Publication Information
ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Effect of Seed Dressing With DA6 on Seed Vigor of Maize
Qian Yang+, Aiju Meng+, Yang Gao, Linmao Zhao, Chunqing Zhang*
State Key Laboratory of Crop Biology, Agronomy College, Shandong Agricultural University, Tai’an, Shandong Province 271018, People’s Republic of China
Received Date: September 16, 2020; Accepted Date: September 22, 2020; Published Date: October 01, 2020;
*Corresponding Author: Chunqing Zhang, State Key Laboratory of Crop Biology, Agronomy College, Shandong Agricultural University, Tai’an, Shandong Province 271018, People’s Republic of China. Email: cqzhang@sdau.edu.cn
+ Qian Yang and Aiju Meng contributed equally to this work.
Citation: Yang Q, Meng A, Gao Y, Zhao L, Zhang C (2020) Effect of Seed Dressing With DA6 on Seed Vigor of Maize. Adv in Nutri and Food Sci: ANAFS-195.
Abstract
Most of DA-6 were mainly foliar spraying in production, but there were few applications that directly affected the seeds themselves. The effect of DA-6 seed treatment on seed vigor was studied with maize seeds with different vigor. The results showed that 50mg/L DA-6 solution had the best effect, 10% -26% improved seed vigor index, 30%, and 23% increased root vigor of seedling, respectively, and 7.9%, 11.8% and 6.1% improved seed vigor index under drought, salt and low-temperature stress. Therefore, the resistance of maize seeds was improved by using DA-6.
Keywords: Adversity Resistance; DA-6; Enzyme Activity; Seed Dressing; Seed Vigor
Abbreviations:
DA6
Diethyl aminoethyl hexanoate
EDTA
Ethylenediaminetetraacetic acid
SOD
Superoxide Dismutase
POD
Peroxidase
CAT
Catalase
SPSS
Statistical Product and Service Solutions
Background
A Seed is an important basis of agricultural production, and seed quality is the key to the healthy and stable development of agriculture. Seed vigor is an important index used to express seed quality, which is closely related to physiological and biochemical processes such as seed germination, growth, development, storage life, and aging. The seeds with high vigor have neat and rapid emergence, complete seedlings, good resistance to bad environment, high tolerance to storage and crop yield, while low vigor seeds are uneven in emergence, sensitive to bad environment, and waste a lot of human, material and financial resources, which have a serious impact on agricultural production [1,2]. Seed treatment is and advance measure for seed sowing before sowing, and it is an important means to improve the quality, quantity and commodity of seed sowing [3]. The common seed treatment methods are the physical method, chemical method, biological method, and seed coating pelletizing. Seed treatment can improve seed vigor, stimulate seed germination, promote seedling growth, and even facilitate mechanized sowing and seed consumption, reduce agricultural investment costs and increase crop yield.
Seed germination is one of the most important stages of the plant life cycle, contributing to the distribution of wild species, and to increased yield and quality of cultivated crop plants [4-8]. Generally, the emergence of the radicle indicates the completion of seed germination [7, 9]. Immediately following seed germination, seedling establishment is another key developmental stage, whereby the seedling transitions from the heterotrophic to the autotrophic state [10-14]. Consequently, both seed germination and seedling establishment are essential for subsequent plant development. It is worth noting that both processes are powered by the energy that is stored in the seed itself [10-12, 15].
Diethyl aminoethyl hexanoate (DA-6) is a novel artificial plant growth regulator, which can increase leaf chlorophyll content, and increase the photosynthetic rate and the rates of carbon and oxygen metabolism in plants [16-18]. In agriculture, DA-6 has been registered for use on a range of crops, including cabbage, pakchoi, cotton, tomato, soybean, peanut, and maize [18]. Furthermore, DA-6 also increased microalgal growth and simultaneously improved the quality and quantity of microalgal lipid for biodiesel production [19, 20], while treatment with DA-6, in combination with EDTA, appeared to be optimal for the remediation efficiency of Lolium perenne L. (perennial ryegrass) on lead-contaminated soil [21]. DA-6 also plays important roles in improving the defense response of plants under diverse environmental stresses, such as salinity stress, chilling stress and heavy metal stress [22-24]. DA-6 can be used to alleviate salinity stress by inducing the advantageous effects of salinity tolerance and decreasing oxidative damage [25].
In the present study, most of the DA-6 is sprayed on the surface of the plant in a certain stage of the growth and development of the plant, or the compound is sprayed with other agents, and is directly applied to the research of the seeds. In this study, the effect of DA-6 on seed vigor was studied, and the physiological mechanism of seed vigor was explored. Prepare medicament with DA-6 concentration of 50 mg / L, 150 mg / L, 250 mg / L and 350 mg / L respectively, mix seeds according to the ratio of seed to drug of 1:75, and then carry out germination test according to the requirements of standard germination test. The aim of this study was to provide a theoretical basis for improving the vigor of the seed.
