Publication Information
ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Enzyme Supplementation in the Gastrointestinal Tract: Glutenase vs. Lactase
István G Télessy*
Department of Pharmaceutics, University of Pécs, Pécs, 7624, Honvéd u. 3. and MedBioFit Lpt. Gödöllő, 2100, Fácán sor 25. Hungary
*Corresponding Author: István G Télessy, Department of Pharmaceutics, University of Pécs, Pécs, 7624, Honvéd u. 3. And MedBioFit Lpt. Gödöllő, 2100, Fácán sor 25. Hungary. Tel: +36304918192; E-mail: telessyist@vnet.hu
Received Date: October 08, 2019; Accepted Date: October 17, 2019; Published Date: October 25, 2019
Citation: Télessy IG (2019) Enzyme Supplementation in the Gastrointestinal Tract: Glutenase Vs Lactase. Adv Nutri and Food Sci: ANAFS-151.
Abstract
Recently, enzyme supplementation in frequently occurring digestive diseases, like lactose and gluten intolerance became more and more popular. Unfortunately, some myths have born in connection with these treatment options. Etiology of the diseases and the background and mechanism of action of these therapeutic enzymes are addressed in the review.
Keywords: Digestive; Enzyme; Gluten; Glutenase; Lactase
Introduction
Oral supplementation/replacement of enzymes is a well-known therapeutic modality for ages. This means -particularly for the lay population based on traditional examples-decisively digestive treatment products, mostly used after high fat meal supplementation of pancreas lipase or complex exocrine pancreatic enzymes [1]. Similarly in case of high protein load the support of gastric enzymes pepsin (and trypsin) have been required since the 19th century. But professionals are aware of these biologics, and it is known that today many other enzymes are used as well in a wide spectrum of indications. In daily practice pharmacists regularly meet people who do not make difference among enzyme therapies. Here we highlight the main differences of the most frequently used enzymes in enzyme deficiencies focusing on lactase and recently introduced glutenase therapies.
Gluten
Gluten is a complex protein mixture in wheat, barley, rye and oats [2]. It is composed of two peptide components called glutenins and gliadins (Figure 1). In bread-wheat and flour soluble gluten and insoluble gliadins are in a mixture stabilized by hydrogen bonds and disulfide bridges. Glutenins are in size of 10-900T daltons, polymers built up from 19 protein-subunits of high molecular weight (HMW) and light molecular weight (LMW). Gliadins are 30-80T daltons, chain molecules that form globules. The latters are divided to alpha-, beta-, gamma- and omega-gliadins. Protein components similar to gliadins are named hordein in barley, secaline in rye, avenin in oats, zein in corn, etc. Gliadins represent high polymorphism, this explain the different electrophoretic movement even if the molecular weight is similar. The gluten formed from 1:1 ratio of the two components are flexible. This is due to the high (35%) glutamine, high (15%) proline and high (35%) hydrophobic amino acid content of gliadins. All these parameters make gluten resistant to gastric juice. Above mentioned data results in high variability of gluten-composition of different grains and diverse speed and size of intestinal decomposition.
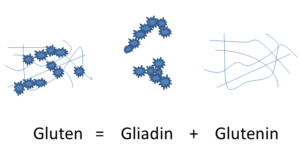
Figure 1: The Composition of Gluten.
Gluten Sensitivity
Gluten, as many other proteins, can trigger allergic and non-allergic reactions in susceptible individuals. To date three different gluten-sensitivity reactions are differentiated out of which celiac disease (CD) is the most serious one. The remaining two, the wheat allergy and the non-celiac gluten sensitivity (NCGS) are less discovered from mechanism of sensitivity point of view [3]. Gluten sensitivity, in general, has reached 5% (range 0, 5 – 8%), highest prevalence can be found in countries consuming wheat bread, i.e. Europe and North-America, however the incidence of the gluten-sensitivity increases all over the world. Accordingly, market-share of gluten-free foodstuff steadily increases over the last two decades.
