Publication Information
ISSN 2692-1529
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Effects of Different Dietary Protein Sources on Water Quality Parameters and Growth Performance of Nile Tilapia (Oreochromis niloticus) Fingerlings
Victor O. Ogweny1*, Merceline Ndinda Ndambuki1, Joyce G. Maina2, Phillip N. Nyaga1, Shimaa E. Ali3, 4
1University of Nairobi, Department of Veterinary Pathology, Microbiology and Parasitology, P.O. BOX 29053-00625, Kangemi.
2University of Nairobi, Department of Animal Production, P.O BOX 29053-00625, Kangemi.
3WorldFish, Abbassa, Sharkia, Egypt
4Department of Hydrobiology, Veterinary Research Institute, National Research Centre, Giza, 12622, Dokki, Egypt
Received Date: December 04, 2023; Accepted Date: December 29, 2023; Published Date: January 27, 2024;
*Corresponding author: Victor O. Ogweny, University of Nairobi, Department of Veterinary Pathology, Microbiology and Parasitology, P.O. BOX 29053-00625, Kangemi. Email: vogweny@gmail.com
Citation: Ogweny VO, Ndambuki1 MN, Maina JG, Nyaga1 PN, Ali SE (2023) Effects of Different Dietary Protein Sources on Water Quality Parameters and Growth Performance of Nile Tilapia (Oreochromis niloticus) Fingerlings. Jr Aqua Mar Bio Eco: JAMBE-122.
DOI: 10.37722/JAMBE.2024201
Abstract
Freshwater aquaculture is the dominant type of aquaculture in Kenya, with Oreochromis niloticus favored due to its adaptability to a wide range of environmental conditions and rapid growth. However, the success and economic viability of aquaculture is significantly impacted by the type and quality of fish feeds in the culture systems. To address this crucial aspect, a study was done to investigate the effects of different sources of protein in fish diets on water quality and growth performance of O. niloticus fingerlings fed with diets containing three different protein sources. Three locally sourced protein sources, namely, Black Soldier Fly larvae (BSF) meal, Freshwater shrimp meal (FSM), and Soybean meal (SBM), and a commercial feed locally purchased were fed to groups of 25 fish replicated 3 times in a completely randomized design. The study was done for a period of 10 weeks at the fish holding unit at the Department of Veterinary Pathology, University of Nairobi. The results showed that the weight gain of juvenile O. niloticus fed on BSF (103.7%), FSM (93.7%), and SBM (82.8%) were higher than the control (59.2%). There were statistically significant effects of the different diets on the growth rates of fish (p < 0.05) with the highest growth rate and survival reported for BSF (SGR = 1.295) and 82.2% respectively during the study period. Water quality parameters remained within acceptable ranges, with temperature and phosphorus levels varying significantly across treatments (p = 0.002) and (p = 4.3e-05) respectively. Specifically, water from fish fed on diets based on BSF had relatively higher Phosphorus levels than the other feeds.
This study demonstrates the advantages of BSF as a superior protein source for the culture of O. niloticus. In it highlights the importance of BSF larvae as a partial replacement of fishmeal in aquaculture as well as the importance of suitable feed formulations in order to maximize fish performance and reduce costs. The study’s outcome can improve food security for millions of people through the use of BSF as an alternative protein source to fish feed.
Keywords: Freshwater Aquaculture, Fish feeds, Food security
Introduction
Fisheries and aquaculture support the livelihoods of more than 800 million people worldwide, providing protein and superior polyunsaturated fatty acids to approximately three million people in underdeveloped nations (FAO, 2015). Aquaculture production globally in 2016 constituted 80 million tons of fish used as food with 54.1 million tons composed of finfish (FAO, 2018). About 70% of aquaculture is produced in countries that are food insecure (LIFDCs), with an initial 8% mean growth rate annually, which has been reported to be declining as a result of insufficient use of resources such as water and feed for fish growth (FAO, 2015).
Freshwater aquaculture accounts for 98% of Kenya's reported aquaculture production of 24,096 MT, representing 15% of total national fish production (KMFRI 2017). The importance of aquaculture is growing, according to the World Review of Aquaculture and Fisheries report (FAO 2014). The surge in fish farming is propelled by the rise in human population which has prompted the growth of aquaculture to satisfy the expanded food need. Fish farming started in Kenya during the 1900s through the introduction of trout in streams for sport fishing (Munguti et al., 2014). The practice became more prominent during the 1960s yet had a slow until the year 2006 when the hydroponics area creation rose from 4,218 metric tons (MT) to a pinnacle of 24,096 MT in 2014 (Mukasa, 2012; Orina et al., 2018). This positioned Kenya as the fourth significant aquaculture producer in Africa after Nigeria, Uganda, and Ghana (Mbowa et al., 2017). Nile tilapia (Oreochromis niloticus) represents about 75 % of the cultured fish (Njiru et al., 2019; Omasaki et al., 2013) with other species such as African catfish (Clarias gariepinus), Trout (Oncorhynchus mykiss) representing the remaining percentage (Orina et al., 2018).
O. niloticus is the most preferred cultured species owing to several factors. The species can tolerate a wide range of environmental factors compared to other fish species (Kibria & Haque, 2018), it grows fast and is resistant to most diseases (Rahman et al., 2017). Other characteristics include the capacity to produce in captivity, the capacity to feed at a low trophic level, and the acceptance and utilization of formulated diets (Ombwa et al., 2018). Tilapia can endure a more extensive scope of natural conditions—including elements like salinity, low oxygen concentrations, temperature, pH, and alkali levels— than most freshwater fishes can (Cifuentes-Torres et al., 2021). Nile tilapia is believed to be the most intolerant to changes in salinity (not more than, 18 ppm) (Heise et al., 2021). Tilapia are, by and large, exceptionally tolerant to low oxygen concentrations, even down to 0.1 mg/L, however, ideal growth and development are usually realized at oxygen concentrations above 3 mg/L (Ross, 2000). Tilapia can endure a pH scope of 3.7 to 11, yet the best development rates are accomplished between pH 7 to 9 (Rebouças et al., 2016).