Materials and Methods
Test Materials
The corn seeds with water content between 11.0% and 12.5%, i.e. Huamei 3 with a germination rate of about 95%, Huamei 2 with germination a rate of about 90%, Zhengdan 958 with a germination rate of about 80%, and Tiantai 33 with a germination rate of about 90%.
Test Method
Evaluation of Seedling and Seed Vigor
Standard germination tests were conducted according to the International Seed Testing Association with minor modifications [26]. Germination boxes (17cm length, 11cm width and 7cm height) were used in this study. Sprouting bed consisted of fine silica sand with a diameter of 0.05 to 0.8 mm. In per germination box, base sand was consisted of 4cm height silica sand with 60% saturation moisture content [27]. Randomly selected 50 maize seeds were sowed in the surface of base sand, and then the seed were covered with 2 cm height silica sand with 60% saturation moisture content. All germination boxes were placed in a growth chamber at 25±1 °C for maize, 70% relative humidity, illumination conditions of 4000 lux and 24 h light photoperiod for seven days maize. Seed germination potential was calculated as the number of seeds germinating normally within the first 4 days/number of seeds tested for germination × 100. Germination rate was calculated as the number of seeds germinating normally within the first 4 days/number of seeds tested for germination ×100. Germination index (GI) was calculated according to the formula GI = ΣGt /At, where Gt is the number of seeds germinated after 10 days and At is the number of days. Vigour index (VI) was calculated according to the formula VI = GI ×S, where S is seedling dry weight.
Measurements of Seedling Dry Weight, Seedling Root Length and Seedling Length.
The root length and seedling length of 15 plants were measured randomly from each repeated normal seedling. Seedling dry weight was eaten in an air oven at 105 °C for 30 min, and then dried to constant weight at 80 °C.
DA-6 Seed Dressing Treatment
Prepare medicament with DA-6 concentration of 50 mg / L, 150 mg / L, 250 mg / L and 350 mg / L respectively, mix seeds according to the ratio of seed to drug of 1:75, and then carry out germination test according to the requirements of standard germination test.
Dealing with Adversity
Drought Treatment: After seed dressing treatment, the sand bed water content is 30% of the saturated water content of the sand. For example, if the saturated water content of one kilogram of sand is 300ml, when one kilogram of sand is used in the sand bed, the water demand of the dry sand bed is 90ml, and the water demand is maintained until the end of germination.
Salt Treatment: After seed dressing, the germination test was carried out with NaCl solution of 180 mmol / L instead of water.
Low Temperature Treatment: After seed dressing, the germination experiment was carried out in a low temperature incubator with 13 ℃ and 12 hours of light and 12 hours of darkness for 14 days.
Determination of Related Enzyme Activity
The determination of dehydrogenases in 24 h seed germination by reference to the improved TTC. The activity of α - amylase and amylase in seeds germinated for 0, 24, 48, 60 and 72 hours was determined by 3,5-Dinitrosalicylic acid method [28].The activities of SOD, POD and CAT were determined according to the research of cakmak [29]. NBT photochemical reduction method, guaiacol method and UV absorption method, respectively. All of the above measurements were repeated three times.
Statistical Analysis
Correlation analysis and regression analysis were performed using SPSS 19.0 software (SPSS, Inc., Chicago, USA).
Results
Effect of DA-6 Seed Dressing on Seed Vigor
Comparison of Seed Vigor under Different Concentrations of Treatment
For Zhengdan 958, about 80% of the germination rate, the Huamei2 and Tiantai 33,95% Huamei 3 of the left and right are respectively 50 mg/L,150 mg/L,250 mg/L and 350 mg/L DA-6. It was found that the seed vigor index of the four varieties was higher than that of other treatments at the concentration of 50 mg/L. Compared with the control, the germination percentage, germination potential, germination index and vigor index of Zhengdan 958 seeds increased by 8.7%, 9.9%, 8.9% and 21.8%, respectively, and the germination percentage, germination potential, germination index and vigor index of Tiantai 33 seeds increased by 4.3%, 2.7%, 4.0% and 10.1%, respectively. Compared with the control, the germination percentage, germination potential, germination index and vigor index of Huamei 2 increased by 4.4%, 4.4%, 4.4% and 17.4%, respectively(Table 1,Table 2). Compared with the control, the germination percentage, germination potential and germination index of Huamei 3 were lower and no significant difference, but the vigor index increased by 26.3%. When 50 mg/L DA-6 seed dressing, the germination percentage of Huamei 2, Huamei 3 and Tiantai 33 with higher germination rate were not significantly different from those of the control, but the germination potential, germination index and vigor index were significantly different from those of the control. The germination rate and each index of Zhengdan 958 with low germination rate were significantly different from those of the control. When the seed dressing concentration was more than 50 mg/L, the seed vigor parameters decreased with the increase of seed dressing concentration, and the seed vigor index decreased by 12.2%.