Celiac disease affects ca. 1% of the population worldwide [4]. This is a systemic, life-long lasting autoimmune disease. Basically, gluten-containing food causes T-cell mediated immune-response in genetically susceptible persons. The main feature of the reaction is the gradually propagating inflammatory response of the gut (duodenum and proximal jejunum) with dominant villous atrophy and hypoplasy of crypts that are main diagnostic signs of CD [5]. The celiac disease is actually a gastrointestinal reaction to enteral gluten exposure
But the complex response is not exclusively due to gluten: genetic predisposition, diminished gut barrier function, increased inflammatory and inappropriate immune response and the imbalance of microbiota plays also a decisive role in development of the immunotoxicity. Great majority (>95%) of CD patients is HLA-DQ2 or HLA-DQ8 positive thus the genetic factor is obvious but participation of more than 50 non-HLA gens is also presumptive [6]. Clinical signs and symptoms are very heterogeneous. Earlier diarrhea dominated (>90%) but today dermatitis herpetica and other atypical symptoms (anemia, weight loss, bone symptoms and the malabsorption syndrome with micronutrient-deficits) are more pronounced. Increased incidence of secunder diseases (IBS, T2DM) are to be seen in the celiac population. Diagnosis: usually long lasting, to conventional therapy not responding primer symptoms arise suspicion of the disease even if the symptoms are not specific at all. Proof can be find in circulating antibodies (anti-tissue transglutaminase, anti-deaminated gliadine antibodies, IgA endomysial antibodies and most recently the anti-neo-epitope tTG) [7], in case of positivity of any of the serologic tests verification of villous atrophy is required out of biopsy samples from duodenum. Most appropriate classical serologic tests are summarized in (Table 1).
| Serological marker | Sensitivity (%) | Specificity (%) |
| Anti-tTG IgA | 96.8 | 91.0 |
| EMA-IgA | 93.7 | 100 |
| DPG IgA | 84.4 | 98.5 |
In verified celiac disease strict gluten-free diet is prescribed as effective treatment. In this case symptoms gradually disappear. Should these patient get gluten load again usually unintentionally, symptoms return, in certain cases they persist. According to the observations of Silvester et al. abdominal discomfort (80%), diarrhea (52%), fatigue (33%) and headache (30%) are the primer complains in such situation [8].
The second allergic disease within the gluten-induced diseases is wheat allergy. It is in the 6 top food allergens (milk, egg, soy, wheat, peanut and tree nut) and its prevalence is estimated under 1%, but in children this figure maybe higher [9]. This is a B-cell specific, IgE mediated inflammatory reaction that appears after exposition with components of wheat and wheat-flour. This reaction is attributable to gliadines, HMW-glutenins and sometimes also to non-gluten flour-proteins like profilin, serpin, alpha-purotionin, etc. and depending on the trigger agent causes local (eg. atopic dermatitis, urticaria, angioedema) or systemic symptoms (baker’s asthma and rhinitis, abdominal pain, nausea) are precipitated [10]. Rarely occurring but severe manifestation is the wheat-dependent exercise-induced anaphylaxis (WDEIA). Symptoms usually occur 1-6 hours after ingestion of allergen. Local wheat-protein allergy (contact urticaria) can develop after use of cosmetics containing hydrolyzed wheat-protein components as well.
Third group of gluten-related disorders is the non-celiac gluten sensitivity (NCGS). Several intestinal and extra-intestinal complains may referred to NCGS. Diagnosis can be set after exclusion of CD and wheat allergy. This type of gluten sensitivity has been proposed ca. a decade ago and while the criteria for categorization, the pathomechanism and the differential diagnostic signs are still under research we can say that this is really different from typical autoimmune and allergic forms. Some overlapping has been however, found as according to an Italian study in 14% of patients had a history of autoimmune disease [11]. Moreover in a quarter of the patients IgG type anti-gliadin antibodies were detected and in 22% had IgE-type allergy. This means that certain immune reaction in these patients exist, too. Genetic disposition was not revealed. In contrast to wheat allergy, this type of gluten-intolerance is more frequent among adults than in children. To date most researchers agree that this type of disease is hard to differentiate from functional gastrointestinal disorders and from IBS due to the similar symptoms, like abdominal pain, bloating, bowel discomfort [12-14].
Lactose
Lactose (milk sugar) the main carbohydrate component of mammalian milk, cow’s milk contains ca. 5%, human’s milk (breast milk) contains ca. 7% lactose. Fermented milk products (yoghurt, kefir) contains lactose, too but just ca. half of the natural milk and cheeses contain even less, especially aged cheeses like Parmigiano or Grano Padano or Romano. Lactose-free „cheese” is, however not real cheese because they are not made of milk, except „yoghurt cheese” that is first fermented with yoghurt cultures and afterward aged.
