Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Enriched Science and Technology Communication Economy in Agriculture by Use of Acacia sides as Potential Bio-Agents against Various Pathogens
Subhas Chandra Datta*
Researcher & Headmaster, Eco-Club Research Unit, Kanchannagar D.N.Das High School, West Bengal, India
Received Date: April 13, 2020; Accepted Date: April 19, 2020; Published Date: April 28, 2020
*Corresponding author: Subhas Chandra Datta, Researcher & Headmaster, Eco-Club Research Unit, Kanchannagar D.N.Das High School, Kanchannagar, Purba Burdwan-713102, West Bengal, India. Email: dattasubhas@rediffmail.com; subhaschandra.datta@gmail.com
Citation: Datta SC (2020) Enriched Science and Technology Communication Economy in Agriculture by Use of Acacia sides as Potential Bio-Agents against Various Pathogens. Adv Agri Harti and Ento: AAHE-117.
Abstract
Plant diseases, caused by different pathogens like nematodes, fungus, virus, bacteria and insects etc., infest almost all kinds of cash and vegetables crops affecting the economic value and agri-market of the world. Mulberry is economical plants because silk production depends on the nutritive quality of the leaves which is hampered by different pathogens attack. Pesticides are the most effective means of control, but they are expensive and as well as environmentally unfriendly also. To move forward, it will require new and more efficient solutions, technologies and products. Present investigation have revealed that Acacia sides (A&B) are highly effective at a dose of 1 mg / mulberry plant (Morus alba L., cv. S1) in ameliorating diseases caused by plant pathogens; Meloidogyne incognita root-knot nematodes causing root-knot disease, Cercosporammoricola fungus causing leaf spot disease, Phyllactiniacorylea fungus causing powdery mildew disease, mosaic virus causing mosaic disease and Maconellicoccushirsutus mealy bug causing tukra disease. Acacia sides were highly effective in ameliorating mulberry diseases and were increased the protein content of mulberry leaves. Silkworms larvae feeding on the leaves of treated plants showed improved growth of silkworms, increased cocoon weight and shell weight, fewer feeding to cocoon formation, zero mortality rate and increased the effective rate of rearing, sex ratio percentage and egg laying capacity of mother moth. The results, showed that Acaciasides (A&B) use as “Potential Eco-friendly Bio-agents Against Various Pathogens of Plants Enriching Science and Technology Communication Economy in Agriculture Significantly without Disturbing Biosphere”. It also conserve our biodiversity which will contribute towards “Sustainable Climate, Health and Development” by controlling plant diseases which is sometime devastating to all kinds of -natural and -artificial vegetation and may also provide a unique platform for showcasing the research across the globe and progress the further advanced research in ‘Agriculture, Horticulture and Entomology’ deals with economy. In near future, a clinical study may be arranged for discovery of ‘Vaccine’ by using Saponins from Boi-Agent Acaciasides which may kill the novel Corona virus COVID-19 by boosting our immune system.
Keywords: Acacia sides; Agriculture; Bio-agents; Pathogens; Mulberry; Silkworms; Science and Technology Communication Economy; Vaccine
Introduction
Mulberry (Morus alba L.) is an important economical crop plants in sericulture and it grows under a wide range of ecological condition. It holds a special place as a major foreign exchange earner for many tropical and temperate countries. India secures the second position for the production of raw silk in the world, which is short about 30 % to fulfill the home requirements [1-10]. The reasons for this deficiency as well as low quality of raw silk are, however, generally attributed to build up of the diseases of mulberry and silkworms, inadequate employment of improved culture and rearing practices [1, 9-19]. Right from sprouting and throughout growing seasons, it is largely affected by a number of pathogens like plant parasitic nematodes, fungus, bacteria, virus and insects causing various diseases forming disease complex and break the host resistance [1-21]. These pathogens are the main obstacles causing considerable loss in yield and nutritive value of mulberry foliage. Feeding of the diseased leaves affect the health of the silkworms adversely and the cocoon yield in terms of quality and quantity. The lack of regular and systematic studies on the occurrence of various diseases and epidemic is responsible for the recurring loss in leaf yield [1-21].
Characteristic Features of the Diseases
Root-Knot Disease
Root-knot nematode, Meloidogyne incognita (Kofoid& White) Chitwood, infesting mulberry forming root galls has been reported from all countries and it is serious due to perennial nature of mulberry and endo-parasitic habit of the nematode. The disease reduces 10-12% leaf yield in addition to affecting the leaf quality for silkworms feeding [21-26].
Leaf spots disease
Cercosporammoricola (Cooke) fungus, causing main leaf spot disease, is the most devastating throughout the country and it causes 10-35 % loss in leaf yield reducing moisture, proteins adversely and ultimately the quality and quantity of cocoons. It produces minor circular light brown spots on the leaves which gradually increase in size and turn dark brown [1-11].
Powdery Mildew Disease
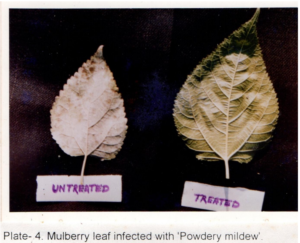
Phyllactiniacorylea (Pers.) Karst fungus, causing powdery mildew disease, is the most common and wide spread economically important disease reducing 10-30 % leaf yield. Biochemically these leaves are poor nutritive value showing reduction in moisture, protein and sugar. Feeding of diseased leaves affect the growth and development of silkworms. Symptoms of disease can easily identified by the appearance of white patches on the lower surface of the leaves. As the disease advances the patches spread to entire leaf surface and turn to blackish colour and become coarser and leathery, reducing the crude protein content by as much as 33 % [1-26].
Mosaic Disease
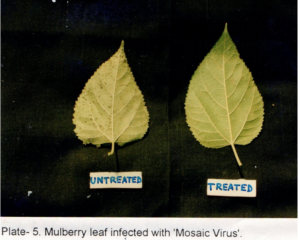
The symptoms of mosaic disease, caused by mosaic virus, are inward curling of leaves, particularly leaf margin and tip with chlorotic lesions on the leaf surface, stunted growth and suppressed leaf size [1-26].
Tukra Disease
Maconellicoccushirsutus (Green) (Pseudococcidae) is commonly known as mealy bug and is associated with mulberry plants showing symptoms popularly known as ‘Tukra disease’. The leaf yield is tremendously reduced the leaves have depleted in nutritive value. The affected apical shoot shows retarded growth. Flattening of the apical shoot and wrinkling of the affected leaves are also associated. The leaves become dark green colour. The disease reduces plant growth, leaf yield and leaf protein content significantly [1-26]. Traditional chemical control using chemical compounds available for few decades is in declining status internationally [1-27]. Thus, there remains a need for developing effective biocompounds, which would be cheap, non-phytotoxic and non-pollutant. Plants offer safe and effective substitutes for chemical control against pathogens [10-16, 27-29]. It has been already observed that the extract from the funicles of Acacia auriculiformis A. Cunn. (Plate 1) is effective in reducing mulberry diseases leaving no residual toxicity in the leaves to affect the growing silkworm larvae [10-19, 27-29]. It has also been isolated the pure compounds acaciasides (A&B) from the crude extract of the funicles of Acacia auriculiformis A. Cunn. [9-12, 24-31].