More than 90 % of fish in Kenya is produced under semi-intensive culture systems especially in earthen fishponds (Wanja et al., 2020). Some other fish culture systems include tanks, recirculating aquaculture systems (RAS), fish cages, and other systems (Wongkiew et al., 2018). The choice of a culture system to adopt is dictated by the available technology, finances, land, and other resources. One of the most important factors to be considered under any system is the ability of the particular system to ensure faster and efficient fish growth and performance (Ronald et al., 2014). The cost and performance of aquaculture production units depend on among other factors the type and source of fish feeds (Tippayadara et al., 2021).
Feed is the most expensive factor of production accounting for over 50% of the total cost of fish production in Kenya (Hua et al., 2019). The type of feed used depends on accessibility, cost, culture system, species under culture, and the technology available to the farmer (Workagegn et al., 2014). The Kenyan fish feed production is still not well developed and many commercial quack-like production units import most of their feeds. Some smallholder farmers have also been utilizing locally available ingredients to formulate and produce fish feeds at the farm level to help reduce the cost of production. The greatest problem associated with these locally-produced feeds is that these feeds do not supply all the nutrients required by fish (Amer et al., 2019). This is mostly because most of these single locally produced feeds are protein-inadequate and have a high amount of unrefined fiber that diminishes their absorbability and satisfactoriness, prompting low utilization by fish (Li et al., 2017). For this reason, many farmers have relied on expensive commercial diets further reducing their returns on investment. High amounts of feed are also lost due to spoilage associated with poor handling and storage at the farm or warehouse level leading to losses. Fish mortalities can also result from feeding the fish on contaminated feeds, especially those that have developed aflatoxin which has been demonstrated to cause death in fish (Kong et al., 2020; Philemon & Rashid, 2019).
A few animals and plant-based materials have been assessed as a possible protein source in the diet of tilapia. Some of the animal-based protein sources under study include hydrolyzed feathers, bone meal, blood meal, insect-based meal, etc. Of recent prominence is the use of Black Soldier Fly as a protein source in aquaculture (Tippayadara et al., 2021)). Different investigations have additionally been directed on the appraisal of plant protein in tilapia diets. Some plant sources include oilseed plants, for example, soybean, cottonseed meal, peanut, and sunflower meals. Some aquatic plants that can be used in aquaculture include, Azolla (Datta, 2011) and duckweed (Philemon & Rashid, 2019). One of the greatest challenges presented by the use of plant-based protein sources is the lack of specific fundamental amino acids required for fish growth and development. Nonetheless, mixing specific oilseed cakes and vegetable feedstuffs can give a reasonable amino profile. However, such a mix might contain inhibiting elements that either limit their utilization in compound feeds or require further handling – expanding feed creation costs (Hua et al., 2019; Kong et al., 2020). The various protein sources are also poised to have different effects on the quality of water and growth performance of fish especially based on the other nutritional elements contained in them (Augustine et al., 2020).
Water quality is an important aspect of aquaculture since it can have an impact on fish growth. The deterioration can be caused by among other factors the type and quality of fish feeds used (Danner et al., 2019). This can be linked to factors like a protein source, frequency of feeding (which might influence feed utilization), and the sinking rate of feeds among many other factors (Devic et al., 2018). Some of the growth parameters that are of importance in aquaculture and which can be affected by changes in water quality are specific growth rate (the estimated growth rate of fish per day), the feed conversion ratio (FCR) (the amount of fish feeds needed to raise a kilo of fish), and survival index (the ability of fish to grow until the end of the culture period) (Abwao et al., 2014; Rahman et al., 2017). The main stressors associated with poor water quality are low dissolved oxygen concentrations, high concentrations of TAN, and other gasses. The stress associated with poor water quality can also increase the susceptibility of the fish to disease and parasites and lead to high aquaculture mortalities (Ombwa et al., 2018). For aquaculture to be successful and continue to provide protein sources to millions of people, the highlighted challenges need to be addressed. Therefore, this study sought to generate information on the influence of fish feeds formulated with different protein sources on water quality and the growth performance of juvenile Nile tilapia reared in aquaria.
Methodology
Experimental Design
The experiment was conducted at the University of Nairobi, Department of Veterinary Pathology, Microbiology and Parasitology wet lab. Three experimental diets were formulated according to NRC (2011), containing approximately 30% crude protein and 3000 Kcal of digestible energy/kg of feed on dry matter basis. All diets were isocaloric and isonitrogenous. Three sources of protein (freshwater shrimp meal, black soldier fly, and soybean meal) were used to formulate the diets (Table 1). A commercial diet sourced from a local feed company was used as a control diet due to its preference in feed markets. Each diet was replicated 3 times in a completely randomized experimental design with 4 treatments, 3 replicates, and 30 fish per replicate. The diets were administered for a period of 10 weeks. The fish were fed at 6% of their body weight until they attained 50 g after which the feeding rate was adjusted to 3% of their body weight.
Table 1. The diet formulated for the experiment containing different proportions of ingredients (BSF = Black Soldier Fly, FSM = Freshwater Shrimp Meal, SBM = Soybean Meal).