Table1: Effect of DA6 seed dressing on seed germination rate and germination potential. We used four DA-6 concentrations: 0 mg/L, 50 mg/L, 150 mg/L, 250mg/Land 350 mg/L.
Seed dressing concentration mg/L
GR%
GP%
Zhengdan958
Tiantai33
Huamei2
Huamei3
Zhengdan958
Tiantai33
Huamei2
Huamei3
0
84.0b±2.292
92.0a±1.633
90.0a±0
95.0a±1.243
81.3b±2.024
92.0ab±1.633
90.0ab±0
95.0a±1.243
50
91.3a±3.055
96.0a±2.309
94.0a±2.828
98.0a±0
89.3a±2.309
94.5a±1.000
94.0a±2.828
97.0a±1.414
150
86.7ab±3.033
90.0a±1.633
90.0a±1.657
93.0a±1.414
83.3b±3.018
90.0b±1.633
90.0ab±1.657
93.0ab±1.414
250
85.3b±3.203
91.0a±4.163
87.0a±1.243
93.0a±2.071
74.7c±2.309
91.0ab±3.163
87.0b±1.243
92.0ab±2.657
350
84.7b±1.155
89.0a±2.582
86.0a±2.828
89.0a±1.414
74.7c±2.163
89.0b±2.852
86.0b±2.828
89.0b±1.414
Seed dressing concentration mg/L
GI
VI
Zhengdan958
Tiantai33
Huamei2
Huamei3
Zhengdan958
Tiantai33
Huamei2
Huamei3
0
10.43ab±0.301
11.50ab±0.204
11.25ab±0
11.88a±0.303
0.577b±0.036
0.566b±0.027
0.547b±0.021
0.861b±0.017
50
11.37a±0.351
11.96a±0.246
11.75a±0.354
12.23a±0.035
0.703a±0.032
0.623a±0.048
0.642a±0.030
1.087a±0.016
150
10.75ab±0.326
11.25b±0.204
11.25ab±0.407
11.63ab±0.177
0.658ab±0.029
0.522bc±0.035
0.609ab±0.031
0.892b±0.022
250
10.40b±0.557
11.38b±0.520
10.88b±0.530
11.60ab±0.485
0.614b±0.034
0.511c±0.032
0.572b±0.039
0.890b±0.010
350
10.33b±0.0289
11.13b±0.323
10.75b±0.354
11.13b±0.177
0.596b±0.023
0.509c±0.022
0.56b±0.023
0.756c±0.018
*Means with the same letter are not significantly different according to Fisher’s LSD test at P ≤ 0.05. GR: Germination rate, GP: Germination potential, GI: Germination index, VI: Vitality index.
Comparison of Morphological Indexes of Seedling under Different Concentration Treatments
Changes of Seedling Morphology Indexes of four kinds of Maize Seeds with different germination percentage treated with DA-6 concentration of 50mg/L, 150mg/L, 250mg/L and 350mg/L (Table 3). The results showed that when the seed concentration was 50 mg/L, the dry weight per plant, seedling length and root length of Zhengdan 958 were 11.7%, 2.0% and 29.6% higher than those of the control, respectively, and the dry weight, seedling length and root length of Tiantai 33 high vigor seeds were 5.8%, 8.7% and 9.9% higher than those of the control, respectively. Compared with the control, the dry weight, seedling length and root length of Huamei 2 increased by 12.3%, 7.0% and 23.4%, respectively, and the dry weight, seedling length, root length and control of Huamei 3 increased by 22.7%, 9.1% and 24.9%, respectively. When the concentration of seed dressing was higher than 50 mg/L, the morphological parameters of seedlings began to decrease with the increase of treatment concentration, and when the concentration was 350mg/L, the difference reached significant difference, which was the most obvious in dry weight and root length per plant, and the highest decrease range was 10% compared with the control. Among the morphological indexes of the four varieties, the root length and dry weight per plant increased the most, which indicated that DA-6 seed dressing mainly promoted the growth and development of roots, was beneficial to the absorption and transport of nutrients, and increased the dry matter quality of seedlings.
Table 3: The comparisons with plant dry weight, root length, seedling length under different concentration. We used four DA-6 concentrations: 0 mg/L, 50 mg/L, 150 mg/L, 250mg/Land 350 mg/L.