The purpose of the present investigation is to further confirm the efficacy of the acaciasides at very low dose, use as bio-agents isolated from the crude extract of acacia funicles, in ameliorating root-knot disease of mulberry (Morus alba L., cv. S1) caused by Meloidogyne incognita (Kofoid& White) Chitwood root-knot nematodes pathogens and also to find out if the pure compounds acacia sides (A&B) can reduce the four foliar diseases, caused by pathogens, under field condition. The foliar diseases were: leaf spot disease caused by Cercosporam moricola (Cooke) fungus pathogens, powdery mildew disease caused by Phyllactiniacorylea (Pers.) Karst fungus pathogens, mosaic disease caused by mosaic virus pathogens and tukra disease caused by Maconellicoccushirsutus (Green) mealy bug pathogens. The effects of the leaves of the acaciasides- treated plants on the leaf consumption, growth of silkworm’s larvae, silk gland weight and effective rate of rearing (ERR) were also observed.
Bio-agents (any biological agents of biological origin derived from either plants or animals source) provide a new class of biological compounds which stand as a suitable and useful alternative to conventional but hazardous methods of chemical control [11]. In course of our experiments with anti-nematode agents, acaciasides (A&B), it was observed that the mulberry plants (Morus alba L., cv. S1) besides being infected with root-knot nematodes, were also naturally infected with above mentioned four foliar diseases (leaf spot, powdery mildew, mosaic viral and tukra disease). Thus both the root-knot and foliar diseases, caused by various plant pathogens, were taken in to consideration during the evaluation of the effects of acaciasides (Figure 1). The result would be more realistic in terms of the potentiality of the Acaciasides (A&B), use as “Eco-friendly Bio-Agents against Various Pathogens of Plants Enriching Science and Technology Communication Economy in Agriculture” is a question as a Phytologist or a general Plant Biologist or Entomologist or Economist.

Material and Methods
Site of the Experimental Plots
The field experiment was carried out at the Sriniketan Sericultural Composite Unit, Government of West Bengal, India where temperature was 28 + 5°C and relative humidity was 75 + 5 % [11-12].
Estimation of the Nematode Pathogen Population
Soil and root samples [11-12, 32-33] were taken at random from a sericulture field spreading over an area of 5.6 acre of land with a view to determining the extent and intensity of Meloidogyne incognita (Kofoid& White) Chitwood nematode pathogen infestation. Later, two areas (in the same locality and climatic condition) each measuring 0.02 ha; one naturally rootknot disease infected untreated field and other naturally root-knot disease infected treated field, were demarcated in the mulberry field where there were no soil differences as well as environmental factor.
Preparation of Fields
The first 0.02 ha nematode infected (2863 + 55 J2 /1 kg of soil) sandy soil area (18889.76x 1066.80 x 45.72 cm3) was mixed with yard manure (2:1 vol / vol). Every day, at least 40 random sampling of moist rhizospheric soil (200g of soil i.e., each sample collected by making a hole of 1.8 cm wide and 6 cm deep) were done in the nematode infected area for 30 days and were assessed the M. incognita population [7-8, 11-12, 14-15, 17, 24-26, 31-35] and this naturally infected soil-filled area, demarking untreated field (Plate-2), was replicated thrice.
The other 0.02 ha (18889.76 x 1066.80 x 45.72 cm3) naturally M. incognita infected sandy soil field was also prepared by mixing yard manure (2: 1 vol / vol), removing weeds, irrigating water and interchanging among the soil for uniform distribution of manure and nematodes in the naturally infected field which was estimated by regular soil sampling like a same process of previous one. This naturally infected soil-filled area, demarking treated field (Plate-2), was also replicated thrice.

Plantation of Mulberry Cutting
Mature three years old mulberry cutting, Morus alba L., cv. S1 (average 25cm length and 20g fresh weight) collected from same sericulture field, were planted with a gap of 45cm throughout the experimental fields where there were no soil difference and climatic conditions. The planted mulberry cuttings were allowed to grow for a period of three months. Regular rhizospheric soil and root sampling (at random) were done for estimation of nematode population during this three month growth period of mulberry in all fields [12-16, 32-33, 36]. At least 80 number at random rhizospheric soil sampling (200g in each sample) were collected from rhizospheric root-soil area of root (10-15cm X 10-15cm) and at least 40 number at random root sampling (2g fresh root in each sample) were collected from newly formed roots (or gall roots) for determining the intensity or presence of nematodes in all the experimental fields.
Division of Groups and Plots
After three months growth of mulberry, M. incognita population were estimated in the rhizospheric soil as well as roots [12-19, 32-33, 36] (at least 40 at random sampling in each area) of mulberry plants in each areas of mulberry field. The M. incognita infected mulberry plants were achieved growth of 50-60 cm in height. The infected mulberry plants were divided in to 16 plots, each measuring the area of 472.44cm X 533.4cm X 45.72cm. The mulberry plants divided into two plant groups; untreated plants group and treated plant group and each group has 8-plots (20 plants / plot). At first all the plants were pruned, manured with NPK and irrigated every 7 days. Rhizospheric soil was interchanged among the plants to keep the nematode infestation as uniform as possible in the naturally infected field. After pruning, the plants were allowed to grow for a period of 135 days when their root-knot, leaf spot, powdery mildew, viral and tukra diseases were assessed [1-3, 37]. The field trial was replicated three times.
Plant Pathogens Caused Mulberry Diseases
Root-Knot Disease
Rhizospheric soil and root sample were taken at random from all the infected plots. Meloidogyne incognita populations (10 samples / plot in each plant group) were estimated in the rhizospheric soil as well as roots [12-19, 23-26, 31-38] of infected mulberry plants. Total number and surface area of leaves of all plant groups were counted [11-19, 23-26, and 31-38]. Total number of root-galls/plant were counted in the infected roots of mulberry plants [11, 19, 33-36, 40]. The total protein content of the leaf and root samples (10 at random sampling / plot) from each of the 16 plots was determined [36, 40-41]. All the data from experiments were counted for statistical analysis by student’s t- test. In this field trial, sacrifices of mulberry plants were not done due to well reported pathological characters from our previous experiments [4-18, 19, 23, 28-29, 33].
Foliar Diseases
The main foliar diseases, observed in the sericulture field, were: leaf spot disease caused by Cercosporammoricola (Cooke) fungus pathogens, powdery mildew disease caused by Phyllactiniacorylea (Pers.) Karst fungus pathogens, mosaic disease caused by mosaic virus pathogens and tukra disease caused by Maconellicoccushirsutus (Green) mealy bug pathogens. All the disease identified according to their characteristic symptoms by the experts concerned [1, 9-16, 20]. Diseased leaves of each type were counted in each plots [1, 11, 20, 23, 31, 34-35].