Ingredients
Ingredient contribution (%)
BSF diet
FSM diet
SBM diet
Maize
4.0
18.4
7.4
Wheat pollard
28.3
28.3
28.3
Black Soldier Fly larvae (BSF)
45.0
0.0
0.0
Freshwater Shrimp Meal (FSM)
17.9
45.0
10.0
Soybean meal sol. Ext. (SBM)
0.0
0.0
45.0
Palm oil
2.3
5.9
6.6
DCP
1.7
1.7
1.7
L-Lysine
0.1
0.1
0.1
DL-Methionine
0.2
0.1
0.4
Salt
0.2
0.2
0.2
Vitamin-Mineral premix
0.3
0.3
0.3
Total
100
100
100
Chemical composition of diets (as fed basis)
DE (kcal/kg)
3000.0
3000.0
3000.0
DM (%)
93.1
90.4
91.2
CP (%)
30.0
30.0
30.0
CF (%)
12.2
6.4
7.2
EE (%)
6.6
8.6
8.2
Ash (%)
10.5
11.3
8.8
Digestible Carbohydrate/ NFE (%)
33.7
34.0
37.0
Ca (%)
3.8
1.2
0.7
P (%)
1.1
0.9
0.9
Na (%)
0.2
0.2
0.2
Cl (%)
0.2
0.2
0.2
Lysine (%)
2.0
2.1
2.0
Methionine (%)
0.9
0.9
0.9
Cost/kg (KSh)
115.0
133.7
128.4
Supply and maintenance of fish
Fingerlings produced from YY tilapia males weighing approximately 8-10 grams were purchased from a fish farm in Nairobi County (Paradise Fish Farm). The fish were acclimatized to experimental conditions for two weeks before the start of the trial. They were then weighed in groups of 30 fish selected at random and stocked in aquariums measuring 50cm long, 25cm wide and 45 cm high. Fish were stocked at a stocking density of 30 fingerlings per aquarium with an initial individual average weight of 8.53 ± 0.03 g. The fish were fed on their prescribed diets 3 times a day at (at 9.00 am, 1.00 pm and 5.00 pm).
Monitoring of water quality in the aquaria
Various physicochemical water parameters such as dissolved oxygen, temperature, and pH, were closely monitored in-situ using a multi-parameter meter with model number H19828 (Hanna Instruments Ltd., Chicago, USA), to ensure the parameters were within the acceptable ranges for Nile tilapia. Ammonium nitrogen determination was done using indophenol blue spectrophotometric method by use of a spectrophotometer (Varian Cary® 50 UV-Vis Spectrophotometer, Varian, Inc., USA) as described by Li et al. (2019). The values obtained were then used to calculate the total ammonia content. Continuous aeration using an electric aerator pump fitted with an appropriate filter was done in order to maintain optimal water conditions in each of the aquarium. Routine removal and cleaning of filters was done twice a day to ensure efficient functioning. The filter was routinely removed and cleaned twice daily, ensuring efficient functioning. Siphoning of solid organic waste comprising of unconsumed feed and fish excreta that settled at the bottom of each aquarium was carried out at least twice a day. To ensure proper management of water quality, approximately two-thirds of the experimental water volume was exchanged with fresh potable water thrice a week.
Data Collection
Fish were sampled randomly every 10 days and body weight (BW)g was taken using a weighing scale. The fish's total length (TL) was taken at the end of the experiment using a measuring board. The total number of fish at the end of the experiment was taken to determine the survival rate. Water quality parameters such as pH, temperature, dissolved oxygen, and total ammonia were determined using a multi-parameter meter. Total phosphorus was determined at water quality laboratory, University of Nairobi.
Evaluation of growth parameters
The effects of diets on growth were determined by evaluating a number of growth indices such as net weight gain, specific growth rate (SGR), feed conversion ratio (FCR), and survival. The following formulas were used:
Weight gain → Final mean fish weight − Initial mean fish weight

The Fulton’s condition factor (K)
The Fulton’s condition factor (K) was calculated for the fish species using the relationship described by Obuya et al. (2018): K =100W/Lb, where: K = Condition factor; W = Weight of the fish (g), L = Total length of the fish (g), and b = the value obtained from the Length-weight equation.
Data Analysis
The growth performance of Nile tilapia in aquaria was investigated in response to different types of feed treatments over a 10-weeks period. A subset of fish was randomly selected from the experiment after every 10 days for weight measurements. The weight data collected was entered in MS. Excel software where it was cleaned and organized for analysis. A repeated measure analysis of variance was conducted using SPSS (IBM SPSS 29) considering the within-subject and between-subject effects. The significance level was set at p < 0.05 to determine statistical significance. Post-hoc analysis using Turkey’s test was performed to identify different feed treatments that exhibited significant differences in growth performance. Prior to the analysis, assumptions of normality and sphericity were examined. To assess the normality assumption, the weight data for each treatment group was subjected to the Shapiro-Wilk test (p > 0.05). In addition, the assumption for sphericity was evaluated using Mauchly’s test, ensuring homogeneity of variance-covariance matrices across time points (p > 0.05).
Results
The growth parameters of O. niloticus fingerlings
The initial weight of fish was consistent and uniform across the treatment with no significant differences observed. However, as the experiment progressed, it was observed that the growth of fish was influenced by the different feeds administered. The final weights demonstrate a non-uniform growth of fish with treatment T3 exhibiting the most substantial net weight gain, indicating superior growth compared to T1, T2, and control. The control performed poorly in terms of fish growth, displaying the least net weight gained among all the treatments (Figure 1). The associated significance value on weight gain for the various feeds is extremely small (p < 0.005), indicating that the effect of the feed on the weight gain is significant. Based on the observed means T3 has a significantly higher net weight gain than T1, T2, and T4 (p < 0.05). On the other hand, T4 had the lowest net weight gain (p < 0.05). The performance of the feeds was as follows: T3>T1>T2>T4. There were significant differences in the specific growth rates (p < 0.05) with the highest growth rate reported for T3. Significant differences were also observed in FCR (p < 0.05) with the highest FCR was reported for T4 (2.787). The percentage survival rates were statistically significant across the different treatments (p < 0.05) although T2 and T3 displayed no significant differences (p > 0.05). The condition factor varied among the treatments with T3 displaying a condition factor less than 1. The multiple comparisons using the Turkey HSD have identified significant differences in the growth parameters and the various treatments as shown in table 2.
