Publication Information
ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Diet, Muscle Protein Synthesis and Autophagy Relationships in Cancer: An Attempt to Understand Where Are We Going, and Why
Francesco Saverio Dioguardi1*, Carol Chen-Scarabelli2, Evasio Pasini3, Giovanni Corsetti4, Tiziano M Scarabelli5
1Department of Internal Medicine, University of Cagliari, Cagliari, Italy
2Division of Cardiology, Hunter Holmes McGuire Veterans Affairs Medical Center (VAMC), Richmond, VA, US
3Maugeri Scientific Clinical Institutes”, IRCCS, Cardiac Rehabilitation Division, Lumezzane (BS), Italy
4Division of Human Anatomy and Physiopathology, Department of Clinical and Experimental Sciences, University of Brescia (BS), Italy
5Center for Heart and Vessel Preclinical Studies, St. John Hospital and Medical Center, Wayne State University, Detroit, Michigan, USA
Received Date: July 31, 2022; Accepted Date: August 10, 2022; Published Date: August 21, 2022
*Corresponding author: Francesco Saverio Dioguardi, Department of Internal Medicine, University of Cagliari, Cagliari, Italy. Tel/ Fax: +39-0238314833; Email: fsdioguardi@gmail.com
Citation: Dioguardi FS, Chen-Scarabelli C, Pasini E, Corsetti G, Scarabelli TM (2022) Diet, Muscle Protein Synthesis and Autophagy Relationships in Cancer. An Attempt to Understand Where Are We Going, and Why. Adv in Nutri and Food Sci: ANAFS-238.
DOI: 10.37722/ANAFS.2022401
Abstract
Protein-based structures are indispensable to maintain life, so identification and removal of worn out structures achieved through proteostasis, the sum of micro and macro-autophagy (autophagy) plus ubiquitin-proteasome system, must balance renewal by new synthesis. Many of the elements controlling dynamically equilibria between protein synthesis and protein degradation have been identified and modalities of activation actively studied, still we are quite far from mastering how this balance is ruled. Failure to maintain a positive balance between protein synthesis and protein degradation would result in sarcopenia, defined as the loss of skeletal muscle mass and function, a major clinical problem frequently accompanying chronic illnesses, but peculiarly spotted in cancer and in elderly patients. Also, how cancer is fed, and how nutrition in cancer patients may affect evolution and therapy effectiveness is another field of opinions and uncertainty. On the other hand, exercise and nutrition tailored to provide adequate amounts of amino acids are widely considered a necessary strategy for prevention and treatment of protein synthetic deficits in muscles. This paper will synthetically review how different nutritional strategies and energy production may interconnect efficiently synthesis and scavenging of aged and overused protein molecules by autophagy. Finally, since energy availability rules life and death of cells and organisms, an hypothesis predicting how energy may control the ratios among protein synthesis and autophagy is proposed: in normal conditions, protein syntheses have a key role in autophagy activation by consuming large amounts of energy when forming peptidic bonds, that is adenosine tri-phosphate (ATP) is consumed to mono-phospahate (AMP), thus decreasing ATP to AMP ratios. Conversely, both protein syntheses and autophagy may be scarcely activated when low availability of ATP would result also in lowest concentrations of AMP. In this peculiar setting, reduced rates of both protein syntheses and autophagy would be observed, resulting in worsening of protein balance and functions.
Keywords: Nutrition; Cancer; Protein synthesis; Autophagy; mTOR; AMPK; ATP; AMP; Amino acids; Sarcopenia
Body Energy Needs and Protein Reservoirs
Two tissues, skeletal appendicular muscles and skin comprise by weight the first and second major organs, and are also containers of the largest amounts of proteins in humans. Indeed, muscles should be considered also as organ acting as a reservoir of amino acids in a dynamic view of human metabolism requirements either in health or disease states, and it is so also in cancer [1].
But, when evaluating metabolism in the human body, and peculiarly in cancer bearing patients, it is often overlooked that the most abundant protein in human body is collagen, with the vast majority stored within the skin dermis, which is indeed significantly dismantled to refuel energy sources utilized by cancer cells [2]. Also maintenance of protein structures require both syntheses and removal of worn-out molecules, which are intertwined. The two main pathways controlling identification, elimination, and partial recycling of protein molecules are autophagy, the sum of both micro and macro autophagy (autophagy) , and the ubiquitin-proteasome system (UPS) whose activities (proteostasis) are needed and balanced with healthy protein synthesis through different mechanisms [3].
Food also should be considered most important “message” through which natural environment communicates with our body, so both quantity and quality of food provide messages that would influence metabolism and manage the balance of synthesis and proteostasis [3]. But, how? There is a lot yet to be understood in how “messages from environment” , that is how materials and energy coming from the environment we are living in [4] convey information’s elaborated by DNA and then adaptively modulated through epigenetic responses in any cell, but energy production is the absolute priority for life [5]. Of notice, all macronutrient may be efficiently funneled to energy production, although from amino acids both carbohydrates and lipids may be synthesized by metabolic pathways, with the unique exception of some non-saturated fatty acids therefore identified as essential for life. Also, only by amino acids nitrogen can be introduced into metabolism of mammals. Carbohydrates, lipids and amino acids have similar carbon skeletons, and energy (ATP) is derived mostly by oxidative breaking of those carbon to carbon covalent bonds [6].Thus, as resumed and outlined in Synoptic Figure, being at roots of life, energy production decides dimensions of ratios between protein synthesis and autophagy.
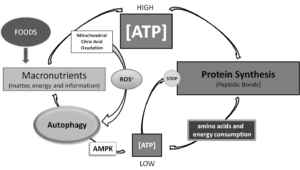
Synoptic Figure: Energy levels rule reciprocal levels of both protein synthesis and autophagy. Of notice, in this system two controlling loops are described, both dependent on ATP production. Production of ATP by mitochondrial oxidation of citrate produces reactive oxygen species, which recently have been shown to be key positive regulators of autophagy. Conversely, low energy levels following ATP consumption for building thousands of peptidic bonds necessary to protein syntheses, would directly and indirectly, by activating AMP dependent kinase (AMPK), which in turn, activates autophagy so providing substrates for replenishing ATP production pathways.