Dry weight of plant
Seedling length
Root length
Seed dressing concentration mg/L
Zhengdan958
Tiantai33
Huamei2
Huamei3
Zhengdan958
Tiantai33
Huamei2
Huamei3
Zhengdan958
Tiantai33
Huamei2
Huamei3
0
0.055b±0.002
0.049ab±0.002
0.049b±0.002
0.072b±0.000
10.3a±0.437
9.7a±0.052
12.9a±0.589
10.8ab±0.028
9.8c±0.316
12.0ab±0.428
10.9b±0.240
12.2b±0.048
50
0.062a±0.002
0.052a±0.003
0.055a±0.003
0.089a±0.002
10.5a±0.343
10.2a±0.332
13.8a±0.231
11.8a±0.453
12.7a±0.580
12.9a±0.306
13.4a±0.317
15.2a±0.255
150
0.061a±0.002
0.046bc±0.002
0.054a±0.003
0.077b±0.001
10.5a±0.353
10.0a±0.169
12.8a±0.539
11.4a±0.651
11.4b±0.398
11.9ab±0.589
11.9ab±0.547
11.6bc±0.516
250
0.059ab±0.002
0.045bc±0.003
0.053a±0.001
0.077b±0.001
10.5a±0.210
9.9a±0.333
12.7a±0.566
11.0a±0.448
11.5b±0.247
11.7ab±0.392
11.0b±0.230
10.8c±0.433
350
0.058ab±0.002
0.046bc±0.001
0.052a±0.004
0.068bc±0.003
10.5a±0.284
9.9a±0.643
12.0a±0.341
10.5ab±0.462
11.2b±0.583
10.7b±0.688
10.6b±0.410
10.9c±0.243
*Means with the same letter are not significantly different according to Fisher’s LSD test at P ≤ 0.05.
Effect of DA6 Seed Dressing on Improving the Resistance of Corn Seedlings
After the seeds with germination rate of about 90% were mixed with 50mg/L DA-6, the germination experiments were carried out under drought, salt and low temperature stress respectively. The results showed that compared with the control, only the vigor index of drought stress reached significant difference and increased by 7.9%, and the germination potential and vigor index of salt stress increased by 57.1% and 11.8%, respectively, and there were significant differences (Fig 1). The germination potential and vigor index of low temperature stress were increased by 3.67 times and 6.1%, respectively. The morphological changes of seedlings under each stress were as follows: compared with the control, the dry weight per plant under drought stress and salt stress increased by 7.2%, 13.2%, seedling length and root length did not reach significant difference, and the dry weight per plant, seedling length and root length under low temperature stress increased by 5.0%, 13.0% and 7.4%, respectively. The results showed that 50mg/L DA-6 seed dressing could effectively improve the stress resistance of seeds. (Fig 2)
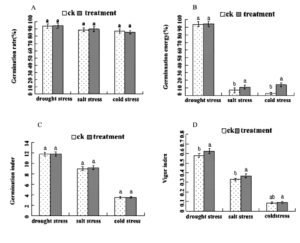
Figure 1: Comparison of germination rate of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing (A) Comparison of germination energy of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing (B).Comparison of germination inder of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing(C).Comparison of vigor inder of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing (D).
 Figure 2: Comparison of plant dry weight of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing(A) Comparison of seeding length of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing(B).Comparison of root length of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing(C).
Figure 2: Comparison of plant dry weight of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing(A) Comparison of seeding length of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing(B).Comparison of root length of Tiantai 33 under different adversities after 50mg/L DA6 seed dressing(C).
Effect of DA-6 Seed Dressing on Physiological Indexes
Effect of DA-6 Seed Dressing on α-amylase Activity
The seeds of Tiantai 33 with about 90% germination rate were treated with 50mg/L DA-6 seed dressing for 0 h, 24 h, 48 h, 60 h and 72 h. The changes of α-amylase activity in these five periods were determined (Fig 3). Compared with the control, the enzyme activity at 60h and 72h increased by 27.5%, 13.0%, 3.1% and 0.7%, respectively, at 24h, 48h, and 72h, respectively, and the enzyme activity increased by 27.5%, 13.0%, 3.1% and 0.7%, respectively.
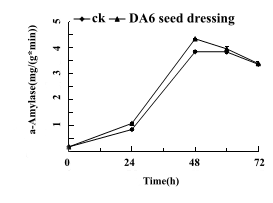
Figure 3: Seed dressing treatment of Tiantai 33 "seed" with about 90% germination rate in 50mg/L DA-6, The activity of α-amylase was measured at 0 h, 24 h, 48 h, 60 h and 72 h after germination.