The percentage of disease infection based on diseased leaf surface area [9-16, 37, 42-44]. Preparation of crude Acacia auriculiformis extracts Air-dried and powdered funicles of Acacia auriculiformis A. Cunn. Were extracted with 90% ethanol at room temperature (25 + 2°C) for 15 days and were filtered for collecting extract. Later, the ethanol from the extract was removed by evaporation at room temperature (25 + 2°C). The residue, obtained after removal of the solvent under reduced pressure, was dried in a desicator over anhydrous calcium chloride [11, 19, 23, 26, 30, 34].
Isolation of Acaciasides
Crude residue of Acacia auriculiformis- extracts from the funicles of Acacia uriculiformis A. Cunn. Were again successively extracted with petrol (60-80° C) and 90% ethanol. The ethanol extract, on removal of the solvent under reduced pressure, yielded a viscous dark brown mass. The extract was chromatographed on silica gel with petrol, petrol-chloroform (1:1), chloroform, chloroform-methanol (9:1, 7:3, 3:2, 1:1 and 2:3) as successive eluents. The chloroform-methanol (7:3 and 3:2) eluates were then combined fraction was found to be composed mainly of two compounds which were separated by repeated preparative HPLC employing Spherisorb S-10-ODS reversed phase column with the solvent system methanol water (7:3) at a flow rate of 4 ml / min and refractive index detector as white amorphous solids.
These two solid compounds, designated as acaciaside A and acaciaside B (Figure 1) according to their increasing order of polarity, were found to be triterpenoidsaponins by LiebermannBurchard, molisch and forth tests [11, 19, 23, 26, 30, 34].
Mortality Test
Ten sets of cavity block with 1ml sterile tap water containing 50 larvae (J2) of Meloidogyne incognita were taken; five set was treated as control and other five were treated as treatment set. The acaciasides (A&B) were dissolved in sterile tap water at 0.0125 mg / ml forming acaciasides-test solution. To assess the direct effect of acaciasides- test solution, the water was removed by pipette and in all the treatment sets, immediately replaced by 1ml of test solutions (0.0125 mg acaciasides / ml concentration) were added, except the control and observed with every one hour interval for a period of 24 hours exposure period at room temperature (25±2°C). Immediately after observation of each block, nematodes were transferred to sterile tap water again to see if any recovery occurred after 4 hours [4-18, 45]. This mortality test was replicated five times.
Preparation of Acaciasides-Test Solution
In our experiment, the mixture of acaciaside A and acaciaside B (3:2) was dissolved in sterile tap water at 0.0125 mg / ml forming acaciasides-test solution and used for treatment plots [12-13, 19].
Treatment
Seventy six days after pruning, of mulberry plants, all the plots (treated groups and untreated groups) were done by foliar spray and soil drench @ 20ml / plant in each type of treatment (0.0125 mg acaciasides / ml concentration in case of treated groups) twice at an interval of 15 days with acaciasides- test solution and sterile tap- water respectively. Treatments were given in such a way that all the leaves and rhizospheric soil of the plants were completely drenched with test-solutions and tap-water. During spraying, the soil surface underneath each plant was covered with polyethylene sheet. All the acaciasides- treated groups were received 80ml / plant test solutions (1 mg / plant) and other untreated- plant groups were similarly received 80 ml / plant sterile tap water respectively [12-13, 19]. It was told about untreated (control); these controls were only treated with the sterile tap water (i.e. without acaciasides-test solution). At fifteen days after the second treatment all the parameters of diseases were assessed again for each group [9-14, 17, 19]. All the data were used for statistical analysis by student’s t-test.
Analysis of Residue
Mulberry leaves, collected fifteen days after second treatment were homogenized in a blender and extracted with ethanol. The residue runs in thin layer chromatography plate (TLC) with the standard from the acaciasides- test substances. The test substances were acaciasides- test solution [9-15, 17, 19].
Rearing of Silkworms
The eggs of a mother moth of the multivoltine ‘Nistari’ race (Bombyxmori L.) supplied by Regional Sericultural Research and Training Institute, Berhampore-742101, India, after hatching (93 % hatching rate) and brushing 1st stage silk worm larvae in the rearing tray, the larvae were divided into two batches (180 silkworm larvae / batch) and reared [9-15,17-18, 46]. The larvae of infected untreated batch (control) were fed with the leaves of pathogens infected diseased leaves of mulberry plants from infected untreated (control) plots and the larvae of infected treated batch were fed with the leaves of acaciasides-treated leaves of mulberry plants from infected treated. Fresh leaves were given to the larvae 4- times daily. Mulberry leaves were used for feeding fifteen days after the last treatment with acaciasides. The larvae were kept inside the rearing chamber at 27±2°C and 70 + 15% RH. The fresh weight of the larvae and that of the leaves served were recorded daily for each batch until the larvae started spinning. The consumption of fresh leaves (Fresh leaves served – Dry leaves residues - Fresh leaves initially consumed) X Moisture loss, number of feeding and number of feeding day to cocoon formation, number of escaping feeding during moulting, moulting span days and mortality rate were recorded. The fresh silk gland weight of mature 5th instar larvae (Plate 6) (before start spinning), starting time to spinning (Plate 7), span of spinning, fresh cocoon weight, fresh shell weight, silk layer ratio (SR % = Shell weight / Cocoon weight X 100), effective rate of rearing (ERR % =Number of cocoon harvested / Number of silk worm hatched X 100), sex ratio percentage (Number of male adult emerged / Number of female adult emerged X 100) and egg laying capacity of mother moth were determined [9-15, 17-18, 46]. For statistical analysis by student’s t- test, ten mature 5th instar silkworm larvae for fresh silk gland weight and ten cocoons for fresh shell weight were dissected out in each batches including replica of all batches [9-15, 17-18, 46]. All the data from rearing trial were used for statistical analysis by student’s t- test.
Science and Technology Communication Economy
The activity of students, researchers, teachers, staff, community, photographers, visitors, different scientist, administrators, institutions, farmers, NGO and media personnel, -campaign or -aware or -make news or -publication regarding the importance of “Acaciasides use as potential Bio-Agents against various plant pathogens: Enriching Science and Technology Communication Economy in Agriculture and Biodiversity Conservation issues” in different audio visual media (TV channels), social media, webpages, news papers and journals is recorded [9-13, 18-26, 47].
Results
Mortality Test
It was observed that acaciasides (A&B) had direct toxic effects on nematodes mortality within the exposure period of 24 hours because all the nematodes died and any recovery of nematodes occurred after 4 hours and no mortality occurred in the control.
Analysis of Residues
Mulberry leaves collected fifteen days after the second treatment, did not contain any toxic residue of the acaciasides- test substance.