Protein Synthesis Entangled Relationships among Substrates and Energy Production
Some amino acids may be synthesized by intermediates of metabolism, but nitrogen necessary for those syntheses is always derived from some amino acids, and those amino acids have to be introduced in sufficient amounts since mammals are unable to synthesize them. Matching amino acids needs is a life-threatening requirement that brought to distinguish amino acids in two categories: non-essential and essential ones [7]. Notably, the ratio between essential (E) and non-essential (NE) amino acids (AA) both in human [8] orin alimentary proteins [9] is largely <0.9, thus NEAA are largely prevalent in foods .This imbalanced ratio underlines that variations both of quantity or quality of nitrogen provided by food may elicit significant adaptive mechanisms targeting any tissue or organ and, finally, influencing phenotype and life span more or less successfully [10].
Therefore, both quality (EAA/NEAA) and quantity of amino acids introduced by nutrition would evidently regulate a more or less efficient balance between protein synthesis and protein turnover [11].
Adequate protein intakes are necessary to match AA needs and as a countermeasure to sarcopenia was suggested, so that meeting a protein threshold (25–30 g/meal) could be a promising yet still largely unexplored dietary strategy to help maintain muscle mass and function [12]. Conversely, some peculiar molecular link among metabolism of amino acids and muscle dismantling and connected to cancer energy needs and thus sarcopenia genesis, has been finally identified: high mobility group box-1 (HMGB1) released during tumor genesis recruits muscle proteins to supply glutamine to cancer cells as an energy source, and for this purpose proteolysis by autophagy is triggered, and amino acids from muscles proteins are generated [13].
Another point is that protein syntheses depend on energy production since peptidic bonds are extremely energy consuming [14] , but another important consideration is that total energy costs for the body are also proportional to physical activity, which increases energy requirements necessary for maintenance of integrity of the body [15]. Epidemiological studies have demonstrated that even short, regular periods of non-intense exercise reduce mortality, and also prolong life span in various populations [16],[17].On the contrary, inactivity, especially with aging and even for medical reasons, may cause permanent damage to muscles [18], along with muscle mass loss which “is predictive of all- cause mortality in older men regardless of age, BMI, lifestyle, physical performance, health status and body composition” [19], and also in cancer patients muscle integrity correlates with outcomes [20]. Those observations suggest some favorable modifications being associated with systematically increasing energy expenditure and stressing contractile proteins. The costs of exercise, in turn, require matching nutritional intakes with exercise-linked requirements, or exercise linked metabolic stress may not exert the expected advantages peculiarly in the elderly [21]. Therefore, muscles integrity is a balance among workloads indispensable to activate syntheses, removal of aged proteins and food intake sufficient to match energy and synthetic requirement precursors.
Some Considerations on Molecular Signaling By Nutrition, And Effects of Caloric Restriction
Protein synthesis is indispensable for life, and at molecular levels control of synthesis involves activation of the two signaling pathways, extracellular signaling regulated kinase/mitogen activated protein kinase (ERK/MAPK) [22], and mammalian or mechanistic target of rapamycin components (mTORCs, commonly identified as C1 and C2), which are necessary to promote translation also in cancer cells [23]. After so many years of studies, yet a major question is at what extent protein synthesis would be associated witha shorter or longer lifespan. The question arises from studies linked to pharmacological mTORC1 inhibition [24] and observations that caloric restriction both reduced mTORC1 phosphorylation, and prolonged lifespan in some animal models [25]. Since then, many studies have explored different severity levels and techniques of caloric restrictions effects, but with time much has changed from the enthusiasm at the beginning of those studies, and caloric restriction in humans most recently has been defined as “a reduction in energy intake below the amount that would be consumed ad libitum while maintaining adequate intake of essential nutrients”, and finally, increased proportions of proteins intakes has been suggested to be necessary for maximal safety and prevention of malnutrition [26]. Also, caloric restriction as well as mTORCs pharmacological inhibition by rapamicin has been shown to be contra-indicated in aging [27]. If caloric restriction has to be considered quantitative, that is maintaining intakes of all macronutrients in normal ratios [28], or should be qualitative, that is a strictly monitored caloric intake summed to marked restriction of at least one among carbohydrates, lipids or proteins, would be discussed in the following paragraphs.
Energy Metabolism, Carbohydrates and Lipids Restriction in Cancer
Carbohydrates, lipids and amino acids of proteins represent the largest percentage of what necessary for life of mammals. Any change in quantity of one single macronutrient would influence either total calories or the percentages of total calories provided by just the other two macronutrients, which would be proportionally increased to match shortage of one of them. This statement may seem obvious, but implications on quality and amounts of macronutrients provided by selective restrictions seem also too often underestimated when significant modifications are applied. Those considerations should be kept in mind any time that diets are modified, or consequences may be extremely significant, as shown by studies with altered EAA/NEAA ratios, as an example [10].
The main task of cancer cells is to duplicate, and this requires spending energy. Production of energy should be fueled by availability of substrates, but duplication also needs substrates for matching completion of syntheses and make a copy of all cell structures (i.e.: enzymes, nucleotides, membranes).
Near 100 years ago improvement of knowledge of chemistry allowed facing cancer metabolism, and peculiarities of energy production linked to glycolysis were documented by Warburg [29], Cori and Cori, and discussed by Greenstein in a pivotal paper [30]. Energy production was identified as a central task for survival and duplication. Otto Warburg identified the abundant amounts of energy necessary for duplication as derived in tumors from cytoplasmic glycolysis to lactate even under aerobic conditions, but the finalistic advantages of incomplete glycolysis for cancer cells and reduced mitochondrial glycolysis, the so-called “Warburg effect”, are still unclear [31]. Also, extent of contribution of glycolysis to energy needs of cancer is still debated [32]. Since carbohydrates and lipids metabolic pathways are strictly intertwined [33], how carbohydrates interface with fats and amino acids metabolism and are utilized by cancer cells is an evolving knowledge, recently revised [34]. On the other hand, in plasma insulin concentrations are strictly connected to glucose levels, and since 5.6% of all cancers are now attributed to hyperinsulinemic conditions such as obesity and type 2 diabetes due to the worldwide increasing prevalence of these metabolic diseases [35], therefore if and how many carbohydrates introduced by diets may be dangerous are controversial items [36].