Lactose is a disaccharide composed of two monosaccharaides: glucose and galactose and lactase enzyme is needed to cleave lactose to the components (Figure 2). The disaccharide is not absorbable in the intestine but its components are absorbed from the small intestine. Lactase enzymes locate in the brush border of the small intestine and it is responsible for the digestion of milk sugar after consumption of lactose-containing foodstuff. Undecomposed disaccharide will be digested in the colon where gut microbiota cleave it to short-chain fatty acids and gas.
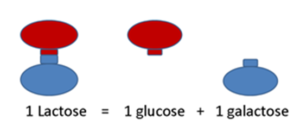
Figure 2: Composition of lactose.
Lactose Allergy, Malabsorption and Intolerance
One of the most frequent occurring food-related disorder is the lactose-intolerance (LI). Lactose intolerance encompasses a set of complex symptoms. In contrast to lay persons’ belief lactose (milk) allergy set out just 5-10% of the intolerance cases and typically presents with symptoms like urticaria, itching, swelling in the mouth/lips, etc. Lactose allergy is an immune-reaction and is in no connection to other, non-allergic diseases. Lactose malabsorption syndrome is often mentioned as synonym of lactose intolerance although it can also be separated from LI, viz. malabsorption means that lactase deficit makes failure in digestion and absorption of lactose moreover makes absorption deficits for other nutrients (mainly micronutrients) but physical symptoms are not present [15]. Most popular diagnostic test for lactose malabsorption is the H2 breath test. This is a non-invasive test performed within 3-6 hours, but there are a lot more tests as well. A comparative summary of lactose-malabsorption tests recently has been published by Deng and coworkers [16].
Lactose intolerance is malabsorption with physical symptoms. It’s most common cause is the genetically determined loss of intestinal lactase production called lactase non-persistence, however the dose of lactose, the microbiome, gastrointestinal motility and the sensitivity of GI tract to the lactose fermentation products, incl. the gas are also mentioned. Lactase deficiency results in high amount of non-digested disaccharides and they cause lactase-intolerance symptoms. Lactose intolerance is the most frequently occurring food-intolerance. Non-digested disaccharides cause the small bowel osmotic diarrhea, the bacterially digestion leads to secretory diarrhea. Both types associate with further local alterations like abdominal pain, bloating, etc. But lactose malabsorption precipitate systemic abnormalities as well including chronic fatigue, rheumatologic complains, etc. Inborn lactase deficit is very rare but relative lactase deficit is present in 5-65% of population. Lowest incidence is in North-Europe and highest in West-Africa, in Arabic countries and within Europe Greece and Italy are the leading countries. Relative lactase deficiency can be traced back to low level of lactase production or to low level of enzyme activity. In the background of deficit in lactase production usually wrong expression of LCT gene is present that is due to the change of nucleobases thymine/cytosine in the regulator locus 2q21. This failure to date can be detected but not cured. In case of diminished activity, in general, accelerated non-persistence is hidden because lactase activity reaches the maximum by the birth and gradually declines with aging. The speed and extent of this process is individually different. Beside the above mentioned there are several secondary lactose intolerances, e.g. all disorders with atrophy of gut mucosa. It is noteworthy that primer lactose intolerance does not destroy gut structure and the symptoms can be reversed by administration of exogenic lactase in a dose-dependent manner.
Enzyme Therapy
For the time being enzyme therapy became a standard constituent of the healthcare services. Two main types are the enzyme substitution/replacement and the facilitation of enzyme production. The former one is the more experienced as substitution of digestive enzymes (digestive enzymes of the stomach, pancreas enzymes, papain, lactase, etc.) has a long history. This type of enzymes are administered orally however there are many others, too. Parenterally the thrombolytic enzymes (streptokinase, urokinase) and locally desquamating/wound cleansing enzymes (trypsine, chimotrxpsine) were the pioneers but today eg. glucerases support patients with metabolite-storage disease (Gaucher-disease) [17].
Enzyme Therapy for Lactose Intolerance
After lactose intolerance/malabsorption has been diagnosed (non-invasive, reliable hydrogen exhalation test after 50 g lactose test-dose) regular enzyme supplementation is required. The deficient lactase production successfully can be treated by exogenous lactase (=beta-galactosidase). The therapeutic lactase supplementation goes back to more than 35 years and at beginning several microbially produced lactases were used to find out the optimal ones. The yeast Kluyveromyces lactis and fungi Aspergillus niger and Aspergillus oryzae were the initial organisms to produce beta-galactosidase for human therapeutic purpose [18, 19, 20]. Some 7 years later the dose-dependent effect and effectiveness of beta-galactosidase supplementation has been proved in humans as well [21]. Since that exogenous administration of lactase enzyme to patients having lactose-digestive problems is commercially available. In case of symptoms of lactose-intolerance (abdominal pain, distension, bloating, etc.) after eating lactose-containing food, one can successfully take this kind of medicine or food supplement just prior or together with the meal.