Root-Knot Disease
Table 1 shows the effects of acaciasides on Meloidogyne incognita pathogens infected mulberry plants in a field trial replicated thrice (P<0.01 by‘t’- test). All naturally infected plants (treated plant group) treated with acaciasides (A&B) showed increase number and surface area of leaves, and higher protein content in leaves and root than infected untreated (control) plants (untreated plant group). In all infected acaciasides- treated plants, the population of root-knot nematodes decreased significantly in rhizospheric soil and as well as in roots than infected untreated (control) plants. The number of root galls also decreased significantly after acaciasides- treatment.
| Treatment groups (20 plants/ Plot & 8 plots / group ) |
Average number of leaves / plant |
Average surface area of leaves (sq.cm) |
Average protein content (%) | Average nematode population | Average number of root galls/plant |
||||||||||
|
Leaf |
Root |
Soil(200g) |
Root (2g) |
||||||||||||
| Day-0 | Day-30 | Day-0 | Day-30 | Day-0 | Day-30 | Day-0 | Day-30 | Day-0 | Day-30 | Day-0 | Day-30 | Day-0 | Day-30 | ||
| Infected
Untreated |
389ax | 373ay | 7998ax | 8003ax | 2.98ax | 2.01ay | 4.39ax | 3.29ay | 1931ax | 2406ay | 638ax | 2084ay | 1233ax | 2078ay | |
| (Control) | ±15.71 | ±14.21 | ±179.64 | ±177.61 | ±0.12 | ±0.07 | ±0.17 | ±0.07 | ±77.10 | ±96.64 | ±24.22 | ±97.40 | ± 49.13 | ±78.62 | |
| Infected | 387ax | 438by | 7992ax | 25246by | 2.98ax | 8.96by | 4.39ax | 7.85by | 1933ax | 69by | 639ax | 49by | 1229ax | 129by | |
| Treated | ±12.81 | ±12.12 | ±179.48 | ±387.96 | ±0.12 | ±0.29 | ±0.17 | ±0.02 | ±77.11 | ±2.33 | ±22.03 | ±1.35 | ±48.91 | ±3.27 | |
| Day-0 means before treatment.
Day-30 means after treatment. a,b,- Significant difference by ‘t’-test (P<0.01) in the same column. x,y- Significant difference by ‘t’- test (P<0.01) in the same row between day-0 and day-30 of each character. |
|||||||||||||||
Foliar Diseases
Table 2 shows the effects of acaciasides on leaf spot, powdery mildew, mosaic viral and tukra diseases of mulberry plants in a field trial replicated thrice assessed initially (Day- 0) and after a period of 30 days (Day -30) by ‘t’- test (P<0.01). Acaciasides (A&B) significantly reduced the number of leaves infected with leaf spot, powdery mildew, mosaic viral and tukra (Plate 3) as compared to the pre-treatment condition (Day -0). The percentage of control achieved were 62.08 for leaf spot, 77.89 for powdery mildew (Plate 4), 64.91 for mosaic virus (Plate 5) and 38.42 for tukra infection as compared to the pre-treatment level (Day- 0). In case of infected untreated plots leaf spot, powdery mildew, mosaic viral and tukra diseases showed naturally 27.80 %, 17.76 %, 29.37 % and 21.20 % reduction respectively, in 30 days (Day -30).
| Treatment groups (20plants/ Plot & 8 plots /group ) |
Average number of disease-infected leaves / plant ( % ) |
|||||||
| Leaf spot | Powdery mildew | Mosaic | Tukra | |||||
|
Day-0 |
Day-30 |
Day-0 |
Day-30 |
Day-30 |
Day-30 |
Day-0 |
Day-30 |
|
| Infected Untreated
(Control) |
70.58ax | 98.38ay | 80.75ax | 98.51ay | 68.68ax | 98.05ay | 57.15ax | 78.35ay |
| ±2.28 | ±3.93(<27.80%) | ±3.23 | ±3.94(<17.76%) | ±2.74 | ±4.10 (<29.37%) | ±2.38 | ±3.26 (<21.20%) | |
| Infected Treated | 70.53ax | 8.45by | 80.86ax | 2.97by | 68.32ax | 3.41by | 57.11ax | 18.69by |
| ±2.71 | ±2.71 (>62.08%) | ±3.11 | ±0.01 (>77.89%) | ±2.62 | ±0.13 (>64.91%) | ±2.37 | ±0.81 (>38.42%) | |
| Day-0 means before treatment. Day-30 means after treatment.
a,b- Significant difference by ‘t’-test (P<0.01) in the same column. x,y- Significant difference by ‘t’- test (P<0.01) in the same row between day-0 and day-30 of each character. ( )- Figures in the parentheses show percentage of reduction on day-30 as compared to the initial level on day-0 in the same row. |
||||||||

Effects on Feeding Silkworms
Table 3 shows the effects of acaciasides on diseased infected mulberry plants in a silkworm rearing and field trial replicated thrice on the feeding, growth and mortality of silkworms (P<0.01 by ‘t’-test). The average consumption of leaves by the 5th instars (Plate 6), average number of feeding to cocoon formation (Plate 7), average number of feeding day to cocoon formation, average number of escaping– feeding during moulting and average moulting span days were less for acaciasides (A&B)- treated plants than for infected untreated (control) ones. The average mortality rate (%) was nil with acaciasides- treated plants groups and 56% with infected untreated (control) one. However, the average fresh weight of the 5th instars larvae were higher with acacia sides- treated plants than with infected untreated (control) one.
| Treatment batches (180 larvae/ batch)* | Average number of | ||||||
| Consumption of leaves(g) (5th instar)* | Feeding to cocoon formation* | Feedingday to cocoon formation* | Escaping feeding during moulting* | Moulting span day (1st to 5th instar)* | Larval fresh weight (g) (5thinstar)*+ | Mortality rate (%)* | |
| Infected Untreated (Control) | 4.03a | 76.00a | 19.00a | 51.00a | 13.00a | 1.48a | 56.00 |
| ±0.15 | ±2.37 | ±0.50 | ±1.75 | ±0.39 | ±0.03 | ±2.43 | |
| Infected Treated | 2.46b | 62.00b | 15.00b | 20.00b | 5.00b | 2.63b |
Nil |
| ±0.09 | ±1.93 | ±0.44 | ±0.68 | ±0.15 | ±0.06 | ||
| a,b- different small letters in a column show significant difference by ‘t’- test (P<0.01).