Indeed, Mediterranean diet, although rich in carbohydrates, is associated to less cancer risk and greater chance of a favorable prognosis at least in some cancer [37], but complexity of Mediterranean diet and the many components it provides (g.e.: insoluble fibers rich vegetables are constantly mixed to carbohydrates rich recipes) complicate epidemiologic evaluations, since some specific component may exert relevant favorable effects that may antagonize eventually negative influences exerted by other food components [38].
Anyway, carbohydrate restriction in diets adapted to cancer patients has been principally planned in terms of caloric restriction, with the simplistic idea that reducing nutrients support to tumor could limit energy production and so cancer cell proliferation. It should be remembered, anyway, that this basic principle should need also to be examined in the light of human’s evolutionary advantages over the frequent and forced long periods of fasting, since through ages, indeed, on scarce resources humans developed being able to survive by using stored fat [39].
Historical studies by Haven, et al. suggested that cancer had peculiar and most elevated fatty acids needs: “the mobilization of fat occurred early in the growth of the tumor while the food intake was normal and before the carbohydrate and protein stores of the animal were exhausted. Both our experiments and those of Smedley-MacLean indicate that fat may be mobilized for the purpose of providing a plentiful supply of the essential unsaturated fatty acids for building tumor tissue” [40]. One hundred years later, still there is much interest but scattered consensus about the impact of diets modified by reducing carbohydrates and so necessarily increasing lipids, and also generally on which sort of caloric restricted diets may favorably act on cancer evolution. In our opinion, there is no clear cut indication about what macronutrient should be the main target of systematic restriction: carbohydrates, lipids or proteins? Conversely, it is not controversial that mortality is most prevalent in malnourished cancer bearing patients, and that malnutrition and sarcopenia are often coupled [41, 42]. Thus, caloric restriction (restriction of all macronutrients still in balanced ratios) and fasting [43] peculiarly in case of chemotherapy [44] have been cautiously explored as potentially helpful in reducing speed of cancer growth. Increased tolerability and effectiveness of chemotherapy are supported by some preclinical data, but always authors convene that yet unequivocal clinical evidences are lacking, so great caution is always necessary to carefully prevent malnutrition [42-44].
Similar conclusions are reported by papers reviewing advantages of ketogenic diets [45], peculiarly if associated with strict carbohydrate restriction [46]. In obese patients, success of those diets in treating obesity by reducing weight has been proposed as potentially correlated with any possible improvement in efficiency of chemotherapy [47].
Of notice, also by clinicians exploring carbohydrates restricted ketogenic diets in cancer patients, it was always underlined that a most individualized nutritional support is crucial in avoiding malnutrition in those delicate patients [48]. Although contribution of carbohydrates to cancer cells metabolism is established, some concern exist also for contribution of fats to cancer cells viability. Recently, advancements in understanding biology and contribution of fatty acids to cancer cell survival in hypoxic and acidic tumor microenvironment have been revised [35]. Fatty acid availability and metabolism support proliferation and invasiveness, and metabolic addiction of cancer for fatty acids was underlined, noticing also that this peculiarity may account for cancer progression associated with high fat diet. But, also, those authors suggest that fatty acid addiction for metabolism of tumor could represent “an Achilles heel”. They proposed thatn-3 polyunsaturated fatty acids may represent a class of lipids that can exert cytotoxic effects in tumors and therefore may possibly represent attractive diet supplementation improving cancer patient outcomes [39].
It can be concluded by all those studies that epigenetic modifications following nutritional modifications of diet both by carbohydrates restriction and increased fats contribution to total calories, so ketogenic diets and /or fasting may be somehow useful, but with a constant warning to avoid malnutrition since this would correlate with sarcopenia, early discontinuation of chemotherapy and increased cancer mortality [49].
At this point, it should be also remembered that if avoiding malnutrition is universally recommended, it should be kept in mind by the skilled clinician the constant possibility of a general risk of caloric malnutrition in those patients, and so this risk should be also carefully and regularly monitored. But, it should be recognized that malnutrition is not easily diagnosed, since both markers of syntheses or inflammation and measures of oral intake commonly performed routinely although are associated with clinical outcomes, alone, they poorly distinguish between risk of malnutrition, sarcopenia and cachexia, which often co-exist in patients with cancer [41,42].
In our opinion, present knowledge is insufficient to indicate if restricting carbohydrates or lipids, or total calories would be advantageous in all cancer patients. Also, we argue if a diet providing all macronutrients and matching patient’s need of all calories so sufficient to maintain a normal weight and body composition should be defined a normal diet, or instead a caloric restricted diet. Excess or deficiency of any component of diet would alter weight or body composition, and so should not be identified as a normal diet: any diet deficient in amino acids would waste muscles, any diet providing excess calories by carbohydrates or fats would increase adipose tissue.
Cancer Metabolism and Nutrition: The Multiple Roles of Amino Acids
Yet in 1951, Mider anticipated the complexity of the need to understand how body metabolism has to adapt to “neoplasia, which introduces into the body a new race of cells derived from tissues common to ontogeny but differing biologically, structurally, and chemically from their ancestors. The new members of the community of organs and tissues intrude themselves on the nicely balanced economy of the organism. Adjustments are necessary.” For the first time energy production, amino acids, carbohydrates and lipids needs of cancer cells were systematically discussed and linked to appearance of sarcopenia and cachexia [50]. Following studies on cancer development identified 12 amino acids as indispensable for cancer cells survival: isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine and in addition, arginine, cyst(e)ine, tyrosine, and histidine [51]. Most wisely, Eagle supposed “it may be that these cells are unable to make these 4 amino acids from their normal precursors. In such case, on a diet limited to the 8 amino acids essential for nitrogen balance in man (of notice: histidine was recognized as an essential amino acid only years later), these 4 would presumably be provided either by the breakdown of other body cells, or by their overproduction in some other tissue” [51]. Also, it was shown that “sarcoma developed slower in animals well fed, where normal tissue developed maximally” [52], a result conflicting with data obtained by most limiting versions of caloric restrictions. Several decades later, we should notice that many researchers studied and discussed effects amino acids of proteins as a whole [53], and very few have tried to separate effects of EAA and NEAA, although since NEAA are most represented than EAA in food proteins, matching EAA needs is certainly more difficult than matching NEAA ones [54].