Effect of DA-6 Seed Dressing on Total Amylase Activity
The changes of total amylase activity in the five periods of germination 0h, 24h, 48h, 60h and 72h are shown (Fig 4). Analysis of the whole time period of seed germination found that the enzyme activity of seed-mixing increased by 32.7%, 0.6%, 2.4% and 9.0%, respectively, compared with the control.
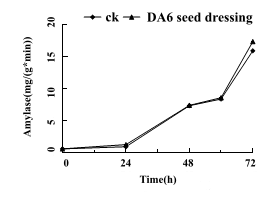
Figure 4: Seed dressing treatment of Tiantai 33 "seed" with about 90% germination rate in 50mg/L DA-6, The changes of total amylase activity were measured at 0 h, 24 h, 48 h, 60 h and 72 h after germination.
Effect of Seed Dressing of DA-6 on the Activity of Dehydrogenase.
The results showed that the activity of seed dressing was increased by 5.0%, the activity of dehydrogenase was increased by DA-6 treatment, the germination of seed embryos was promoted, and the activation of physiological and biochemical processes in the seeds was facilitated(Fig 5).
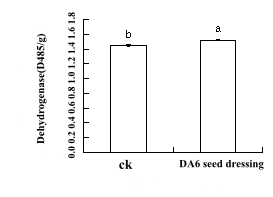
Figure 5: Seed dressing treatment of Tiantai 33 "seed" with about 90% germination rate in 50mg/L DA-6, Determination of dehydrogenase activity in 24 hours of germination.
Effect of Seed Dressing of DA-6 on SOD Activity
The SOD activity of Tiantai 33 high-activity corn seeds with 0h, 24h, 48h, 60h and 72h of germination were determined. The results are shown in (Fig 6). The enzyme activity of the seed mixing treatment increased by 7.7%, 3.1%, 5.2%and 2.3%compared with the control.
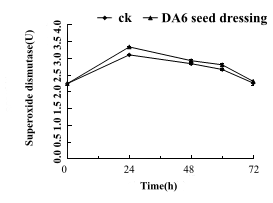
Figure 6: Seed dressing treatment of Tiantai 33 "seed" with about 90% germination rate in 50mg/L DA-6, The changes of SOD were measured at 0 h, 24 h, 48 h, 60 h and 72 h after germination.
Effect of DA-6 Seed Dressing on POD Activity
The results of POD activity of the high-energy corn seeds of Tiantai 33 at 0h, 24h, 48h, 60h and 72h were measured, and the results are shown (Fig 7), where A shows the difference between the POD activity and the POD activity at the time of the 2 min reading in the determination of the activity of the POD, and can be recorded as the activity of the POD A4701; B. The difference between the POD activity and the POD activity at the time of the 3-minute reading in the measurement process can be recorded as the difference of the activity of the POD at the time of 2 min. The enzyme activity of high activity seeds in figure A was the same as that in chart B. the enzyme activity of high activity seeds in figure A was increased by 2.2%, 9.2%, 12.8% and 15.9% respectively at 48 h, 60 h and 72 h compared with the control, respectively. In figure B, the enzyme activity increased by 18.0%, 11.1%, 17.4% and 25.0%, respectively, at 48 h, 60 h and 72 h compared with the control, and the enzyme activity at 60 h and 72 h was 18.0%, 11.1%, 17.4% and 25.0%, respectively, compared with the control at 24 h, 11.1%, 17.4% and 25.0%, respectively. DA-6 treatment can increase the activity of POD enzyme in seeds, reduce the effect of tissue aging on seed germination, and assist SOD and CAT to protect the membrane system.

Figure 7: Tiantai 33 reacted to the value of POD enzyme activity within 1 min to 2 min at different time after 50mg/L DA6 seed dressing(A).Tiantai 33 reacted to the value of POD enzyme activity within 2 min to 3 min at different time after 50mg/L DA6 seed dressing(B).
Effect of Seed Dressing of DA-6 on CAT Activity
The CAT activity of Tiantai 33 high vigor corn seeds germinated for 0h, 24h, 48h, 60h and 72h was determined (Fig 8). Where A denotes the difference between CAT activity at 2 min reading and CAT activity at 1 min during the determination of POD activity, it can be recorded as Δ A2401 ≤ B indicating the difference between CAT activity at 3 min reading and 2 min CAT activity, and Δ A2402. The enzyme activities of high activity seeds in figure A and B were basically the same. The enzyme activities of 60 h and 72 h in figure A were 7.6%, 2.7%, 1.3% and 17.3%, respectively, compared with the control at 24 h, 14.1%, 18.8% and 12.5%, respectively, at 48 h, 14.1%, 18.8% and 12.5%, respectively, at 48 h, 14.1%, 18.8% and 12.5%, respectively, compared with the control at 24 h, 60 h and 72 h after seed dressing in fig. B, the enzyme activity at 60h and 72h was 8.7%, 14.1%, 18.8% and 12.5% higher than that of the control, respectively. The activities of SOD, POD and CAT enzymes were increased after DA-6 treatment compared with the control, which indicated that the treatment reduced the accumulation of peroxide, oxygen free radicals and other substances, thus using seed germination.
