Publication Information
ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Effect of Liraglutide 3.0 mg 3.0 on Body Weight & Quality of Life Among Patients With Obesity Disease at King Fahad Medical City Retrospective Study
Al Amri K1*, Hijazi L, AlamoudiF2, Rabie A S2, BazaidW2, Alanazi A2
1Consultant of obesity medicine, King Fahad Medical City, Riyadh, KSA
2Pharma D graduate, Princess NouraUniversity, Riyadh, KSA
Received Date: October 27, 2020; Accepted Date: January 27, 2021; Published Date: January 27, 2021;
*Corresponding Author: Al Amri K, Consultant of obesity medicine, King Fahad Medical City, Riyadh, KSA.
Email: kalamri@kfmc.med.sa
Citation: Al Amri K, Hijazi L, AlamoudiF, Rabie A S, BazaidW et al. (2021) Effect of Liraglutide 3.0mg 3.0 on Body Weight & Quality of Life Among Patients With Obesity Disease at King Fahad Medical City Retrospective Study. Adv in Nutri and Food Sci: ANAFS-202.
DOI: 10.37722/ANAFS.2021102
Introduction
Obesity is defined as a condition of abnormal or excessive fat accumulation in adipose tissue, and its distribution in the body resulting in detrimental health hazard. The most commonly used definitions, established by the World Health Organization (WHO) classify the values as follows; Class 1, Class 2 and Class 3.
Classification
BMI Kg/m2
Obesity
≥ 30
Obesity class I
≥ 30 and < 35
Obesity class II
≥ 35 and < 40
Obesity class III
≥40
The prevalence of obesity increases worldwide. Therefore, it determined by genetic, social, and environmental factors, obesity has been officially recognized as a chronic diseases.
One of the newest weight loss medications is liraglutide 3.0mg an analog of the incretin hormone glucagon-like peptide-1 (GLP-1) with 97% homology to human GLP-1. Improvements in HRQoL with liraglutide 3.0mg 3.0 mg were observed using both disease-specific and generic instruments across physical and mental HRQoL subscales; however, the greatest improvements were seen in the physical aspects of HRQoL and self- esteem.
Assessing the impact of weight loss happened with the patient in a clinical practice would augment on the benefit of weight loss in improving quality of lives of patients.
Objectives
The primary objective in our study was to evaluate liraglutide 3.0 mg 3.0 mg effectiveness in weight loss by measuring the change in body mass index (BMI) ≥ 4- 6 months, before and after treatment in obesity unit at King Fahad Medical City, in addition to determine the quality of life for participants after initiation of treatment, evaluating adverse effects of liraglutide 3.0mg, and compared to lifestyle modifications.

Method
This observational retrospective study was conducted at King Fahad Medical City in Riyadh, Saudi Arabia. Data had been collected from November 2018 to January 2019.
The study was approved by King Fahad medical city institutional review board. Participants were divided into three core groups: group 1 = liraglutide 3.0mg therapy for four to six months or longer; group 2 = participants following lifestyle modifications; group 3 = participants who underwent surgical operations based on body mass index and quality of life prior and after treatment.
Inclusion/Exclusion Criteria
Inclusion criteria included participants aged (≥ 18 years) who presented with a measured body mass index (BMI)
≥ 30 kg m2 and completed an adapted WHOQOL-BREF questionnaire and experienced a brief examination in King Fahad Medical City’s obesity clinics.
The survey integrated an assessment of multiple variables such as:
- physical health
- social relationships
- environmental factors.
Furthermore, the feedback forms were shared randomly or concluded in a one-to-one interview.
Participants were involved if they had been able to understand and sign informed consent, cognitively functional and able to express an opinion in the Arabic or English language.
Exclusion criteria disqualified patients if they:
- Unwilling or unable to participate.
- Had been on liraglutide 3.0mg therapy for less than four months.
- Weighed less than 27 kg/m2.
- Pregnant patients.
- Concurrent drug regimen contraindicated with liraglutide 3.0mg therapy like DPP-4s.
- Hypersensitivity.
Personal, or family history of medullary thyroid carcinoma, and multiple endocrine neoplasia syndrome II.