Syntheses of proteins in cancer cells have the same qualitative requirements of amino acids than in normal cells, but some feature in amino acid metabolism in cancer cells has been identified. Historic studies identified the source of amino acids used by cancer in the same tissues that serve as a reservoir during prolonged starvation [55], so amino acids identified as peculiarly important both for cancer energy metabolism and syntheses included proline, glutamine, glycine and serine, with the latter three being the most abundant amino acids in food proteins [52, 54]. Of notice, proline and glycine are peculiarly stored into collagen, and skin is a major reservoir for those amino acids [2]. Actually, there is no NEAA that could be considered useless in promoting cancer metabolism, either if roles in energy metabolism or utilization for protein synthesis are considered [56]. Also, when comparing the effects on cancer development of amino acids mixtures were one single NEAA was subtracted, some misleading interpretation was also originated by initially not recalculating how relevant were modifications of ratios among EAA and NEAA connected with this not compensated subtraction [57, 58]. Indeed, EAA have effects on cancer, and peculiarly if EAA are given in amounts sufficient to rise the ratio among EAA and NEAA usually found in foods. Leucine supplementation proved effective in both maintaining muscular masses and improving exercise effects on sarcopenia, while slowing cancer development [59], and in agreement, epidemiological studies showed that incidence of liver cancer in cirrhotic patients wasreduced by chronic assumption of branched chain amino acids (BCAA= leucine, isoleucine and valine) and some molecular mechanism of action was also identified [60].
Also supplemental histidine intake has been suggested as positively correlating with chemotherapy outcomes, at least in experimental settings [61]. Conversely, although methionine- free diets may be toxic beyond a 24-hour regimen, appropriate methionine and cysteine/cystine restriction regimens have been recently revised and improved quantitative indications proposed, althoughby those restrictions in humans drop of anti-oxydant systems (N-Acetyl-Cysteine and Glutathione) was recorded [62]. By now, only in animals an increased efficiency of chemotherapy (and specifically 5Fluor-Uracyl) on tumor growth was identified [63].
It is suggested that limiting to about 2-2.92 mg/kg-1 methionine intakes that is a safe dietary methionine restriction but not depletion, may favorably improve chemotherapy tolerance and efficacy [62-64]. Also, pharmacological attempts of interfering with methionine metabolism in cancer are ongoing [65]. Of notice, methionine and cysteine/cystine metabolism are strictly interconnected, in absence of dietary cysteine/cystine, a substantial part of methionine should be transformed in cysteine/cystine unloading folates of methyl groups and forming homocysteine as metabolic intermediate. Cystine and cysteine being indistinguishable, since cystine is formed by two moieties of cysteine linked by a very labile hydrogen bond, spontaneously loosening up [66]. Certainly, focusing on single amino acids metabolism may be easiest and modulation of some amino acid intake helpful, but nitrogen intake in humans has also be considered in its complexity [67]. Since cancer cells evolve on a nutritional supply providing EAA/NEAA ratios that are largely < 0.9, and since increasing EAA/NEAA proved in vitro protective toward chemotherapy damages on mitochondria [68], a question was if inversion of EAA/NEAA ratio would have fed cancer cells favoring duplication and what kind of epigenetic modifications this would have triggered. What was observed leads to change many paradigms: increasing EAA over NEAA (that is: if EAA/NEAA ratios are substantially modified to be <1) powerfully activate autophagy instead of inhibiting as previewed by EAA driven signaling leading toMTORC1 activation. Autophagy is activated so efficiently rapidly triggering apoptosis and killing cancer cells evidently overwhelming cancer adaptive mechanisms, also simultaneously inhibiting ubiquitin-proteasome system [69]. Studies on cancer development in animals are now ongoing, once that safety on healthy animals was established [10].
Essential amino acids, and peculiarly leucine, on the contrary, are still considered uniquely as positive effectors for protein syntheses and MTORC1, thus negative effectors for autophagy: this simplistic paradigm, proven to be false also in cancer cells [69] dates the beginning of studies on caloric restriction and rapamycin effects on MTORCs [70]. A theory that may predict all those apparently contrasting findings is therefore proposed in the following paragraphs.
Connecting Energy Metabolism, Protein Synthesis and Autophagy by Epigenetic Modifications
A main positive effect ascribed to caloric restriction is activation of autophagy consequent to reduced energy availability, ATP utilization and increased AMP concentrations. Reduction of ATP/AMP ratios would in turn both inhibit mTORC1 dependent protein synthesis and activate autophagy [71]. Autophagy is a complex cellular function aimed both to removal of aged structures and to recycle cellular components after dismantling, also funneled into maintaining energy production [72]. Autophagy, in turn, would be inhibited when mTORC1 and protein syntheses are fully active and ATP is abundantly available, but it has been always omitted that, in turn, protein synthesis has huge costs and thus consumes enormous ATP amounts. The latter observation is of extreme relevance, since in our opinion this links energy production to protein synthesis and autophagy. For instance, it has been calculated that up to 80% of energy production in cells is dedicated to complete protein synthesis [73].
Of notice, physical exercise, indispensable to promote muscular protein synthesis is not only associated to mTORC1 dependent activation of protein synthesis [74], reduction in mortality and extended lifespan, but also is associated to increased autophagy [75], in evident contradiction with the predicted rapamycin/caloric restriction and blunted protein synthesis linked benefits. How to solve those apparent antinomies? To answer this question, we have to remember that physical exercise, if coupled with an adequate increase in food intake, not only improves protein synthesis throughout the body (muscles, heart, bones, tendons and ligaments), but exercise also increases needs of ATP, and ATP production by oxidation of citrate into mitochondria, in turn, increases production of reactive oxygen species (ROS) in proportion to energy requirements (that is of ATP molecules) necessary for matching both demands of workload and energetic costs of protein syntheses [76]. A clue to disentangle those elements, has been the important finding that ROS produced by mitochondria while producing ATP are also indispensable mediators tuning efficiently autophagy, and this kind of activation evidently links energy production to most efficient proteostasis [77].