* - average values of 180 silk worm larvae in triplicate. + - average values of 10 silk worm larvae were dissected in triplicate. |
|||||||


Effects on Silk Production and Rearing Practices
Table 4 shows the effects of feeding acaciasides- treated mulberry leaves on silk production, spinning characters and rearing practices in a silkworm rearing (Plate 7) and field trial replicated thrice (P<0.01 by ‘t’-test). The average fresh silk gland weight, average fresh cocoon weight, average fresh shell weight and average shell ratio (SR %) were higher with acacia sides- treated plants than with infected untreated (control) one. It is notable that average starting time to spinning day and average span of spinning day (i.e. duration of span) were fewer with the acaciasides- treated plants than with infected untreated (control) ones. Average effective rate of rearing (ERR %), average sex ratio percentage and average egg laying capacity were significantly higher with all acaciasides- treated groups.
| Treatment batches (180 larvae/ batch)* | Average number of | ||||||||
| Silk gland fresh weight (g) (5thinstar)+ | + Starting time to spinning (at day-) * | Span of spinning day * | Cocoon fresh weight (g)* | Shell fresh weight (g) + | Shell ratio (SR%) + | Effective rate of rearing (ERR%)* | Sex ratio (Male / Female%) | Egg laying capasity | |
| Infected Untreated (Control) | 0.98a | 34.00a | 10.00a | 0.85a | 0.11a | 12.94a | 21.37a | 76.00a | 320.00a |
| ±0.03 | ±1.30 | ±0.45 | ±0.03 | ±0.01 | ±0.49 | ±0.63 | ±1.94 | ±13.91 | |
| Infected Treated | 1.98b | 20.00b | 3.00b | 1.09b | 0.24b | 22.01b | 97.43b | 68.00b | 540.00b |
| ±0.07 | ±0.51 | ±0.09 | ±0.02 | ±0.01 | ±0.67 | ±2.16 | ±1.74 | ±11.73 | |
| a,b- different small letters in a column show significant difference by ‘t’- test (P<0.01).
* - average values of 180 silk worm larvae in triplicate. + - average values of 10 silk worm larvae and cocoon were dissected in triplicate. |
|||||||||
Here, Key Findings
- Here, acacia sides (A&B), isolated from the funicles of Acacia auriculiformis A. Cunn., were used against root-knot, leaf spot, powdery mildew, mosaic viral and tukra diseases caused by pathogens in a mulberry field trial. It was also observed the responses of silkworms feeding on mulberry leaves.
- Acacia sides were soluble in water and applied to 6 plots twice at an interval of 15 days @ 1 mg / plant infected with above mentioned pathogens by foliar spray and soil drench using 20ml solution for each type of treatment serving treatment plots. The remaining 6 plots treated with tap water serving control plots.
- Acacia sides were highly effective in ameliorating mulberry diseases and were increased the protein content of mulberry leaves. Silkworms larvae feeding on the leaves of treated plants showed improved growth, increased cocoon weight and shell weight, fewer feeding to cocoon formation, zero mortality rate and increased the effective rate of rearing.
- The results showed that acaciasides use as potential eco-friendly bio-agents against various pathogens of plants enriching agriculture significantly without disturbing biosphere.
Science and Technology Communication Economy
The students, researchers, teachers, staff, community, photographers, visitors, different scientist, administrators, institutions, farmers, NGOs and media personnel campaign, aware, discussion, arrange workshops and seminars, make news and publish as abstract regarding the importance of “Acaciasides use as Potential Bio-Agents Against Various Plant Pathogens: Enriching Science and Technology Communication Economy in Agriculture and Biodiversity Conservation Issues” in different national- and local- audio visual media (TV channels), different social media, web pages, news papers and different -national and –international Journals as well as Congress Proceedings also. We are amazed for use of Acaciasides - directly or -indirectly help the society in various ways and may also provide a unique platform for showcasing the research across the globe and progress the further advanced research in Agriculture, Horticulture and Entomology deals with economy. Common people also realize the meaning of new and more efficient solutions, technologies, products and it has to improve Science and Technology Communication Economy forming joyful environment and fulfill its food and nutrition requirement which resist any kinds of chemical thresholds or natural infections for the climate change and resource productive economies enriching quality of environment.
Discussion
The pure compounds acacia sides (A&B), isolated from the funicles of Acacia auriculiformis A. Cunn., Bio-Agents not only reduced root-knot, leaf spot, powdery mildew, viral and tukra diseases but also improved the nutritive value of the treated leaves of infected plants [11-13, 19]. Acacia sides treatments directly influences on the consumption of leaves, number of feeding and number of feeding day to cocoon formation, and indirectly affects on moulting stage in the infected treated groups from this trial. And due to ill development of infected untreated (control) batches silkworm larvae took more time to moult which is proved from the number of escaping feeding during moulting. Higher nutritive value of treated plants contributed to higher growth of silkworm larvae, silk gland weight, cocoon weight and shell weight which increase silk production significantly for commercial purpose [11-13, 19]. The improved health of the larvae, cocoon weight, silk gland and shell weight from the acacia sides- treated groups of infected plants might have resulted in the fewer starting time to spinning and span of spinning day and the total elimination of the mortality rate. Or, the acacia sides might have infused in to mulberry leaves a substance that has conferred disease resistance on growing silkworm larvae by releasing defence-related natural products by plants [2-13, 17-18, 31, 34-35, 49]. The effective rate of rearing (ERR %) is very high in all acacia sides- treated treatment batches which enriches the sericulture industry in many ways, especially for commercial purpose. The mulberry leaves did not contain any toxic residues of the acacia sides- test substances by the thin layer chromatography (TLC). Rather, the acacia sides might have induced natural defense response in the test plants against all above mentioned pathogens and has conferred defense response on growing larvae [2-13, 17-19, 31, 34-35, 48-49, 52].
The present study clearly showed that acacia sides (A&B) were treated as effective or potential bio-agents and it had no direct toxic effect on plants but to the pathogens of mulberry plants. The bio-agents, acacia sides (A&B), could induced some resistance in mulberry against pathogens infection. It can be assumed that acacia side A and acaciaside B could induce synthesis of some antagonistic substance in the treated plants. Lectins accumulated in gall regions of root of Hibiscus esculentus infected with M. incognita [50]. Systemic acquired resistance can be induced by in different crop plants by localized virus infection, non-pathogenic and pathogenic microorganisms or their culture filtrates or by salicylic acid [2-18, 34, 51-54]. Plant-derived natural products have important functions in ecological interactions. In some cases these compounds are deployed to sites of pathogen challenged by vesicle-mediated trafficking. Polar vesicle trafficking of natural products, proteins and other, as yet uncharacterized, cargo is emerging as a common theme in investigations of diverse disease resistance mechanisms in plants [49]. Sequestration implies the involvement of specific transport process. For example, the defence-related triterpene glycoside avenaacin A-1 is synthesized [55].
It is reported that a plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoidsecrerion [56]. Functional analysis has confirmed a role for this transporter in disease resistance [57]. Though, M. incognita is known to share common antigens with its host plants [58]. It appeared that during natural infection with the nematode, host plants showed minimal defense responses to the nematode because of this antigenic similarity. Acacia sides (A&B) contain two triterpenoidssaponins [12, 30, 34] and these saponins provide defence to the test plants against pathogens [59-63]. Acacia sides must be responsible for defense resistance of the mulberry. Acacia sides (A&B) may synthesis various antigens particularly (low molecular weight proteins) and induce defense responses involving a number of pathogenesis related protein in which the naturally infected plant pathogens fails to tolerate [12, 18, 31, 34, 59-63]. It is observed that in lady’s finger plants treated with NE (nematode extract) showed the highest number of root proteins (no. 24) but in inoculated untreated root was 18 number and uninoculated untreated root was 11 number [12,18]. Those showed that NE served as a stimulus for the expression of may proteins particularly the defense – related proteins which later provide resistance to nematode infection. However, in the test plant were treated with NE after inoculation with live nematodes did not show much increase in number of proteins in root [23].