Table 2 . Growth performance Oreochromis niloticus under different protein sources
Parameter
Control
T1
T2
T3
P
Initial weight (g)
8.53a±0.011
8.51a±0.003
8.53a±0.009
8.54a±0.014
0.278
Final weight (g)
13.58d±0.01
16.48b±0.01
15.58c±0.009
17.40a±0.015
.000
Weight gain (g)
5.05d±0.006
7.97b±0.01
7.06c±0.003
8.86a±0.003
.000
SGR (% d−1)
0.85d±0.001
1.20b±0.001
1.10c±0.001
1.29a±0.002
.000
FCR
2.79a±0.006
1.76c±0.003
1.99b±0.002
1.59d±0.003
.000
Survival (%)
60.0c±1.92
75.56b±1.11
72.22b±1.11
82.22a±1.11
.000
CF
1.09a±0.01
1.02b±0.009
1.00b±0.0006
0.92c±0.008
.000
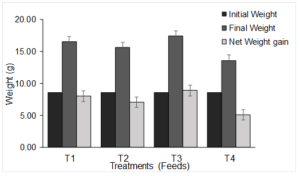
Figure 1. The mean initial weight, mean final weight and net gain in body weight of juvenile Nile tilapia (O. niloticus) fed on different feeds (T1 = Freshwater Shrimp Meal, T2 = Soybean Meal, T3 = Black Soldier Fly, and T4 = Commercial feed)
Water Quality Parameters
The effects of feed on five distinct water quality parameters, namely temperature, pH, dissolved oxygen (DO), Phosphorus, and ammonia (NH3), were also examined. There was no significant difference (p > 0.05) for pH, DO and Ammonia (NH3) and the various treatments, suggesting that the feeds did not significantly impact these parameters (Table 4). In contrast, there were significant differences (p < 0.05) observed for temperature and P suggesting that feed choice had a significant impact on these two parameters. A post-hoc Tukey HSD test revealed distinct differences in temperature, with T1 and T2 recording higher temperatures than other treatments (Figure 3). Phosphorus levels also varied significantly across treatments, with T3 displaying higher levels of P when compared to other parameters (Figure 4).
Table 3. Water quality parameters in the culture units
Parameter
Control
T1
T2
T3
P
pH
7.71a±0.05
7.53a±0.06
7.67a±0.05
7.62a±0.04
0.073
Temperature
26.50b±0.06
26.80a±0.07
26.74a±0.06
26.50b±0.09
0.002
DO
5.35a±0.06
5.25a±0.07
5.37a±0.07
5.33a±0.06
0.569
Ammonia
1.54a±0.04
1.55a±0.04
1.54a±0.04
1.50a±0.04
0.797
Phosphorus
0.14d±0.005
0.21b±0.005
0.17c±0.005
0.33a±0.05
0.000043
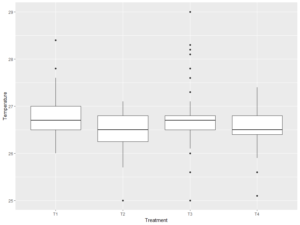
Figure 2. Boxplot showing variation in water temperature across the treatments
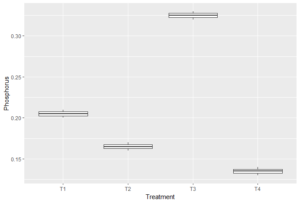
Figure 3. Boxplot showing variation in NP across the treatments
Correlation between the water quality parameters
Positive correlation existed among the parameters (Table 5). pH and temperature showed a strong positive correlation, indicating that as one increased, so did the other. A strong positive correlation was also found between NH3 and dissolved oxygen (DO) and phosphorus (P), suggesting that higher levels of NH3 corresponded to higher levels of DO and P. However, DO levels tended to decrease as temperature increased, implying a negative correlation with temperature. There was also a strong negative correlation between temperature and NH3 and P, indicating that higher temperature corresponded to lower levels of NH3 and P.
Table 4. Correlation matrix between the water quality parameters
Parameters
PH
Temperature
DO
Ammonia
Phosphorus
PH
1
0.679
0.333
0.107
-0.579
Temperature
0.679
1
-0.226
-0.656
-0.937
DO
0.333
-0.226
1
0.608
0.02
Ammonia
0.107
-0.656
0.608
1
0.692
Phosphorus
-0.579
-0.937
0.02
0.692
1
Discussion
The growth parameters for O. niloticus fingerlings varied across the different protein sources used during the experiment. The utilization of BSF larvae as a feed source reported remarkable outcomes with the fingerlings displaying the highest net weight gain, specific growth rates, and survival while also exhibiting the lowest feed conversion ratios compared to the other tested feeds. The low FCR reported demonstrates the efficient utilization of the feed for growth which could be attributed to the nutritional quality of the feed (Xu et al., 2020; Belghit et al., 2019). The higher performance of BSF larvae points to their nutritional profile, digestibility and energy content which plays a vital role in achieving optimal growth performance with minimal ecological costs (Colombo et al., 2022). Pinotti and Ottoboni (2021) reported a concentration of about 2.1–2.8 of protein and five times the lipids from the substrate to the BSF biomass through the bioconversion process suggesting its desirable nutritional quality for fish production.
Fish require protein for their growth and development, as it provides the amino acids necessary for tissue formation and repair (Tran et al., 2022). The protein source used in fish feed formulation can have an impact on their growth and performance, according to a study by (Sinaga & Mukti, 2021). Black Soldier Fly larvae are an emerging and sustainable alternative to conventional protein sources in animal and fish feeds due to their high protein content and favorable amino acid profile (Panikkar et al., 2022). Villazana, 2018 reported that tilapia fed with BSF-based diets showed comparable growth performance to those fed with fishmeal-based diets. The results highlight the potential of BSF as a partial or total replacement for fishmeal in tilapia diets in aquaculture.