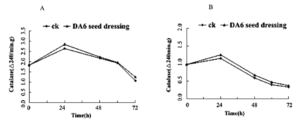
Figure 8: Tiantai 33 reacted to the value of CAT enzyme activity within 1 min to 2 min at different time after 50mg/L DA6 seed dressing(A).Tiantai 33 reacted to the value of CAT enzyme activity within 2 min to 3 min at different time after 50mg/L DA6 seed dressing(B).
Discussion
Effect of DA6 Seed Dressing on Improving Corn Seed Vigor
Studies have shown that DA-6 treatment can improve the quality and yield of crops, mainly because the treatment promotes chlorophyll synthesis, improves the leaf color value, net photosynthetic rate, stomatal conductance of seedlings, and enzymes related to photosynthesis Activity, protective enzymes and metabolic enzyme activities improve plant root activity, plant dry weight and nitrogen metabolism [30-32].
DA-6 is an artificial plant growth regulator with multiple biological functions that can affect the production of field crops [17-21].This study extends the practical use of DA-6 and proves that DA-6 can reverse the germination loss and seedling establishment activities associated with corn seed aging. Seed germination and subsequent seedling growth are important steps in the plant life cycle, helping to determine plant distribution in wild species and crop yield. Studies have shown that with the increase of seed age, the decline of seed germination capacity is positively correlated with the length of storage period [33-35]. In tillage agriculture, farm seeds preserved by farmers are usually stored under less than ideal conditions for different periods of time, resulting in seed aging and reduced germination characteristics. Therefore, in the basis and application of plant biology and crop production research, enhancing germination and seedling growth of aging seeds is worth exploring. Soaking seeds with appropriate concentration DA-6 can significantly improve the germination potential and vigor index of rice seeds, greatly improve the vigor of rice seedlings, and have a great effect on further improving the uniformity of rice field emergence and increasing rice yield [36]. In this test, the alpha-amylase and total amylase activities of corn seeds after DA-6 seed dressing were higher than that of the control group, indicating that the activity of amylase after treatment increased, which can accelerate the conversion of endosperm material and promote Seedlings grow early and grow quickly, laying a solid foundation for the further cultivation of strong corn seedlings.
Effect of DA6 Seed Dressing on Improving the Resistance of Corn Seedlings
The damage to maize seedlings caused by adversity is widespread in China, which seriously affects the production of maize. After maize is hurt by adversity again, the survival rate of seedlings is one of the most basic indicators to measure the resistance of maize population. The results of this experiment showed that the survival rate of corn seedlings seeded with the appropriate concentration of DA -6 after being damaged by adversity was higher than that of the control, indicating that DA -6 can enhance the resistance of the corn population, which is related to the resistance of cytokinin The impact of sex is consistent.
SOD and CAT are important membrane protective enzymes in plants. SOD catalyzes the disproportionation reaction of superoxy groups to produce H2O2 and O2, and CAT can scavenge H2O2, so they can reduce the accumulation of free radicals caused by low temperature, defend the process of lipid peroxidation, and reduce the accumulation of lipid peroxidation product MDA, The membrane plays a protective role. This experiment shows that the appropriate concentration of DA-6 seed dressing can increase the activity of SOD, POD and CAT of corn seedlings and reduce the accumulation of MDA. Leshen [37] and Pauls [38] pointed out that cytokinins can directly or indirectly scavenge free radicals and reduce lipid peroxidation. It can be seen that the anti-stress effect of DA -6 may be related to the promotion of SOD and CAT activity. Relevant, cytokinins can inhibit the reduction of photosynthetic pigment content [39], improve Rubisco activity and photosynthetic efficiency [40], thereby improving the resistance of corn. In addition, DA-6 also has the effect of improving the vitality of the maize seedling root system, so that the maize seedling can still absorb a certain amount of nutrients under adversity and maintain the normal metabolism.
This study showed that under stress, the seed vigor after the appropriate concentration of DA6 after seed dressing increased by 7.9%, 11.8% and 6.1% under drought stress, salt stress and low temperature stress respectively, indicating that the seed dressing treatment can be effective Improve seed resistance.