Therefore, there are two very different mechanisms that can activate autophagy, 1) AMPK phosphorylation and MTORC1 inhibition, sensible to ATP consumption and driven by AMP rise, and 2) ATP production by active citric acid cycle. Most of ATP is produced by mitochondrial oxidation of citrate, and this is a ROS producing activity that in specific parts of cells is spatially limited by structured endogenous antioxidants systems. On the other hand, mitochondrial glucose oxidation would also trigger by ROS an efficient signaling that activates autophagy and is so indispensable for maintenance of protein synthesis and protein degradation by autophagy tightly balanced [78,79].
As an example, recently the need of ATP production and the linked ROS dependent balanced activation of both autophagy and protein synthesis has been identified as indispensable in successful cardiac remodeling after myocardial infarction [80].
Additionally, it should be noticed that nutrition per se cannot overcome or substitute for exercise in the maintenance of muscle synthesis integrity. This is documented by recent research which demonstrated that immobilization activates a block of mTORC1 by blunted phosphorylation of one of its component, DEP domain-containing mTOR interacting protein, DEPTOR, which is very poorly activated by refeeding only, in absence of exercise [81]. A similar mechanism has been described for albumin synthesis by the liver, deeply influenced positively by EAA availability, but blunted by “inhibition of specific mRNA-synthesis in the hepatocellular nuclei induced by the direct interaction of the cell with the acute-phase cytokines” [82]. In authors opinion, those data suggests that in conditions where synthetic drive of proteins is blunted, more frequent and abundant loads of EAA are necessary to positively modify plasma concentrations and to favorably oppose epigenetic blocks of syntheses [67].
Authors Hypothesis: Protein Synthesis and Autophagy Regulation by Amino Acids
It is widely accepted that protein synthesis and autophagy, are both necessary to maintain a healthy muscle and to prevent or treat sarcopenia. Protein synthesis and autophagy are dynamically related processes ruled either by substrate availability necessary for syntheses (i.e.: peculiarly amino acids) and by changes in concentrations of ATP, since in turn protein syntheses control the amount of ATP consumed. Indeed, in cells, the most significant metabolic event consuming ATP and turning it into AMP is protein synthesis, which requires large amounts of energy necessary to match the costs of huge numbers of new peptidic bonds. Consequently and proportionally to the extent of protein synthesis dependent ATP consumption, AMP concentrations raise. In turn, changes in concentrations of ATP and AMP establish depth, frequency and duration of protein synthesis and autophagy by controlling the tightly regulated mTORC1 and AMPK reciprocal intensity of inhibition and activation, as pictorially resumed in (Figure 1A, 1B and 1C). Thus, we suppose and propose that supplementation of consistent amounts of EAA would promote protein synthesis, and peculiarly the inversion of EAA/NEAA usual ratios provided by foods (< 0,9), reaching EAA/NEAA concentrations > or >> 1, may provide a metabolic strain to which cells would respond epigenetically by both increasing protein syntheses and linked ATP consumption. When abundance of EAA would promote protein assembly, the process may be restrained by the reduced availability of enough NEAA necessary to complete synthesis. Synthesis of NEAA needed to match completion of protein synthesis would further trigger an increased ATP consumption to AMP [83], and so promote autophagy [69]. At the end, ATP to AMP shift is therefore linked also to the huge energetic costs of forming the many peptidic bonds necessary for completing syntheses of huge proteins, but also, as represented in (Figure 1 A), to different concentrations of ATP would correspond different amounts of AMP generated. Therefore, it is not surprising that physical exercise both triggers autophagy, but exercise may also rescue protein synthesis and reverse signaling triggering autophagy that is activated by starvation-induced decrease in insulin/IGF-I and serum amino acids, both effectively suppressing the mammalian target of rapamycin (mTor) [84]. Autophagy may be triggered also by a third mechanism, that is by the mTORC1-independent mTORC2 signaling described by Vlahakis et al [85], who demonstratedmTORC2 being a positive regulator of autophagy acting through its downstream target kinase, Ypk1.Vlahakis et al., indeed, demonstrated by starvation that that NEAA reduced availability, specifically of those NEAA required for maintaining endogenous non-essential amino acids for protein syntheses, as glutamine, induces autophagy in a manner independent of TORC1 inactivation, but instead requiring the general AA control (GAAC) response regulated by the eIF2α kinase, Gcn2 , via the Ca2+-dependent phosphatase calcineurin [82]. Glutamine synthetic needs, if not fully matched by adequate EAA intakes, are also implicated in cancer linked sarcopenia, as already mentioned [13].
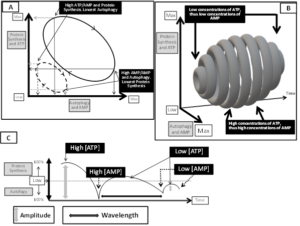
Figure 1. In (A): Cycling of dimensions of protein synthesis and autophagy according to reciprocal variations of energy consumption and ATP to AMP drive. Of notice, this is a typical pray/predator cycle diagram. In B: conditioned by the number of molecules of ATP, [ATP], also concentration of AMP, [AMP] will change, and accordingly both the amounts of proteins synthesized or efficiency of autophagy triggered by AMP dependent AMPK activation will change. High [ATP] and high protein synthesis would drive to largest amounts of [AMP], and the rise of [AMP], in turn, would activate maximally the largest number of AMPK. In those conditions, high protein synthesis would be matched by high autophagy efficiency. On the contrary, as peculiarly evidenced in (B) and in (C), low [ATP] would allow low protein synthesis and since low [AMP] would be generated, therefore less AMPK would be activated, and less autophagy triggered. In (C) it is also shown that fluctuations of [ATP]/[AMP] may control not only relative dimensions of protein synthesis and autophagy (amplitude of pattern), but also wavelength, that is the time span necessary to perform protein synthesis or autophagy. Our hypothesis suggest therefore that not only variations of [ATP/AMP] control protein synthesis and autophagy reciprocal fluctuations, but also a most variable speed of modification of any element of this fraction may influence synthesis and autophagy dynamics. A corollary of those observations is that energy production is the most important regulator of balance between protein synthesis and autophagy. Also by those dynamics energy production controls life efficiency and span.