The European Union has recently approved the utilization of insect-processed animal protein in aquafeeds with BSF considered the most relevant species for the production of insect-based feed for fish, poultry and pigs (Xu et al., 2020). BSF larvae contain approximately 42% protein/dry weight and lipid levels of about 28%/ dry weight making it the most suitable for use in different fish and developmental stages (Belghit et al., 2019). In aquafeeds for Jian carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss), it has been successfully demonstrated that substituting black soldier fly larvae oil for fish oil or soybean oil has no adverse effects on growth performance or intestinal morphology on fish, hence improved fish growth.
Fish fed with the FSM meal-based diet on the other hand recorded a commendable growth rate, although lower than those fed with BSF. Although shrimp meal contains a relatively high protein content, it might lack specific essential amino acids in quantities required for the growth of tilapia (Villazana, 2018). This could limit its growth-promoting potential. The presence of chitin in shrimp exoskeletons could also lower nutrient absorption and utilization in fish (Anne et al., 2022). A study by Seck et al., 2023 reported that Nile tilapia fed a diet that contained 50% fishmeal had a significantly higher growth rate than those fed a diet that contained 50% soybean meal. The fish fed the fishmeal diet had a final body weight that was 20% greater than the fish fed the soybean meal diet. This supports the findings regarding its slight inferiority as a protein source for tilapia recorded in the study.
Soybean is rich in essential amino acids, like lysine and arginine although it is typically low in methionine which promotes growth and protein synthesis in fish (Craig et al., 2017). The amino acid must thus be supplemented in the diet of feeds formulated with soybean to match the nutritional requirements of tilapia for higher feed utilization and growth (Jatta et al., 2022). In a study by (Shaw et al., 2022), tilapia fed with a diet containing 40% soybean meal recorded significantly higher growth rates compared to diets with other protein sources. This, however, does not agree with the findings of the current study as other factors might have influenced the observed growth. The digestibility of SBM might be lower than BSF, which could explain the lower growth rate observed in the present study. In addition, the processing and preparation of SBM for aquafeed plays a crucial role in its effectiveness hence poor processing and improper formulation could result in reduced nutrient bioavailability and fish growth performance (Woodgate et al., 2022). Based on these results, it is feasible therefore to do partial rather than complete replacement of fishmeal in fish feeds while also considering proper processing and handling in feed formulations.
The commercial diet recorded the least growth in the treated tilapia fingerlings. This could be attributed to many possible factors. Commercial diets are mostly formulated for a wide range of fish species, and they might not be optimized for the specific nutritional requirements of tilapia (Sinaga & Mukti, 2021). These diets can be expensive, and cost-cutting measures may lead to the inclusion of less optimal protein sources. This could affect the overall growth performance of fish. In a study by Lusiana et al., 2021, tilapia fed with a commercial diet exhibited suboptimal growth rates compared to fish fed with a species-specific formulated diet. This research highlights the importance of formulating diets tailored to the nutritional needs of each fish species under culture.
The observed condition factor (K) of 0.92 in fish that consumed Black Soldier Fly (BSF) larvae indicates a potential state of nutritional stress or insufficient feeding circumstances (Muin et al.,2022). The finding of this study, however is not consistent with other studies such as Limbu et al., 2022 who noted a higher K (K >1) in O. niloticus fed on BSF larvae. This value signifies that the fish are relatively thin or less well fed in relation to their size which may be attributed to environmental variables like the elevated phosphorus levels as observed in the experimental unit in the current study. While factors such as season, sex, type of food organism consumed, age, amount of fat reserves, and environmental conditions can influence the fish condition (Youssef et al., 2023), these factors were not accounted for in the present study. Additional investigation is necessary to fully understand the underlying factors contributing to these variations and to come up with effective approaches for enhancing the well-being and productivity of fish in relation to their nutritional consumption.
Water quality parameters, which included pH, dissolved oxygen (DO), temperature, phosphorus, and ammonium (NH4), remained within acceptable ranges for tilapia culture. The results of the study revealed no significant differences among most of the various dietary treatments. BSF however, recorded significantly higher phosphorus levels compared to the other feeds. This could be due to a phosphorus-rich diet for BSF. A wide range of organic substances, including nutrient-rich organic waste like food scraps, are known to be consumed by BSF larvae (Basri et al., 2022). If their feed contains a lot of phosphorus, the larvae could absorb and retain this nutrient and when formulated into feeds, it might release extra phosphorus into the water (Basri et al., 2022). This could in part explain the observed variations although such factors were not considered in the present study.
pH values remained stable and within the optimal range of 6.5 to 8.5, which is considered suitable for tilapia culture (Adams et al., 2019). Dissolved oxygen levels also remained at an appropriate range of 5 to 7 mg/L, which is essential for supporting aerobic respiration and preventing stress in tilapia as reported by (Adams et al., 2019). Water temperature also remained within the recommended range of 25 to 30°C, which is conducive to tilapia growth and metabolism. Phosphorus levels were managed to ensure they remained within the range of 0.05 to 0.5 mg/L, as excessive phosphorus can lead to eutrophication and water quality deterioration. Ammonium levels were maintained below 1 mg/L to prevent toxicity to fish (Su et al., 2020).
The lack of significant differences in water quality parameters between dietary treatments suggests that all tested protein sources had minimal impact on water quality when properly managed. This is crucial as stable water quality is essential for the health and growth performance of fish (Yang & Kim, 2019). The stable water quality observed in this study could be attributed to several factors that could include efficient nutrient utilization from all tested protein sources. The other possible factor could be potential biological filtration facilitated by beneficial microorganisms in the aquaculture system that helped maintain the water quality (Guttman, 2019). Since the water was also exchanged regularly, this kept the water fresh and of good quality throughout the period of the experiment. A study on different feed ingredients, including soybean meal, fishmeal, and poultry by-product meal, were compared in Nile tilapia diets, and researchers found no significant effect on water quality parameters, ensuring a suitable culture environment for tilapia (Kong et al., 2020).