Treatment Options for Gluten Sensitivity
Contrary to lactose gluten dominantly generate an allergic reaction in susceptible individuals. Gluten contain indigestible protein fractions that in the GI-tract permanently preserve the immunogenicity. In the course of long-term exposition, especially if further environmental factors were present as well, allergens can break natural resistance and induce immunological reactions.
Gliadin is definitively able to dilate tight junctions in the epithel. This associate with zonuline release, which further increase the paracellular permeability. Similar action can be seen after binding of gliadin to chemokin-3. The rise in permeability favors migration of gliadin through the gutwall-barrier and after deamination aggressively initiate inflammatory immune-reaction in the gut wall and in lesser extent systemically in other organs [22]. Translocated gliadin results in the typical celiac destructions of the intestinal villi because the gliadin accumulated in the gutwall (endomysium of lamina propria) is deaminated via tissue transglutaminase 2 (tTG2). The deaminated product of the reaction is very resistant to proteolytic enzymes and gliadin plus tTG2 complex act as antigen in the sub epithelial region (Figure 3). The autoimmune reaction starts here. T-cell mediated adaptive and humoral immune response results in B-cell activation and subsequent specific B-cell complex formation. Typical local celiac mucosa damage is made by the immune marker, inflammatory cytokine and matrix metalloprotein production. (Maybe the homology of epitopes in different tTG is responsible for the development of autoimmune diseases like gluten ataxia or dermatitis herpetiformis).
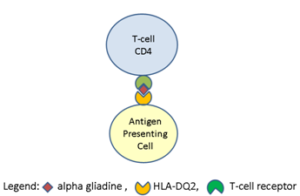
Figure 3: Composition of the antigen unit.
In the case at issue exclusion of the gluten-containing food and nutrients is the only way to ensure the proper therapy however this assume a reliable behavior on the part of patients, needing high level of self-control and the economic burden is also quite high, additionally quality of gluten-free food usually fail to reach the one of traditional food [23]. Besides, under the best notions, some glutens will come into the food víz. Due to the cross-contamination of foods and packaging processes it may happen. According to an Italian measurement moderate pollution occurs in 4, 5% and serious pollution occurs in 9% in such products [24]. Moreover, labeling may sometimes also be incorrect and adherence to the gluten-free diet is often not 100%. A recent research demonstrated that just 6, 3-7% of patients could successfully keep the gluten-free diet [25, 26]. During the average eating habits people ingest 15-20 g gluten but according to the above mentioned findings patients on gluten-free diet usually are unintentionally loaded with 1-3 g gluten per day. This low dose gluten is responsible for the low grade inflammation persists in many CD patients. Alternative solutions to avoid gluten expositions are the attempts to spoil the protein allergen motive. The most obvious techniques would be the quick decomposition of peptides. As the endogenous enzymes are able to digest gluten-peptides in a small portion only, maybe additional exogenous enzymes are required. Research in recent two decades resulted in finding (in plants), in fortification (selection of highly active glutenases/ glutaminase produced by bacteria from human microbiome) or in production (let produce glutenases by non-human origin bacteria or fungi) of peptidases suitable to decompose glutens in human GI tract.
Plant-origin endoprotease were isolated from barley (Hordeum vulgare; cistine- endoprotease B2) [27] and from the viscoelastic digestive fluid of the Nepenthes genus [28]. A research group detected 7 bacteria from human-origin (from mouth-mucosa) that can digest gluten and gluten-peptids [29]. Out of these bacteria Rothia mucilaginosa is under further investigations [30]. To date several, endoprotease producing non-human bacteria, like Actinoallomurus A8, Streptomyces lividans TK24, Myxococcus xanthus, Sphyngomonas capsulata, Flavobacterium meningosepticum and Pyrococcus furiosus are under investigation [31, 32]. Out of fungi Aspergillus niger has been substantially investigated and its prolyl endoprotease (AN-PEP) belonging to the serine peptidase family S28 has been successfully developed to be remedy to treat celiac disease [33]. Recently a randomized clinical study also underpinned the results [34]. Today AN-PEP in various brand name is on the market in several countries as nutrition supplement.