Those observations reinforce the hypothesis that altering EAA to NEAA ratios by supplementing EAA may definitively control and regulate both protein synthesis and autophagy, so favoring muscle health maintenance. Since EAA improve mitochondrial biogenesis and function, and both are paralleled by endogenous anti-oxidants expression [86], an improved ATP availability and a most focused activation of autophagy (and mitophagy)also dependent by ATP production linked ROS signaling may contribute to ameliorate a balanced protein synthesis and renewal, resulting finally in a prolonged lifespan [10]. We believe, indeed, that ROS would be detrimental if spread throughout cell, but funneled and constrained by walls of endogenous anti-oxidants may exert a pivotal role in balancing ATP concentrations increased yields and autophagy [76-79].
On the contrary, or preferably in agreement with those data, as shown in cancer HCT-116 cells, EAA supplementation triggers apoptosis by activating autophagy , without providing any damage to normal cells [69].
Conclusion
EAAare indispensable substrates for promoting and completing protein synthesis in muscles, but also may be driven to energy production into mitochondria, if ATP production becomes a priority issues. We should focus on providing adequate amounts of EAA, and not just providing nitrogen by undefined EAA/NEAA ratios, if we want to support protein metabolism integrity in our patients. Nutritional intake of EAA in adequate amounts must be associated with exercise to switch off the multiple epigenetic controls that inhibit muscle synthesis and so to activate muscle protein syntheses exercise is indispensable as well as adequate EAA introduction to antagonize sarcopenia.
Protein synthesis and autophagy are tightly regulated processes which alternate reciprocally in cells, but dimensions of those activities may vary significantly. Protein synthesis, although regulated at various levels, some yet to be elucidated, provides an intrinsic message activating autophagy by consuming enormous amounts of ATP necessary for completing all peptidic bonds and to accomplish synthesis of proteins, especially those of high molecular weight. As an example, large proteins, as enzymes often are, are formed by thousands of AAs linked at the cost of huge energy expenditure, and have short or even very short half-lifes. ATP and ADP consumption, and the resultant increase in AMP concentrations are therefore main effectors of AMPK phosphorylation and activation of autophagy. Also, the role of mitochondrial ATP and ROS linked production in activation of autophagy and protein remodeling has only recently been described. Thus, we propose, for the first time, that synthesis of proteins controls increased production of a second messenger, AMP, and this in turn triggers autophagy activation. This would be followed by blunted protein synthesis due to a reduction in energy rich compounds and necessity to replenish ATP availability necessary for new protein syntheses, which would be paralleled by ROS production, also triggering autophagy. Therefore, autophagy and the EAA/NEAA ratios are in continuous and tightly coordinated dynamical balance. Only large EAA availability may promote protein syntheses, which promote reduced ATP/AMP, while NEAA comprise the vast majority of protein sequences in mammals, and so reduced ratios of NEAA would activate autophagy by MTORC2, independently by MTORC1 inactivation. The long term consequences of those dynamics, that in vitro have been shown to be linked to induction of apoptosis in cancer cells and not in normal ones, should be evaluated at best by measuring cohort lifespan and evaluating the specific changes in organs after prolonged intakes of selected EAA based formulations.
Author Contributions: Conceptualization: Francesco Saverio Dioguardi. Validation, data curation and discussion, writing, review and editing:Carol Chen-Scarabelli, Evasio Pasini, Giovanni Corsetti, Tiziano M Scarabelli. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Wolfe RR(2006)The underappreciated role of muscle in health and disease. Am J ClinNutr84: 475–482.
- Phang JM, Donald SP, Pandhare J, Liu Y (2008)The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 35: 681-690.
- Korovila I, Hugo M, Castro JP, Weber D, Höhn A, et al (2017)Proteostasis, oxidative stress and aging. Redox Biol13:550-567.
- Miller JL, Miller JG (1993) Greater than the sum of its parts. II. Matter-energy processing subsystems. BehavSci 38:1-73.
- Lane N, Martin W (2010) The energetics of genome complexity. Nature 21:929-34.
- Fulghum K and Hill BG (2018) Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling. Front. Cardiovasc. Med.. 5:127.
- Lopez MJ (2020) Mohiuddin SS. Biochemistry, Essential Amino Acids. Apr 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–. PMID: 32496725.
- Tessari P (2019) nonessential amino acid usage for protein replenishment in humans: a method of estimation. Am J ClinNutr 110:255-264.
- Gorissen SH and Witard OC (2018) Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proceedings of the Nutrition Society 77: 20–31.
- Romano C, Corsetti G, Flati V, Pasini E, Picca A, et al. (2019) Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan 11: 1367.
- LaymanDK (2009) Dietary Guidelines should reflect new understandings about adult protein needs. Nutrition & Metabolism 6:12.
- Paddon-Jones D, Campbell WW, Jacques PF, Kritchevsky SB, Moore LL, et al. (2015) Protein and healthy aging. Am J ClinNutr 101:1339S -1345S.
- Luo Y, Yoneda J, Ohmori H, Sasaki T, Shimbo K, et al.( 2013) Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014 Jan 1;74(1):330-340.
- Kaleta C, Schäuble S, Rinas U, Schuster S (2013) Metabolic costs of amino acid and protein production in Escherichia coli. Biotechnol J 8:1105-14.
- O’Leary TJ, Wardle SL and Greeves JP (2020) Energy Deficiency in Soldiers: The Risk of the Athlete Triad and Relative Energy Deficiency in Sport Syndromes in the Military. Front. Nutr 7:142.
- Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, et al (2011) Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet378:1244-1253.
- Hupin D, Roche F, Gremeaux V, Chatard JC, Oriol M, (2015) Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: a systematic review and meta-analysis. Br J Sports Med 49: 1262–1267.
- Kortebein P, Symons TB, Ferrando A, Paddon-Jones D (2008) Functional Impact of 10 Days of Bed Rest in Healthy Older Adults. J Gerontology: MEDICAL SCIENCES 63: 1076–1081.
- Szulc P, Munoz F, Marchand F, Chapurlat R, and Delmas PD (2010) Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J ClinNutr 91: 1227–1236.