Those showed that nematode infestation somehow serve as a repressor for the expression of defense gene in plant [12, 18]. From this point of view, we must assume that acaciasides serve as a stimulus for the expression of many new induced defence-related PR-proteins by systemic acquired resistance which provides defense-resistance to various pathogens causing major diseases of plants. It can be told that acacia sides (A&B) acquiring systemic resistance could serve very effective eco-friendly bio-agents and promoted growth of test plants by inducing their defense responses of the host plants by expression of some new proteins against many plant pathogens infection causing major diseases and this bio-agents conserved our biodiversity and makes pollution free environment.
Now the key question is, whether plant-derived natural products (acaciasides A&B), can be used as potential Bio-Agents by inducing defence- response against various plant pathogens causing major mulberry diseases in a mulberry field trial and silkworms rearing. It is surprising that all infected acaciasides-treated plants not only are less affected by pathogens but also have a better growth than infected untreated (control) plants. The positive effects of growth may be responsible for defense resistance against pathogens. Acaciasides (A&B) might have induced synthesis of many new proteins which have stimulated increase photosynthesis rate, stomatal activity and water retaintion capacity of acacia sides- treated plants [2-9, 12-16, 64]. The positive effects of growth on disease-infected treated plants might not only be responsible for defense resistance to pathogens but also improved growth of silkworm larvae and silk gland weight, cocoon weight, shell weight, effective rate of rearing (ERR %), sex ratio percentage and egg laying capacity of mother moth with zero mortality rate were higher with all acacia sides- treated groups which increase silk production for commercial purpose. It is proved from the results that silk production is higher in all acaciasides- treated plants than infected untreated treated (control) plants. Now the answer is, bio-agents acaciasides was not only highly effective in ameliorating mulberry diseases but also enriched sericulture industry as well as agriculture industry.
Future Approach in Research
It is reported that the Bio-Agent acacia sides (A&B) is being used traditionally to overcome various medical complications like sore eyes, aches, rheumatism, allergy, itching, and rashes. Besides, it has also been proven for many pharmacological activities like central nervous system depressant activity, antioxidant, antimicrobial, antimalarial, anti-filarial, cestocidal, antimutagenic, chemopreventive, spermicidal, wound healing, hepatoprotective and antidiabetic activity and high efficacy. It has been used to treat several medical ailments due to its low toxicity and the presence of bioactive phytoconstituents [65]. Recently, in ‘India Today’ discuss regarding the “Trade in the time of COVID-19: The economic impact of corona virus on India and beyond” shows the estimates of India’s aggressive 21-day lockdown could bring the country’s growth down to 2.5 % from the 4.5 %. The effect of corona virus is likely to be seen long after medical science offers a cure or at least a vaccine [66, 67]. In a joint report from the World Health Organization (WHO) and the World Bank estimates the impact of such a pandemic at 2.2 per cent to 4.8 per cent of global GDP (US$3 trillion) [66, 67]. In the Drug Target Review 2020, NovavaxInc, which contributed to the development of other epidemic vaccines, has announced it is currently in pre-clinical animal trials for several multiple nanoparticle COVID-19 vaccine candidates. The biotechnology company has announced its efforts to help in creating a vaccine against SARS-CoV-2. “Our previous experience working with other corona viruses, including both MERS and SARS, allowed us to mobilize quickly against COVID-19 and successfully complete the critical preliminary steps to engineer viable vaccine candidates,” said Stanley Erck, President and Chief Executive Officer of Novavax which adjuvant is saponin-based and it has shown a “potent and well-tolerated effect” [68, 70]. It is already reported that the presence of chief constituents as flavonoids and two acylatedtriterpenoidbisglycosidesaponins present in Acaciasides (A&B) [3, 5, 11-12, 18, 30-31, 59, 66, 69, 71-75] (vide World Economic Forum’s COVID Action Platform). So, in near future, a clinical study may be arranged for discovery of ‘Vaccine’ by using saponins from Boi-Agent Acaciasides which may kill the novel Corona virus COVID-19 by boosting human immune system and -generating superoxide anions, -initiating lipid peroxidation and -dissolving the fatty layer that coats of corona viruses.
Conclusion
It can be concluded that plant diseases, like root-knot disease, leaf spot disease, powdery mildew disease, mosaic disease and tukra disease, might be effectively controlled by the cost effective acaciasides (A&B) by using as potential Bio-Agents against various plant pathogens in a mulberry field trial and silkworms rearing at an extremely low dose and commercially increases silk production which directly enriches sericulture industry as well as agriculture sector. And it would not only easily available, non-phytotoxic and non-pollutant but also Acaciasides enrich “Science and Technology Communication Economy in Agriculture significantly without disturbing biosphere”.
It also conserve our biodiversity which will contribute towards “Sustainable Climate, Health and Development” by controlling plant diseases which is sometime devastating to all kinds of -natural and -artificial vegetation and may also provide a unique platform for showcasing the research across the globe and progress the further advanced research in Agriculture, Horticulture and Entomology deals with economy. And it would go a long way in tackling various pest of crops in a safe way by inducing their defense-responses of host plants against pathogens. In near future, a clinical study may be arranged for discovery of ‘Vaccine’ by using Saponins from Boi-Agent Acaciasides which may kill the novel Coronavirus COVID-19 by boosting our immune system and the whole world may retain in normal forms.
Acknowledgements
The work described here has been supported by CSB and UGC, Government of India to Rtd. Prof. N.C.Sukul and Prof. S.P.Sinha Babu, Department of Zoology, Visva-Bharati and Joint Director, Sriniketan Sericultural Composite Unit, Sriniketan, Govt. of West Bengal. I am thankful to Rtd. Prof. Dr. Sukanta Sen and Prof. Dr. Nirmalya Banerjee, Department of Botany, Visva-Bharati, Santiniketan-731235, for confirming the identity of the foliar diseases. I like to thanks Mr. Rakesh Khan, Secretary and Mr. Subhendu Bose, President with all young green-members of “NGO named Burdwan Green Haunter and Students’ Goal” for arranging several awareness programme on “Biodiversity Conservation and Enriching Science and Technology Communication Economy in Agriculture Issue”. Last but not the least; I am thankful to the eminent educationist Sri Tapaprakash Bhattacharya for inspiration and guidance.
References
- Teotia RS, Sen SK (1994) Mulberry disease in India and their control. Sericologia 34: 1-18.
- Datta SC, Datta R (2007a) Intercropping amaranth with mulberry for managing rootknot nematodes and improving sericulture. Sericologia 47: 297-302.