Safety Warning
Treatment of the gluten-intolerance is a challenge. It is clear that the best chance to avoid gluten-intolerance is to keep full gluten-free nourishment. As described above, even under these conditions 100% protection permanently does not exist. Moreover, in the scientific literature one can read about non-responsive celiac disease (NRCD) and refracter celiac disease (RCD). Incidence of the first one is between 7 – 30% and the incidence of the latter one is ca. 10% [35].
The second hazard is that many users think glutenase therapy ensures a total protection. Unfortunately not! The human studies proved that gluten levels in the duodenum and small intestine were jut reduced but not abolished even in higher doses of enzymes taken [34]. This confirms that patients are not on the safe side if they take glutenase products only. Moreover the commercially available glutenase-containing products are very different from quality and reliability point of view [36]. It means patients should know the limitations of the various treatment options and must be informed by the pharmacists accordingly.
Conclusions
Lactose intolerance is mainly of non-allergic origin. Gluten sensitivity mainly results of allergic reaction. Both disease should be primary treated with exclusion of the allergen, i.e. the lactose and the gluten. Additional treatment can be oral administration of exogenous enzymes like lactase and glutamase supplementation, however they are not able to prevent or treat lactose- or gluten-intolerance symptoms alone.
Competing Interests: The author has no competing interests.
Reference
- Ianiro G, Pecere S, Giorgio V, Gasbarrinni A, Cammarota G (2016) Digestive enzyme supplementation in gastrointestinal diseases. Curr Drug Metabol 17: 187-193.
- Shewry PR, Halford NG, Belton PS, Tatham AS (2002) The structure and properties of gluten: an elastic protein from wheat grain. Phil Trans R Soc Lond B 357: 133-143.
- Elli L, Branchi F, Tomba C, Villalta D, Norsa L, et al. (2015) Diagnosis of gluten related disorders: celiac disease, wheat allergy and non-celiac gluten sensitivity. World J Gastroenterol. 21: 7110-7119.
- Ludvigsson JF, Murray JA (2019) Epidemiology of celiac disease. Gastroenterol Clin North Amer 48: 1-18.
- Lebwohl B, Sanders DS, Green PHR (2019) Coeliac disease. Lancet 391 (10115): 70-81.
- Van Heel DA, Franke L, Hunt KA, Gwilliam R, Zhernakova R, et al. (2007) A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat. Genet 39: 827-829.
- Lerner A, Ramesh A, Matthias T (2019) Serologic diagnosis of celiac disease: new biomarkers. Gastroenterol Clin North Am 48: 307-317.
- Silvester JA, Graff LA, Rigaux L, Walker JR, Duerksen DR (2016) Symptomatic suspected gluten exposure is common among patients with celiac disease on a gluten-free diet. Aliment Pharmacol Ther 44: 612-619.
- Ricci G, Andreozzi L, Cipriani F, Giannetti A, Gallucci M, et al. (2019) Wheat allergy in children: a comprehensive update. Medicina (Kaunas) 55: pii: E400
- Balakireva AV, Zamyatnin AA (2016) Properties of gluten intolerance: gluten structure, evolution, pathogenicity and detoxification capabilities. Nutrients 8: 644- 670.
- Volta U, Bardella MT, Calabro A, Troncone R, Corazza GR, et al. (2014) An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. MBC Med 12: 85-92.
- Catassi C, Alaedini A, Bojarski C, Bonaz B, Bouma B, et al. (2017) The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): an update. Nutrients 9: 1268-1283.
- Barbaro MR, Cremon C, Stanghellini V, Barbara G (2018) Recent advances in understanding non-celiac gluten sensitivity. F1000Res 7: 1631.
- Kabbani TA, Vanga RR, Leffler DA, Villafuerte-Galvez J, Pallav K, et al. (2014) Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol. 109: 741-746.
- Misselwitz B, Butter M, Verbeke C, Fox MR (2019) Update of lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut 68: 2080-2091.
- Deng Y, Misselwitz B, Dai N, Fox M (2015) Lactose intolerance in adults: Biological mechanisms and dietary management. Nutrients 7: 8020-8035.
- Brady RO (2003) Enzyme replacement therapy: conception, chaos and culmination. Phil Trans R Soc Lond 358: 915-919.