- Iwase T, Wang X, Shrimanker TV, Kolonin MG, Ueno NT (2021) Body composition and breast cancer risk and treatment: mechanisms and impact. Breast Cancer Res Treat 186: 273-283.
- Carlsson M, Littbrand H, Gustafson Y, Lundin-Olsson L, Lindelöf N, et al. (2011) Effects of high-intensity exercise and protein supplement on muscle mass in ADL dependent older people with and without malnutrition: a randomized controlled trial. J Nutr Health Aging 15:554-60
- Cao W, Li J, Yang K, Cao D (2021) An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer. Jan 7:S0007-4551(20)30501-4.
- Harachi M, Masui K, Okamura Y, Tsukui R, Mischel PS, Shibata N (2018) mTOR Complexes as a Nutrient Sensor for Driving Cancer Progression. Int J MolSci(10):3267.
- Papadopoli D, Boulay K, Kazak L et al. (2019) mTOR as a central regulator of lifespan and aging [version 1; peer review: 3 approved]. F1000Research, 8(F1000 Faculty Rev):998
- Heilbronn LK, Ravussin E (2005). Calorie restriction extends life span--but which calories?PLoS Med 2:e231.
- Dorling JL, van Vliet S, Huffman KM, Kraus WE, BhapkarM,et al. (2021); CALERIE Study Group. Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: highlights from CALERIE phase 2. Nutr Rev 79:98-113.
- Goldberg EL, Romero-Aleshire MJ, Renkema KR, Ventevogel MS, Chew WM, etal. (2015) Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell 14:130-8.
- Trumbo P, Schlicker S, Yates AA, Poos M; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc102:1621-30.
- Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 11:325-37.
- Greenstein JP(1956) Some biochemical characteristics of morphologically separable cancers. Cancer Res 16: 641-53.
- Chen X, Qian Y, Wu S (2015) The Warburg effect: evolving interpretations of an established concept. Free Radic Biol Med 79: 253-263.
- Zu XL, Guppy M (2004) Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun 313: 459-465.
- Galton DJ (1968) Lipogenesis in humnan adipose tissue. J Lipid Res 9: 19-26.
- Chiaradonna F, Ricciardiello F, Palorini R (2018) The Nutrient-Sensing Hexosamine Biosynthetic Pathway as the Hub of Cancer Metabolic Rewiring. Cells 7: 53.
- Vigneri R, Sciacca L, Vigneri P (2020) Rethinking the Relationship between Insulin and Cancer. Trends Endocrinol Metab 31: 551-560.
- Yang J, Nishihara R, Zhang X, Ogino S, Qian ZR (2017) Energy sensing pathways: Bridging type 2 diabetes and colorectal cancer? J Diabetes Complications 31: 1228-1236.
- Di Maso M, Maso LD, Augustin LSA, Puppo A, Falcini F, et al. (2020) Adherence to the Mediterranean Diet and Mortality after Breast Cancer. Nutrients 12: 3649.
- Siddique AB, Kilgore PCSR, Tajmim A, Singh SS, Meyer SA, et al. (2020) (-)-Oleocanthal as a Dual c-MET-COX2 Inhibitor for the Control of Lung Cancer. Nutrients 12: 1749.
- Dierge E, Larondelle Y, Feron O (2020) Cancer diets for cancer patients: Lessons from mouse studies and new insights from the study of fatty acid metabolism in tumors, Biochimie 178: 56-68.
- Haven FL, Bloor WR, Randall C (1951) The nature of the fatty acids of rats growing Walker carcinoma 256. Cancer Res 11: 619-623.
- Álvaro Sanz E, Garrido Siles M, Rey Fernández L, Villatoro Roldán R, Rueda Domínguez A, Abilés J (2019) Nutritional risk and malnutrition rates at diagnosis of cancer in patients treated in outpatient settings: early intervention protocol. Nutrition 57: 148-153.
- Bullock AF, Greenley SL, McKenzie GAG, Paton LW, Johnson MJ (2020) Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: systematic review, narrative synthesis and meta-analysis. Eur J Clin Nutr 74:1519-1535.
- Alidadi M, Banach M, Guest PC, Bo S, Jamialahmadi T, Sahebkar A (2020) The effect of caloric restriction and fasting on cancer. Semin Cancer Biol. 2020 Sep 22: S1044-579X(20)30200-5.
- Caccialanza R, Cereda E, De Lorenzo F, Farina G, Pedrazzoli P, AIOM-SINPE-FAVO Working Group. (2018) To fast, or not to fast before chemotherapy, that is the question. BMC Cancer 18: 337.
- Plotti F, Terranova C, Luvero D, Bartolone M, Messina G, et al. (2020) Diet and Chemotherapy: The Effects of Fasting and Ketogenic Diet on Cancer Treatment. Chemotherapy 65: 77-84.
- Grandl G, Straub L, Rudigier C, Arnold M, Wueest S, Konrad D, Wolfrum C (2018) Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet. J Physiol 596: 4597-4609.
- Barrea L, Caprio M, Tuccinardi D, Moriconi E, Di Renzo L, et al. (2020) Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Could ketogenic diet "starve" cancer? Emerging evidence. Crit Rev Food Sci Nutr 4: 1-22.
- Oliveira CLP, Mattingly S, Schirrmacher R, Sawyer MB, Fine EJ, Prado CM (2018) A Nutritional Perspective of Ketogenic Diet in Cancer: A Narrative Review. J Acad Nutr Diet 118: 668-688.
- Caillet P, Liuu E, Raynaud Simon A, Bonnefoy M, Guerin O, Berrut G, et al.(2017) Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin Nutr 36: 1473-1482.
- Mider GB (1951) Some aspects of nitrogen and energy metabolism in cancerous subjects: a review. Cancer Res 11: 821-829.
- Eagle H (1955) The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture. J Exp Med 102: 37-48.
- Allison JB, Wannemacher RW, Prosky L Jr, Crossley ML (1995) Nutritive Value of Protein and Tumor-Host Relationship in the Rat. The Journal of Nutrition 60: 297-307.
- Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, et al. (2002) Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 51: 599-605.
- Young VR, Bier DM (1987) Amino acid requirements in the adult human: how well do we know them? J Nutr. 117: 1484–1487.
- Sherman CD, Morton JJ, Mider GB (1950) Potential sources of tumor nitrogen. Cancer Res 10: 374-378.