- Datta SC, Datta R (2007b) Increased silk production by effective treatment of naturally infected root-knot and black leaf spot diseases of mulberry with acacia sides. Journal of Environment & Sociobiology 4: 209-214.
- Datta SC, Datta R (2011a) Homeopathic Medicines Protect Environment, Health and Development by Controlling Mulberry Diseases. J Journal of Homeopathy and Ayurvedic Medicine 1:104.
- Datta SC, Datta R (2011b) Control of Root-Knot Disease of Mulberry by Homeopathic Medicines: Aakashmoni [Mt, 30c, 200c & 1000c] Prepared from the Funicles of Acacia Auriculiformis. HpathyEzine.
- Datta SC, Datta R (2012a) Homeopathic Medicines Protect Environment, Health and Development by Controlling Mulberry Diseases. Journal of Traditional Medicine & Clinical Naturopathy 1: 104.
- Datta SC, Datta R (2012b) Homeopathic medicine Aakashmoni 200C controls mulberry diseases enriching sericulture. Journal of Current Chemistry Pharmacological Science 2: 37-49.
- Datta SC, Datta R (2012c) Efficacy of pure compound- acaciasides A and B as potential bioagents against various plant pathogens. Journal of Environment & Sociobiology 9: 17-26.
- Datta SC (2007) Mulberry disease: Problem in sericulture. SEBA NEWSLETTER, Environment & Sociobiology 4: 7-10.
- Datta SC (2008) Enriched sericulture by controlling major mulberry diseases with some cost effective ways-a review. Journal of Environment & Sociobiology (In press).
- Datta SC, Sinhababu SP, Sukul NC (1997) Improved growth of silkworms from effective treatment of mulberry diseases by Acacia auriculiformis extract. Sericologia 37: 707-712.
- Datta SC (1999) Bio-nematicides in the control of root-knot nematode. Ph.D. thesis, Department of Zoology, VisvaBharati, Santiniketan-731235, West Bengal, India (unpublished).
- Datta, SC (2005a) Plant Parasitic nematodes – an agricultural problem and its solutions. Visva-Bharati Quarterly 11: 89-100.
- Datta, SC (2005b) Possible use of amaranth as catch crop for root-knot nematodes intercropped with mulberry. Journal of Environment & Sociobiology 2: 61-65.
- Datta, SC (2006a) Effects of Cina on root-knot disease of mulberry. Homeopathy 95: 98-102.
- Datta, SC (2006b) Possible use of amaranth as catch crop for root-knot nematodes intercropped with okra. Phytomorphology 56(3&4): 113-116.
- Datta SC, Datta R (2006a) Liquid homeopathic medicine Cina enriches sericulture industry. Journal of Environment & Sociobiology 3: 55-60.
- Datta SC, Datta R (2006b) Defence resistance of okra against root-knot disease by bionematicides. Proceedings of the Zoological Society 59: 75-82.
- Sukul NC, Sinhababu SP, Datta SC, Nandi B, Sukul A (2001) Nematotoxic effect of Acacia auriculiformis and Artemisia nilagirica against root-knot nematodes. Allelopathy Journal 8: 65-72.
- Powell NT (1971) Interaction between nematodes and fungi in disease complexes. Annual Review Phytopathology 9: 253-274.
- Datta SC, Datta R (2013) Efficacy of homeopathic medicine-Aakashmoni as potential bio-agent against various plant pathogens. Journal of Biochemistry & Pharmacology 2: 4.
- Govindaiah, Sharma DD (1994) Root-knot nematode, Meloidogyne incognita infesting mulberry - a review. Indian Journal of Sericulture 33: 110-113.
- Datta SC, Das R, Chatterjee K, Mondal B, Das R (2016) Amaranth Plant: Protects Climate, Health and Development by Controlling Root-Knot Disease. Journal of Environmental & Analytical Toxicology 6: 341.
- Datta SC, Datta R (2016) Prevention and control of root-knot disease of mulberry plants using bioagents amaranth plants: improving sericulture by protecting climate health, health and development. Journal of Environment & Sociobiology 13: 191-200.
- Datta SC, Datta R (2017) Acaciasides use as Potential Bio-Agents against Various Plant Pathogens. Book: New Innovations in Environmental Biotechnology. Publisher: Lenin Media Delhi Pvt.Ltd. Delhi, Chapter 14, 2016; Pages-20, Editors: Dr. M.M. Abid Ali Khan (India), MurtazaAbid (India), Dr. Abdeen Mustafa Omer (United Kingdom), Dr.Binna Rani (India).
- Datta SC (2019a) Enriched Sericulture from Effective Treatment of Mulberry Diseases by Homeopathic Medicines. Advances in Biochemistry and Biotechnology 7: 084.
- Ouassat S, Allam L (2020) Toxicity of Three Pesticides to The European red mite Panonychusulmi and its predator, Typhlodromus (T.) setubali (Acari: Phytoseiidae, Tetranychidae). Advances in Agriculture, Horticulture and Entomology 2020: 1-6. AAHE-106.
- Sukul NC (1992) Plants antagonistic to plant parasitic nematodes. Indian Review of Life Science 12: 23-52.
- Sukul NC (1994) Control of plant parasitic nematodes by plant substances. Allelopathy in Agriculture and Forestry, (Edited by S.S. Narwal and P. Tauro), Scientific Publisher, Jodhpur, India pp.183-211.
- Mahato SB, Pal BC, Nandi AK (1992) Structure elucidation of two acylatedtriterpenoid bioglycosides from Acacia auriculiformisCunn. Tetrahedron 48: 6717-6728.
- Datta SC, Datta R, Sinhababu SP, Sukul NC (1998b) Acaciasides and root-knot nematode extract suppress Melodogyne incognita infection in lady’s finger plants. Proceeding of the National Seminar on Environmental Biology 98: 205-209.
- Christie JR, Perry VG (1951) Removing nematodes from soil. Proceeding of Helminthological Society, Washington 18: 106-108.
- Sukul NC (1987) Soil and plant nematodes. West Bengal State Book Board Publisher pp.1-271.
- Datta SC, Sinhababu SP, Banerjee N, Ghosh K, Sukul NC (1998a) Melodogyne incognita extract reduces Melodogyne incognita infestation in tomato. Indian Journal of Nematology 28: 1-5.
- Datta SC, Datta R, Sukul A, Sukul NC, Sinhababu SP (2000) Relative attractiveness of four species of vegetable crops for Meloidogyne Incognita. Environment and Ecology 18: 233-235.
- Chatterjee A, Sukul NC (1981) Total protein of galled roots as an index of root-knot nematode infestation of lady’s finger plants, Phytopathology 71: 372-274.
- Gunasekhar V, Govindaiah, Datta RK (1994) Occurrence of Altemaria leaf blight of mulberry and a key for disease assessment. International Journal of Tropical Plant Disease 12: 53-57.
- Datta SC (2019b) Improved Environment by Identification of More Susceptible Plant between Cowpea and Mulberry for Root-Knot Disease. Open Access Journal of Environmental Soil Science 2: 242-245.