Conclusion and Recommendations
This study highlights the significant impact of protein source selection on the growth performance of tilapia. BSF meal emerged as the most effective protein source for promoting tilapia growth, followed by FSM meal and SBM meal. The commercial diet, being a generic option, recorded the lowest growth rate. These results are consistent with existing literature and case studies, emphasizing the importance of formulating species-specific diets to achieve optimal growth and feed efficiency in tilapia aquaculture.
Author contributions
VO conceptualized the study, performed the experiment, analyzed and interpreted data, wrote the first draft, MN analyzed and interpreted data, JG conceived and designed the experiments, analyzed and interpreted data, PN analyzed and interpreted data and SE conceived and designed the experiments, analyzed and interpreted data.
Declaration of competing interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment statement
This work was carried out as part of the AHA project (Increased Sustainability in the Aquaculture Sector in SSA, through Improved Aquatic Animal Health Management, RAF-19/0051), which was funded by the Norwegian Agency for Development Cooperation (Norad). The research was conducted by the University of Nairobi in collaboration with WorldFish and the Norwegian Veterinary Institute.
References
- Abwao, J. O., Boera, P., Safina, M., Munguti, J. M., & Ogello, E. O. (2014). Effect of Different Artificial Diets on the Culture Potential of Labeo victorianus (Boulenger, 1909) Reared in Aquaria. International Journal of Advanced Research, 2(11), 246–252.
- Adams, T. E., Yakubu, A. F., Eke, M., Okabe, O. R., Usulor, A. F., Nwangwu, M. C., & Nwefia, E. J. (2019). Interaction of Temperature, Dissolved Oxygen and Feed Energy on Growth Performance of All Male Tilapia Fingerlings. International Journal of Forest, Animal and Fisheries Research, 3(3), 81–85. https://doi.org/10.22161/ijfaf.3.3.2
- Amer, S., Osman, A., Naif, A.-G., Shafika, E., El-Rahman, G., Elabbasy, M., Ahmed, S., & Ibrahim, R. (2019). animals The Effect of Dietary Replacement of Fish Meal with Performance, Fish Health, and Immune Status of Nile tipaia. Animals, 9(1003), 1–22.
- Anne, M., Jonathan, M., Joshua, M., kasozi Nasser, David, L., & Rekha, S. (2022). Effect of replacing freshwater shrimp meal (Caridina nilotica) protein with a mixture of plant protein on growth, apparent digestibility, and economic returns of Nile tilapia (Oreochromis niloticus L.). Journal of Agricultural Extension and Rural Development, 14(3), 140–147. https://doi.org/10.5897/jaerd2022.1328
- Augustine, O., Augustine, F., Adesina, E., & Bolanle, S. (2020). Practical Growth Performance and Nutrient Utilization of Catfish Clarias gariepinus Fed Varying SInclusion Level of Fermented Unsieved Yellow Maize. Journal of Natural Sciences Research, 43–50.
- Basri, N. E. A., Azman, N. A., Ahmad, I. K., Suja, F., Jalil, N. A. A., & Amrul, N. F. (2022). Potential applications of frass derived from black soldier fly larvae treatment of food waste: A review. Foods, 11(17), 2664.
- Belghit, I., Liland, N. S., Gjesdal, P., Biancarosa, I., Menchetti, E., Li, Y., ... & Lock, E. J. (2019). Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture, 503, 609-619.
- Cifuentes-Torres, L., Correa-Reyes, G., & Mendoza-Espinosa, L. G. (2021). Can Reclaimed Water Be Used for Sustainable Food Production in Aquaponics? Frontiers in Plant Science, 12(June), 1–10.
- Colombo, S. M., Roy, K., Mraz, J., Wan, A. H., Davies, S. J., Tibbetts, S. M., ... & Turchini, G. M. (2022). Towards achieving circularity and sustainability in feeds for farmed blue foods. Reviews in Aquaculture.
- Craig, S. R., Helfrich, L. A., Kuhn, D., & Schwarz, M. H. (2017). Understanding fish nutrition, feeds, and feeding.
- Danner, R. I., Mankasingh, U., Anamthawat-Jonsson, K., & Thorarinsdottir, R. I. (2019). Designing aquaponic production systems towards integration into greenhouse farming. Water (Switzerland), 11(10).
- Datta, S. N. (2011). Culture of Azolla and its efficacy in the diet of Labeo rohita. Aquaculture, 310(3–4), 376–379.
- Devic, E., Leschen, W., Murray, F., & Little, D. C. (2018). Growth performance, feed utilization and body composition of advanced nursing Nile tilapia (Oreochromis niloticus) fed diets containing Black Soldier Fly (Hermetia illucens) larvae meal. Aquaculture Nutrition, 24(1), 416–423.
- Elshaer, F. M., Azab, A. M., & El-Tabakh, M. A. M. (2022). Effect of Replacing Fish Meal in Fish Diet with Shrimp by-Product Meal on Growth Performance, Feed Utilization, Length-Weight Relationship and Condition Factors of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758). International Journal of Morphology, 40(1), 261–269. https://doi.org/10.4067/s0717-95022022000100261
- FAO (2014). The state of world fisheries and aquaculture. ISBN 978-92-5-108275-1. FAO Fisheries Department, Rome.
- FAO (2015). Voluntary Guidelines for Securing Sustainable Small-Scale Fisheries in the Context of Food Security and Poverty Eradication. Rome (available at www.fao.org/3/i4356en/I4356EN.pdf.
- Guttman, L. (2019). Periphyton For Biofiltration And Fish Feeding in An Integrated Multi-Trophic Aquaculture System: A Case Study in The Gulf of Aqaba. Open Access Journal of Environmental and Soil Sciences, 3(5), 413–418. https://doi.org/10.32474/oajess.2019.03.000171
- Heise, J., Müller, H., Probst, A. J., & Meckenstock, R. U. (2021). Ammonium Removal in Aquaponics Indicates Participation of Comammox nitrospira. Current Microbiology, 78(3), 894–903.