- Choi BH, Coloff JL (2019) The Diverse Functions of Non-Essential Amino Acids in Cancer. Cancers (Basel) 11: 675.
- Dioguardi FS, Flati V, Corsetti G, Pasini E, Romano C (2018) Is the Response of Tumours Dependent on the Dietary Input of Some Amino Acids or Ratios among Essential and Non-Essential Amino Acids? All That Glitters Is Not Gold. Int J Mol Sci 19: 3631.
- Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, et al. (2017) Corrigendum: Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 548: 122.
- Emilianne M. Salomão, Aline T. Toneto, Gisele O. Silva & Maria Cristina C. Gomes-Marcondes (2010): Physical Exercise and a Leucine-Rich Diet Modulate the Muscle Protein Metabolism in Walker Tumor-Bearing Rats, Nutrition and Cancer 62: 1095-1104
- Hagiwara A, Nishiyama M, Ishizaki S (2012) Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J Cell Physiol 227: 2097-2105.
- Frezza C (2018) Histidine degradation boosts cancer therapy. Nature 559: 484-485. doi:10.1038/d41586-018-05573-4.
- Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, et al. (2019) Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572: 397-401. doi: 10.1038/s41586-019-1437-3.
- Sanderson SM, Gao X, Dai Z, Locasale JW (2019) Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer 19: 625-637. doi: 10.1038/s41568-019-0187-8.
- Durando X, Farges MC, Buc E, Abrial C, Petorin-Lesens C, et al. (2010) Dietary methionine restriction with FOLFOX regimen as first line therapy of metastatic colorectal cancer: a feasibility study. Oncology 78: 205-209. doi: 10.1159/000313700.
- Hoffman RM, Han Q, Kawaguchi K, Li S, Tan Y (2019) Afterword: Oral Methioninase-Answer to Cancer and Fountain of Youth?. Methods Mol Biol 1866: 311-322. doi: 10.1007/978-1-4939-8796-2_24.
- Brosnan JT, Brosnan ME (2006) The sulfur-containing amino acids: an overview. J. Nutr. 136: 1636S-1640S.
- Dioguardi FS (2011) Clinical use of amino acids as dietary supplement: pros and cons. J Cachexia Sarcopenia Muscle. 2: 75-80. doi: 10.1007/s13539-011-0032-8.
- Flati V, Corsetti G, Pasini E, Rufo A, Romano C, et al. (2016) Nutrition, Nitrogen Requirements, Exercise and Chemotherapy-Induced Toxicity in Cancer Patients. A puzzle of Contrasting Truths?. Anticancer Agents Med Chem 16: 89-100. doi: 10.2174/1871520615666150824152900.
- Bonfili L, Cecarini V, Cuccioloni M, Angeletti M, Flati V, et al. (2017) Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. FEBS J 284: 1726-1737. doi: 10.1111/febs.14081.
- Speakman JR, Mitchell SE (2011) Caloric restriction. Mol Aspects Med 32: 159-221. doi: 10.1016/j.mam.2011.07.001.
- Tee AR (2018) The target of rapamycin and mechanisms of cell growth. Int J Mol Sci 19: 880. doi: 10.3390/ijms19030880.
- Russell RC, Yuan HX, Guan KL (2014) Autophagy regulation by nutrient signaling. Cell Res 24: 42-57. doi: 10.1038/cr.2013.166.
- Barton MD, Delneri D, Oliver SG, Rattray M, Bergman CM (2010) Evolutionary Systems Biology of Amino Acid Biosynthetic Cost in Yeast. PLoS ONE 5(8):e11935. doi:10.1371/journal.pone.0011935
- Hornberger TA (2011) Mechanotransduction and the Regulation of mTORC1 Signaling in Skeletal Muscle. Int J Biochem Cell Biol 43: 1267–1276. doi:10.1016/j.biocel.2011.05.007.
- Neel BA, Lin Y, Pessin JE (2013) skeletal muscle autophagy: a new metabolic regulator. Trends Endocrinol Metab 24: 635-43. doi: 10.1016/j.tem.2013.09.004.
- Sakellariou GK, Pearson T, Lightfoot AP, Nye GA, Wells N, et al. (2016) Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep 6:33944. doi: 10.1038/srep33944.
- Niforou K, Cheimonidou C, Trougakos IP (2014) Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol 2:323-32. doi: 10.1016/j.redox.2014.01.017.
- Ristow M, Schmeisser S (2011) Extending life span by increasing oxidative stress. Free Radical Biology & Medicine 51: 327–336.
- Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251-262. doi: 10.1038/nrm3311.
- Jia D, Hou L, Lv Y, Xi L, Tian Z (2019) Post infarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC‐1α/PI3K/Akt signaling. J Cell Physiol 234: 23705–23718.
- Shimkus KL, Jefferson LS, Gordon BS, Kimball SR (2018) Repressors of mTORC1 act to blunt the anabolic response to feeding in the soleus muscle of a cast-immobilized mouse hindlimb. Physiol Rep 6: e13891. doi: 10.14814/phy2.13891.
- Ramadori G (2020) Hypoalbuminemia: an underestimated, vital characteristic of hospitalized COVID-19 positive patients? Hepatoma Res 6: 28.
- Raiford DW, Heizer EM Jr, Miller RV, Akashi H, Raymer ML, et al. (2008) Do amino acid biosynthetic costs constrain protein evolution in Saccharomyces cerevisiae?. J Mol Evol 67: 621-30. doi: 10.1007/s00239-008-9162-9.
- Zheng DM, Bian Z, Furuya N, Oliva Trejo JA, Takeda-Ezaki M, et al. (2015) A treadmill exercise reactivates the signaling of the mammalian target of rapamycin (mTor) in the skeletal muscles of starved mice. Biochem Biophys Res Commun 456: 519-526. doi: 10.1016/j.bbrc.2014.11.118
- Vlahakis A, Graef M, Nunnari J, Powers T (2014) TOR complex 2-Ypk1 signaling is an essential positive regulator of the general amino acid control response and autophagy. PNAS 29: 10586-10591.
- D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, et al. (2010) Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab 12: 362-372. doi: 10.1016/j.cmet.2010.08.016.