Results
The study was conducted from November 2018 through January 2019. The baseline characteristics were:
- Mean age was 40.5 years
- BMI 30 kg/m2
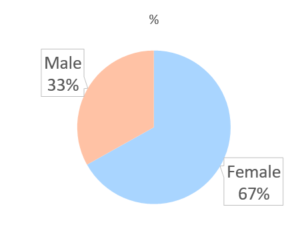
Approximately half of the responders (50.8%) completed postsecondary education (i.e., bachelor’s degree), Sixty-eight (66.7%) females compared with thirty-four (33.3%) males had enrolled.

The primary endpoints measured overall Health-Related Quality of Life (HRQoL) and reduction in body mass index (BMI) amongst obese patients who used liraglutide 3.0mg injections or adhered to surgical or lifestyle recommendations. There were statistically significant differences in the amount of weight loss among the three groups, (P= 0.005).
Participants treated with liraglutide 3.0mg lost weight on average 18.2 kg compared with 34.8 kg loss with the surgery group, and 14.66 kg loss in participants adhering to lifestyle changes.
Quality of life measured by the (WHOQOL)-BREF
Questionnaire showed a response rate in Q1 varied from 51.5
% to 60%. Thus, these results indicated participants were ill, or participants with other disease. The general evaluation showed life quality related to social support alternated from great to moderate in 33 (66%) adults on liraglutide 3.0mg.
Secondary endpoints assessed patient’s reports on liraglutide 3.0mg’s most common side effects, such as gastrointestinal disturbances and subsequently reducing the incidence, Ninety percent of patients who used liraglutide 3.0mg complained of side effects at the start of treatment and at titrated doses.
Nonetheless, patients have recounted that their symptoms faded after a few days. Fifty-eight percent of patients reported vomiting and nausea, forty percent dizziness, and twenty-two percent contemplated cessation of therapy.
Events
Participants No.
Participants %
Nausea
29
58%
Vomiting
29
58%
Dizziness
20
40%
Potential discontinuation of causative drug
8
16%
Planning to continue to take the drug
42
84%
Discussion
The finding from this study suggests that liraglutide 3.0mg administration, safely lead to weight loss, in agreement with previous studies that mentioned the good effect of liraglutide 3.0mg in weight loss. In addition, to its effect in improving patient’s quality of life.
Liraglutide 3.0mg treatment led to weight loss means average (18.2 kg) at intervals of four to six months among our patients . Around 90% of patients who used liraglutide 3.0mg complained from side effects at the start of the treatment and at the titrated increment doses. Nonetheless, these symptoms faded after a few days.
Conclusion
Liraglutide 3.0mg administration safely improves the quality of life by facilitating weight loss, it must be borne in mind that this study was only conducted on a small group of obese patients over a short period of time. Further research is hence needed to determine the long-term effects of the use of liraglutide 3.0mg in enhancing weight loss before generalized conclusions can be drawn.
References
- Mancini MC, de Melo ME (2017) The burden of obesity in the current world and the new treatments available: focus on liraglutide 3.0mg 3.0 mg. Diabetology & Metabolic Syndrome 9.
- Ofei F (2005) Obesity - A Preventable Disease. Ghana Medical Journal 39:98-101.
- Wadden TA, Hollander P, Klein S, Niswender K, Woo V et al. (2013) Weight maintenance and additional weight loss with liraglutide 3.0mg after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 37:1443-1451.
- Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L et al. (2009) Effects of liraglutide 3.0mg in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374:1606-1616.
- Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A et al. (2015) A Randomized, Controlled Trial of 3.0 mg of liraglutide 3.0mg in Weight Management. N Engl J Med 373:11-22.
- Wadden TA, Hollander P, Klein S et al. (2013) Weight maintenance and additional weight loss with liraglutide 3.0mg after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 37:1443-1451.