Conclusion
The ratio of 50mg/L DA-6 solution to the seed dressing ratio of 1:75 is the best. The results showed that the optimum concentration of DA-6 seed dressing can improve the living force of the seed in the adversity, the increase of the vigor of the seed is mainly reflected in the vigor index, the dry weight of the single plant and the root length of the seedling form index are obviously increased in both treatments, and the DA-6 treatment improves the vitality of the root system of the seedling, the method is beneficial to the increase of the quality of the seedling, and the related metabolic enzymes (enzyme-amylase, amylase and dehydrogenase) and the protective enzyme (SOD, POD and CAT) of the high-activity corn seeds are carried out in different time periods, and all the measured enzyme activity of the treatment is higher than that of the control treatment, The DA-6 treatment improves the decomposition and utilization rate of the material in the seed, enhances the metabolism capacity of the seeds, promotes the germination of the embryo, reduces the accumulation of the active oxygen or the oxide, and is beneficial to the germination of the seeds.
Acknowledgements: We thank the funding support.
Authors’ Contributions
QY, AM and CZ conceived the study. QY, AM, YG and LZ performed the experiments and analysed the results. QY, AM and CZ wrote the manuscript, with feedback from all authors. All authors have read and approved the manuscript.
Funding
This work was supported by the National Key Research and Development Plan of China (number 2018YFD0100900), SDAIT-02-02 and SYL2017YSTD02. The funding bodies were not involved in the design of the study, collection, analysis, interpretation of data, and in writing the manuscript.
References
- Sun Q, Wang J, Sun B (2007) Advances on seed vigor physiological and genetic mechanisms. Chinese Agricultural Sciences 40:48-53.
- Vanstraelen M, Benková E (2012) Hormonal interactions in the regulation of plant development. Annual Review of Cell and Developmental Biology 28:463-487.
- Liangang M, Dongdong Y, Zhuanfang W, Taotao M, Qiuxia W et al. (2013) Research progress of seed treatment technology. Chinese vegetables 1:9-15.
- Shu K, Zhang H, Wang S, Chen M, Wu Y et al. (2013) ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in arabidopsis. PLoS Genetics 9:e1003577.
- Shu K, Meng YJ, Shuai HW, Liu WG, Du JB et al. (2015) Dormancy and germination: how does the crop seed decide?Plant Biology 17:1104-1112.
- Shu K, Chen Q, Wu Y, Liu R, Zhang H et al. (2016a) ABSCISIC ACID-INSENSITIVE 4 negatively regulates flowering through directly promoting Arabidopsis FLOWERING LOCUS C Journal of Experimental Botany 67:195-205.
- Shu K, Liu XD, Xie Q, He ZH (2016b) Two faces of one seed: hormonal regulation of dormancy and germination. Molecular Plant9:34-
- Rubio de Casas R, Willis CG, Pearse WD, Baskin CC, Baskin JM et al. (2017) Global biogeography of seed dormancy is determined by seasonality and seed size: a case study in the legumes. New Phytologist 214:1527-
- Bewley JD (1997) Seed germination and dormancy. The Plant Cell 9:1055-1066.
- Eastmond PJ (2006) SUGAR-DEPENDENT1encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. The Plant Cell 18:665-675.
- Quettier AL, Shaw E, Eastmond PJ (2008) SUGAR-DEPENDENT6encodes a mitochondrial flavin adenine dinucleotide-dependent glycerol-3-P dehydrogenase, which is required for glycerol catabolism and post germinative seedling growth in Arabidopsis. Plant Physiology 148:519-528.
- Chen M, Thelen JJ (2010) The plastid isoform of triose phosphate isomerase is required for the postgerminative transition from heterotrophic to autotrophic growth in Arabidopsis. The Plant Cell 22:77-90.
- Theodoulou FL, Eastmond PJ (2012) Seed storage oil catabolism: a story of give and take. Current Opinion in Plant Biology 15:322-
- Eastmond PJ, Astley HM, Parsley K, Aubry S, Williams BP et al. (2015) Arabidopsisuses two gluconeogenic gateways for organic acids to fuel seedling establishment. Nature Communications 6:6659.
- Eastmond PJ (2004) Glycerol-insensitive Arabidopsis mutants: gli1 seedlings lack glycerol kinase, accumulate glycerol and are more resistant to abiotic stress. The Plant Journal 37:617-
- Yokoyama H, Hsu WJ, Poling S, Hayman E (1982) Bioregulation of pigment biosynthesis by onium compounds. ACS Symposium Series181:153-173.
- Zhang H, Xie L, Xu P, Jiang S (2008) Dissipation of the plant growth regulator hexanoic acid 2-(diethylamino) ethyl ester in pakchoi and soil. International Journal of Environmental Analytical Chemistry 88:561-569.