- Rosado JL, Solomons NW, Lisker R, Bourges H (1984) Enzyme replacemenrt therapy for primary adults lactase deficiency. Effective reduction of lactose malabsorption and milk intolerance by direct addition of beta-galactosidase to milk at mealtime. Gastroenterology 87: 1072-1082.
- Solomon NW, Gurrero AM, Torun B (1985) Dietary manipulation of postprandial colonic lactose fermentation: II. adition of exogenous, microbial beta-galactosidases at mealtime. Am J Clin Nutr 4: 209-2021?
- Moskovitz M, Curtis C, Gavaler J (1987) Does oral enzyme replacement therapy reverses intestinal lactose malabsorption. Am J Gastroenterol 82: 632-635
- Corazza GR, Benati G, Sorge M, Strocchi A, Calza G, et al. (1992) beta-galactosidase from Aspergillus nigeri n adult lactose malabsorption: a double-blind crossover study. Aliment Pharmacol 6: 61-66.
- Caio G, Volta U, Sapone A, Leffler DA, Di Gorgio R, et al. (2019) Celiac disease: a comprehensive current review. BMC Med 17: 142-161.
- El Khoury D, Balfour-Ducharme S, Joye IJ (2018) A review of the gluten-free diet: technological and nuritional challanges. Nutrients 10: pii: E1410.
- Verma AK, Gatti S, Galeazzi T, Monachesi L, Patella L. et al. (2017) Gluten contamination in nuturally or labelled gluten-free products marketed in Italy. Nutrients 9: 115-124.
- Cabrera-Chávez F, Dezar GV, Islas-Zamorano AP, Espinoza-Alderete JG, Vergara-Jiménez MJ, et al. (2017) Prevalence of self-reported gluten sensitivity and adherence to gluten-free diet in Argentinian adult population. Nutrients 9: 81-91.
- Ontiveros N, Rodriguez-Bellagarriguie CI, Galicia-Rodriguez G, Vergara-Jiménez MJ, Zepeda-Gómez EM, et al. (2018) Prevalence of self-reported gluten-related disorders and adherence to gluten-free diet in Salvadorian adult population. Int J Environm Res Publ Health 15: 786-796.
- Gass J, Vora H, Bethune MT, Grey GM,Khosla C (2006) Effect of barley endoprotease EP B2on gluten digestion int he intact rat. J Pharmacol Exp Ther 318: 1178-1186.
- Rey M, Yang M, Lee L, Zhang Y, Sheff JG, et al. (2016) Addressing proeolytic efficacy in enzymatic degradation therapy for celiac disease. Sci Rep 6: 30980-31004.
- Wei G, Tian N, Valery AC, Zhong Y, Schuppan D, Helmerhorst EJ (2015) Identification of pseudolysin (lasB) as an aciduric gluten-degrading enzyme with high temperature potential for celiac disease. Am J Gastroenterol 110: 899-908
- Wei G, Tan N, Siezen R, Schuppan D, Helmerhorst EJ (2016) Identification of food-grade subtilisins as gluten-degrading enzymes to treat ceiac disease. Am J Physiol Gastrointest Liver Physiol 311: G571-G580.
- Osorio CE, Wen N, Mejias JH, Liu B, Reinbothe S, et al. (2019) Development of wheat genotypes expressing a glutamine-specific endoprotease from barley and a propyl endoprotease from Flavobacterium meningosepticum or Pyrococcus furiosus as a potential remedy to celiac disease. Funct Integr Genomics 19: 123-136.
- Cavaletti L, Taravella A, Carrano L, Carenzi G, Suguarta A, et al. (2019) E40, a novel microbial protease efficiently detoxifying gluten proteins, for the dietary management of gluten intolerance. Sci Reports 9: 13147
- Stepniak D, Spaenij-Dekking L, Mieta C, Moester M, de Ru A, et al. (2006) High efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol 291: G621-G629.
- König J, Holster S, Bruins MJ, Brummer RK (2017) Randomized clinical trial: effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci Report7: 13100.
- Kelly CP, Bai JC, Liu E, Leffler DA (2015) Advances in diagnosis and management of celliac disease. Gastroenterology 148: 1175-1186.
- Krishnareddy S, Stier K, Recanati M, Lebwohl B, Green PHR (2017) Commercially available glutenases: a potential hazard in celiac disease. Therap Adv Gastroenterol 10: 473-481.