- Hua, K., Cobcroft, J. M., Cole, A., Condon, K., Jerry, D. R., Mangott, A., Praeger, C., Vucko, M. J., Zeng, C., Zenger, K., & Strugnell, J. M. (2019). The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth, 1(3), 316–329.
- Jatta, S., Fall, J., Diouf, M., Dung, L. V., & Sheen, S. S. (2022). The Effects of Substituting Soybean Meal, Wheat Flour and Cassava Flour with Groundnut Cake, Poultry By-product Meal, Brewery Waste and Rice Bran on Growth and Body Composition of Tilapia Fry. European Journal of Agriculture and Food Sciences, 4(3), 30–34. https://doi.org/10.24018/ejfood.2022.4.3.320
- Kibria, A. S. M., & Haque, M. M. (2018). Potentials of integrated multi-trophic aquaculture (IMTA) in freshwater ponds in Bangladesh. Aquaculture Reports, 11, 8–16.
- Kirema Mukasa, C. T. (2012). Regional fish trade in eastern and southern Africa Products and Markets : A Fish Traders Guide. In Smart Fish. Working Papers.
- KMFRI (2017). Kenya’s Aquaculture Brief 2017: Status, Trends, Challenges and Future Outlook. Kenya Marine and Fisheries Research Institute, Mombasa, Kenya. 12pgs.
- Kong, W., Huang, S., Yang, Z., Shi, F., Feng, Y., & Khatoon, Z. (2020). Fish Feed Quality Is a Key Factor in Impacting Aquaculture Water Environment: Evidence from Incubator Experiments. Scientific Reports, 10(1), 1–15.
- Kong, W., Huang, S., Yang, Z., Shi, F., Feng, Y., & Khatoon, Z. (2020). Fish Feed Quality Is a Key Factor in Impacting Aquaculture Water Environment: Evidence from Incubator Experiments. Scientific Reports, 10(1), 1–15. https://doi.org/10.1038/s41598-019-57063-w
- Li, Y., Yang, P., Zhang, Y., Ai, Q., Xu, W., Zhang, W., Zhang, Y., Hu, H., Liu, J., & Mai, K. (2017). Effects of dietary glycinin on the growth performance, digestion, intestinal morphology and bacterial community of juvenile turbot, Scophthalmus maximus L. Aquaculture, 479, 125–133.
- Limbu, S. M., Shoko, A. P., Ulotu, E. E., Luvanga, S. A., Munyi, F. M., John, J. O., & Opiyo, M. A. (2022). Black soldier fly (Hermetia illucens, L.) larvae meal improves growth performance, feed efficiency and economic returns of Nile tilapia (Oreochromis niloticus, L.) fry. Aquaculture, Fish and Fisheries, 2(3), 167-178.
- Lusiana, E. D., Musa, M., & Ramadhan, S. (2021). Determinants of Nile tilapia’s (Oreochromis niloticus) growth in aquaculture pond in Batu, Indonesia. Biodiversitas Journal of Biological Diversity, 22(2). https://doi.org/10.13057/biodiv/d220256
- Mbowa, S., Odokonyero, T., & Munyaho, A. T. (2017). Harnessing floating cage technology to increase fish production in Uganda. Research Series - Economic Policy Research Centre, Research S, 1–44.
- Muin, H., & Taufek, N. M. (2022). Evaluation of growth performance, feed efficiency and nutrient digestibility of red hybrid tilapia fed dietary inclusion of black soldier fly larvae (Hermetia illucens). Aquaculture and Fisheries.
- Munguti, J. M., Kim, J.-D., & Ogello, E. O. (2014). An Overview of Kenyan Aquaculture: Current Status, Challenges, and Opportunities for Future Development. Fisheries and Aquatic Sciences, 17(1), 1–11.
- Njiru, J. M., Aura, C. M., & Okechi, J. K. (2019). Cage fish culture in Lake Victoria: A boon or a disaster in waiting? Fisheries Management and Ecology, 26(5), 426–434.
- Obuya, J. A., Sigana, D. A., Wang'ondu, V., Wambiji, N., Ong'anda, H. O., & Orembo, B. (2018). Length-weight relationship of selected teleost fishes from Kilifi County, Kenya. Western Indian Ocean Journal of Marine Science, 17(1), 125-135.
- Omasaki, S. K., Charo-Karisa, H., & Kosgey, I. S. (2013). Fish production practices of smallholder farmers in western Kenya. Livestock Research for Rural Development, 25(3), 1–16.
- Ombwa, V., Aura, C., Odada, E., Ogari, Z., & Ogik, W. (2018). The socio-economic impact of Cage Culture in Lake Victoria for informed decision making. Technical Report: KMF/RS/2017/C1.8 (Ii), 8(ii), 1–48. www.kmfri.co.ke
- Orina, P. S., Charo-Karisa, H., Munguti, J. M., Boera, P., Abwao, J., Kyule, D., Opiyo, M., Marcial, H., Manyala, J., & Rasowo, J. O. (2018). A comparative study of Labeo victorianus (Bouelenger, 1901) and Oreochromis niloticus (Linnaeus, 1758) grown in polyculture systems. Lakes and Reservoirs: Research and Management, 23(1), 56–62.
- Orina, S., Ogello, E. O., Kembenya, E. M., & Muthoni, C. (2018). State of Cage Culture in Lake Victoria , KMFRI Series (Issue June 2019).