- Datta SC (2019c) Improved midday meal by using cowpea as eco-friendly crop controlling root-knot forming global, green, growth and green economy. International Journal of Advanced Research (Accepted).
- Das S, Sukul NC (1986) Biochemical changes of some crop plants due to root-knot nematode infection. Proceeding of National Symposium of New Dimension in Parasitology, Allahabad, India 86: 122-125.
- Lowry OH, Rossebrough NJ, Farr AR, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. Journal of Biological Chemistry 193: 265-275.
- James WC (1971) An illustrated series of assessment keys for plant diseases, their preparation and usage. Canadian Plant Disease Survey 51: 39-65.
- Allen SJ, Brown JF, Kochman JK (1983) Production of inoculum and field assessment of Atemariahelianthi on sunflower. Plant Disease 67: 665-668.
- Sengupta K, Govindaiah, Pradip K, Murthuza B (1990) Hand book on pest and disease control of mulberry and silkworm- Disease of mulberry and their control. United Nations Economic and Social Commission for Asia and Pacific, Bangkok, Thailand pp. 1-14.
- Fenner LM (1962) Determination of nematode mortality. Plant Disease Reporter 46: 383.
- Krishnaswamy S, Narasimhanna MN, Suryanarayana SK, Kumararaj S (1972) Manual on sericulture , Vol.2. Silkworm rearing , Agric Services Bul.15,FAO, Rome.
- Datta SC, Datta R (2008b) Potentized Artemisia nilagirica Extract (Cina) Increases Silk Production and Effective Rate of Rearing in a Field Trial. HpathyEzine.
- Datta SC (2010) The Role of Cina in the Field of Enriched Sericulture. HpathyEzine, Febuary 15, 2010.
- Field B, Jordan F, Osbourn A (2006) First encounters – development of defence – related natural products by plants. New Phytologist 172: 193-207.
- Das S, Sukul NC, Mitra D, Sarkar H (1989) Distribution of lectin in nematode infested and uninfested roots of Hibiscus esculentus. NematologicaMediterranea 17: 123-125.
- Ross AF (1961) Systemic acquired resistance induced by localized virus infection in plants. Virology 14: 340-358.
- Klessig, Daniel F, Malamy J (1994) The salicylic acid signal in plants. Plant Molecular Biology 26: 1439-1458.
- Mauch-Mani B, Metraux JP (1998) Salicylic acid and systemic acquired resistance to pathogen attack. Annals of Botany 82: 535-540.
- Nandi B, Kundu K, Banerjee N, Sinhababu SP (2003) Salicylic acid –induced suppression of Meloidogyne incognita infestation of okra and cowpea. Nematology 5: 747-752.
- Osbourn AE, Clarke BR, Lunness P, Scott PR, Daniels MJ (1994) An oat species lacking avenacin is susceptible to infection by Gaeumannomycesgraminis var. tritici. Physiological and Molecular Plant Pathology 45: 457-467.
- Jasinski M, Stukkens Y, Degand H, Purnelle B, Marchand-Brynaert J, Boutry M (2001) A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 13: 1095-1107.
- Stukkens Y, Bultreys A, Grec S, Trombik T, Vanham D, Boutry M (2005) NpPDRI, a pleiotropic drug resistance-type ATP-binding cassette transporter from Nicotiana plumbaginifolia, plays a major role in plant pathogen defense. Plant Physiology 139: 341-352.
- McClure MA, Misaghi I, Night Edward LJ (1973) Shared antigens of parasitic Nematodes and Host plants. Nature 244: 306.
- Osbourn AE (1996) Saponins and plant defence– a soap story. Trends in Plant Science 1: 4-9.
- Papadopoulou K, Melton RE, Leggett M, Daniels MJ, Osbourn AE (1999) Compromised disease resistance in saponin-deficient plants. Proceedings of the National Academy of Science, USA 96: 12923-12928.
- Datta SC, Datta B (2018) Improved midday meal by using cowpea as eco-friendly crop controlling root-knot forming global green, growth and green economy. Journal of Recent Science (In Press). ISBN: 978-93-86675-21-7 and ISSN: 2277-2502.
- Osbourn AE, Qi X, Townsend B, Qin B (2003) Secondary metabolism and plant defence. New Phytologist 159: 101-108.
- Sasser JN, Freckman DW (1986) Proceedings of 25th Annual Meeting Society of Nematologists, Orlando, Florida 86: 32.
- Zarter CR, Demmig-Adams B, Ebbert V, Adamska I, Adams WW III (2006) Photosynthetic capacity and light harvesting efficiency during the winter-to-spring transition in subalpine conifers. New Phytologist172: 283-292.
- Rangra NK, Samanta S, Pradhan KK (2019) A comprehensive review on phytopharmacological investigations of Acacia auriculiformisA.Cunn. ex Benth. Asian Pacific Journal of Tropical Biomedicine 9: 1-11.
- IUCN (1993) The International Union for Conservation of Nature and Natural Resources, World Health Organization and World Wide Fund for Nature. Guidelines on the conservation of medicinal plants. Gland, Switzerland: IUCN; 1993.
- Mukewar P. (2020) Trade in the time of COVID-19: The economic impact of coronavirus on India and beyond. India Today , New Delhi March 29, 2020 UPDATED: March 30, 2020 00:04 IST
- Said N (2020) Coronavirus COVID-19: Available Free Literature Provided by Various Companies, Journals and Organizations around the World. Ongoing Chemical Research 5: 7-13.
- Nandy B, Roy S, Bhattacharya S, Sinha Babu SP (2004) Free Radicals Mediated Membrane Damage by the SaponinsAcaciaside A and Acaciaside B. Phytotherapy Research 18: 191-194.
- News Drug Target Review’s (2020) Coronavirus update: recent developments in vaccine Research. Drug Target Review’s round-up of the latest developments in 2019 novel coronavirus (COVID-19 or SARS-CoV-2) therapeutics and vaccines 27th February 2020.
- Sean Fleming (2020) A chemistry professor explains: why soap is so good at killing COVID-19. World Economic Forum. Senior Writer, Formative Content 12th March2020.
- Barreiro P, Duro RJ (2020) From Environmental Robots to Cyber Creatures: A Liberal Prospection on the Future Agroecological Systems. Advances in Agriculture, Horticulture and Entomology 2020: 1-12.
- Crombie L, Crombie WML, Whiting DA (1986) Structure of the oat root resistance factors to take –all disease, avenacins A-1, A-2, B-1 and B-2 and their companion substances. Journal of the Chemical Society Perkin Transactions 1: 1917-1922.
- Datta SC (2019d) Enriched School Health For The Effective Healthcare Bio-Activity of Barn Owls. Research & Review of Health Care Open Access Journal 3: 269-275.
- Datta SC (2019e) Enriched School Environment for the Effective Bio-Activity of Barn Owls. International journal of Horticulture, Agriculture and Food science (IJHAF) 3: 119-126.