- Jiang Y, Jiang Y, He S, Zhang H, Pan C (2012) Dissipation of diethyl aminoethyl hexanoate (DA-6) residues in pakchoi, cotton crops and soil. Bulletin of Environmental Contamination and Toxicology 88:533-
- Salama ES, Kabra AN, Ji MK, Kim JR, Min B et al. (2014)Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresource Technology 172:97-
- Jiang L, Pei H, Hu W, Han F, Zhang L et al. (2015) Effect of diethyl aminoethyl hexanoate on the accumulation of high-value biocompounds produced by two novel isolated microalgae. Bioresource Technology 197:178-
- He S, Wu Q, He Z (2013) Effect of DA-6 and EDTA alone or in combination on uptake, subcellular distribution and chemical form of Pb in Lolium perenne. Chemosphere 93:2782-2788.
- Fu XJ, Maimaiti AS, Mou HM, Yang Q, Liu GJ (2011) Hexanoic acid 2-(diethylamino)ethyl ester enhances chilling tolerance in strawberry seedlings by impact on photosynthesis and antioxidants. Biol Plantarum 55:793-796.
- He S, Wu Q, He Z (2014) Synergetic effects of DA-6/GA3 with EDTA on plant growth, extraction and detoxification of cd by Lolium perenne. 117:132-138.
- Li Z, Zhang R, Zhang H (2018) Effects of plant growth regulators (DA-6 and 6-BA) and EDDS chelator on phytoextraction and detoxification of cadmium by Amaranthus hybridus Linn. Int J Phytoremediat. 20:1121-1128.
- Zhang C, He P, Li Y, Li Y, Yao H et al. (2016) Exogenous diethyl aminoethyl hexanoate, a plant growth regulator, highly improved the salinity tolerance of important medicinal plant Cassia obtusifolia L. J Plant Growth Regul 35:330-344.
- ISTA (ed.) (2010) International rules for seed testing. (ISTA, Switzerland).
- Wen D, Hou H, Meng A, Meng J, Xie L,Zhang C (2018) Rapid evaluation of seed vigor by the absolute content of protein in seed within the same crop. Scientific Reports.
- Zhang C, Jianhua Wang J (2006) Seed testing. Seed germination test, 4rd edn. Chinese.
- Cakmak I, Strbac D, Marschner H (1993) Activities of Hydrogen Peroxide-Scavenging Enzymes in Germinating Wheat Seeds. Journal of Experimental Botany, 44:127-132.
- Han D, Hu H, Wu X, Duan J, Wang D (2012) Regulation effects of plant growth regulators on the nitrogen metabolism of Spinacia oleracea L.by soil drench application. Guangdong Agricultural Sciences 39:49-51.
- Yang Q, Ai S, Ti M, Wang Z (2012) Effects of DA-6 on chlorophyll biosythesis pathway in peach leaves.Journal of Horticulture 39:621-628.
- Yin X, He D, Gupta R, Yang P (2015) Physiological and proteomic analyses on artificially aged Brassica napusFrontiers in Plant Science 6:112.
- Yin G, Xin X, Song C, Chen X, Zhang J et al. (2014) Activity levels and expression of antioxidant enzymes in the ascorbate-glutathione cycle in artificially aged rice seed. Plant Physiology and Biochemistry 80:1-
- Nguyen TP, Cueff G, Hegedus DD, Rajjou L, Bentsink L (2015) A role for seed storage proteins in Arabidopsisseed longevity. Journal of Experimental Botany 66:6399-6413.
- Fleming MB, Richards CM, Walters C (2017) Decline in RNA integrity of dry-stored soybean seeds correlates with loss of germination potential. Journal of Experimental Botany 68:2219-2230.
- Zilong Zhang and Yang Liang.2001. Effect of DA-6 on Rice Seed Germination and Seedling Growth.Journal of Southwest University (Natural Science Edition), 23(3).
- Leshem YY, Wurzburger J, Grossman S, Frimer AA (1981) Cytokinin interaction with free radical metabolism and senescence: Effects on endogenous lipoxygenase and purine oxidation. Physiologia Plantarum 53:9-12.
- Pauls KP, Thompson JE (1981) Effects of cytokinins and antioxidants on the susceptibility of membranes to ozone damage. Pant Cell Physiol 23:821-832.
- Liao XR, He PC, Zhu XC (1997) Effect of zeatin on H202 scavenging system of Vitis vulpina leaf disks under salt stress.Acta Botanica Sinica.39:641-646.
- Chernyad'ev II (2000) Photosynthesis in sugarbeet plants treated with benzyladenine and metribuzin during leaf development. Russian Journal of Plant Physiology 47:161-167.