation. Moreover, this staining could be removed during the rehydration process, without affecting the efficacy of the staining methods on the sections.
In this study, we have developed an innovative method for paraffin embedding, opening new avenues for its application across various fields including disease modeling research, translational medical studies, and pharmaceutical innovation.
Reference
- Hubrecht RC, Carter E. The 3Rs and Humane Experimental Technique: Implemen ting Change. Animals (Basel), 2019; 9(10):754.
- Grimm H, Biller-Andorno N, Buch T, Dahlhoff M, Davies G, Cederroth CR, et al. Advancing the 3Rs: innovation, implementation, ethics and society. Front Vet Sci, 2023;10:1185706.
- Girolami I, Neri S, Eccher A, Brunelli M, Hanna M, Pantanowitz L, et al. Frozen s ection telepathology service: Efficiency and benefits of an e-health policy in South Tyrol. Digit Health. 2022; 8:20552076221116776.
- Jiang F, Xu XW, Chen FQ, Weng HF, Chen J, Ru Y, et al. Extraction, Modificatio n and Biomedical Application of Agarose Hydrogels: A Review. Mar Drugs. 2023;21(5):299.
- Pham HM, Zhang Y, Munguia-Lopez JG, Tran SD. Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing. Biomimetics (Basel). 2021;7(1):5.