- Panikkar, P., Parakkandi, J., Khan, F., Das, B. K., Udayakumar, A., Eregowda, V. M., & Yandigeri, M. (2022). Use of black soldier fly (Hermetia illucens) prepupae reared on organic waste as feed or as an ingredient in a pellet-feed formulation for Nile tilapia (Oreochromis niloticus). Environmental Science and Pollution Research International, 29(48), 72968–72978. https://doi.org/10.1007/s11356-022-20926-3
- Philemon, N., & Rashid, T. (2019). Growth performance of Tilapia sparmanni fed on formulated chicken feeds. International Journal of Aquaculture and Fishery Sciences, 5(4), 027–031.
- Pinotti, L., & Ottoboni, M. (2021). Substrate as insect feed for bio-mass production. Journal of Insects as Food and Feed, 7(5), 585-596.
- Rahman, M. A., Habib, K. A., Hossain, A., Azad, S. O., & Rayhan, M. Z. (2017). Impacts of stocking density and economic returns on the cage culture of stinging catfish, Heteropneustes fossilis. International Journal of Fisheries and Aquatic Studies, 5(4), 198–201.
- Rebouças, V. T., Lima, F. R. dos S., Cavalcante, D. de H., & do Carmo E Sá, M. V. (2016). Reavaliação da faixa adequada de pH da água para o cultivo da tilápia do Nilo, Oreochromis niloticus L. Em águas eutróficas. Acta Scientiarum - Animal Sciences, 38(4), 361–368.
- Ronald, N., Gladys, B., & Gasper, E. (2014). The effects of stocking density on the growth and survival of Nile tilapia (Oreochromis niloticus) fry at son fish farm, Uganda. Journal of Aquaculture Research and Development, 5(2) 456-461.
- Seck, O., Fall, J., Mbaye, N. C., & Diouf, M. (2023). Effects of Substituting Soybean Meal by Unprocessed and Processed Peanut Cake Meal on the Growth, Body Composition and Survival of Tilapia (Oreochromis niloticus). Journal of Biology and Life Science, 14(2), 103. https://doi.org/10.5296/jbls.v14i2.21123
- Shaw, C., Knopf, K., & Kloas, W. (2022). Toward Feeds for Circular Multitrophic Food Production Systems: Holistically Evaluating Growth Performance and Nutrient Excretion of African Catfish Fed Fish Meal-Free Diets in Comparison to Nile Tilapia. Sustainability, 14(21), 14252. https://doi.org/10.3390/su142114252
- Sinaga, V. O. B., & Mukti, R. C. (2021). The Growth of Tilapia (Oreochromis niloticus) with the Addition of Probiotics to Feed in Sakatiga Village, Indralaya District, Ogan Ilir Regency, South Sumatera. Journal of Aquaculture and Fish Health, 11(1), 90–96. https://doi.org/10.20473/jafh.v11i1.26741
- Su, M. H., Azwar, E., Yang, Y., Sonne, C., Yek, P. N. Y., Liew, K., Cheng, C. K., Show, P. L., & Lam, S. S. (2020). Simultaneous removal of toxic ammonia and lettuce cultivation in aquaponic system using microwave pyrolysis biochar. Journal of Hazardous Materials, 396, 122610--. https://doi.org/10.1016/j.jhazmat.2020.122610
- Tippayadara, N., Dawood, M. A. O., Krutmuang, P., Hoseinifar, S. H., Doan, H. Van, & Paolucci, M. (2021). Replacement of fish meal by black soldier fly (Hermetia illucens) larvae meal: Effects on growth, haematology, and skin mucus immunity of Nile tilapia, Oreochromis niloticus. Animals, 11(1), 1–19.
- Tran, T. T. H., Lam, M. L., & Tran, L. C. T. (2022). Effects of dietary protein and lipid levels on growth, feed utilization and meat quality of clown knifefish (Chitala chitala). Can Tho University Journal of Science, 14(2), 61–72. https://doi.org/10.22144/ctu.jen.2022.016
- Villazana, J. (2018). Black Soldier Fly larvae as value-added feed for aquaculture in Maine. December 2018, 100 pp.
- Wanja, D. W., Mbuthia, P. G., Waruiru, R. M., Mwadime, J. M., Bebora, L. C., Nyaga, P. N., & Ngowi, H. A. (2020). Fish Husbandry Practices and Water Quality in Central Kenya: Potential Risk Factors for Fish Mortality and Infectious Diseases. Veterinary Medicine International, 2020.
- Wongkiew, S., Park, M. R., Chandran, K., & Khanal, S. K. (2018). Aquaponic Systems for Sustainable Resource Recovery: Linking Nitrogen Transformations to Microbial Communities. Environmental Science and Technology, 52(21), 12728–12739.
- Woodgate, S. L., Wan, A. H., Hartnett, F., Wilkinson, R. G., & Davies, S. J. (2022). The utilisation of European processed animal proteins as safe, sustainable and circular ingredients for global aquafeeds. Reviews in Aquaculture, 14(3), 1572-1596.
- Workagegn, K. B., Ababboa, E. D., Yimer, G. T., & Amare, T. A. (2014). Growth performance of the Nile tilapia (Oreochromis niloticus L.) fed different types of diets formulated from of feed ingredients. Journal of Aquaculture varieties Research and Development, 5(3), 3–6.
- Xu, X., Ji, H., Belghit, I., & Sun, J. (2020). Black soldier fly larvae as a better lipid source than yellow mealworm or silkworm oils for juvenile mirror carp (Cyprinus carpio var. specularis). Aquaculture, 527, 735453.
- Yang, T., & Kim, H. J. (2019). Nutrient management regime affects water quality, crop growth, and nitrogen use efficiency of aquaponic systems. Scientia Horticulturae, 256. https://doi.org/10.1016/J.SCIENTA.2019.108619
- Youssef, I. M., Saleh, E. S., Tawfeek, S. S., Abdel-Fadeel, A. A., Abdel-Razik, A. R. H., & Abdel-Daim, A. S. (2023). Effect of Spirulina platensis on growth, hematological, biochemical, and immunological parameters of Nile tilapia (Oreochromis niloticus). Tropical Animal Health and Production, 55(4), 275.