Publication Information
ISSN: 2641-693X
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Diabetes Management in Myocardial Infarction Patients - A Retrospective Study and Literature Review in Tertiary Level Hospital of Australia
Neelabh Sharma*, Thakur S, Sutcliffe S, Starmer G
Department of Cardiology, Cairns Hospital, QLD Australia
Received Date: February 04, 2020; Accepted Date: February 12, 2020; Published Date: February 21, 2020
*Corresponding author: Neelabh Sharma, Department of Cardiology, Cairns Hospital, QLD Australia. Email: sharma_neelabh@yahoo.com
Citation: Sharma N, Thakur S, Sutcliffe S, Starmer G (2020) Diabetes Management in Myocardial Infarction Patients - A Retrospective Study and Literature Review in Tertiary Level Hospital of Australia. Int Jr Cardiac Sci and Res: IJCSAR-115.
Introduction
Heart failure has been recognised as a leading cause of hospital admission in Australia with an annual cost of $2.7 billion including inpatient and outpatient based managements. As per the epidemiological data of 2016 it’s estimated that over 61000 adult Australian ages >45 have been diagnosed of heart failure every year. Its prevalence and future burden will continue to increase over the next 10 years with predicted 750000 patients and annual health cost of $3.8 billion [1]. Despite the trends and reforms in the management of chronic heart failure its prevalence, mortality and morbidity is on the rise in recent year. Diabetes is a potent and independent risk factor for heart failure [2]. It affects 30-40% heart failure patients as reported in multicentre heart failure registries of ADHERE-AP, ADHERE and EHFS-II [3]. In the last few years the novel treatment of type II Diabetes mellitus has been revolutionising the treatment of chronic heart failure. The use of Sodium-glucose contransporter-2 inhibitor (SGLT2 inhibitor) in patients with cardiomyopathy has been highlighted in major trials. In EMPAREG outcome, CANVAS and DECLARE trial the composite death from cardiovascular causes, hospitalization for heart failure and death from any cause in DMII has been found to be reduced [4,5]. In recently published DAPA-HF trial these positive effects were equally present in patients irrespective of their diabetes status, highlighting the insignificance of cardiotoxicity secondary to hyperglycaemia and support its therapeutic role beyond Diabetes [6].
In the light of above we have planned a quality assurance retrograde study at Cairns hospital to check the use of SGLT2 inhibitors in Diabetics presenting with ischemic events in inpatient setting. This quality assurance (QA) activity aims to identify the use of SGLT2 inhibitors in diabetics with ischemic cardiomyopathy and to document changes in clinical practice from 2015 to 2018.
Keywords: Ischemic Cardiomyopathy; Myocardial Infarction; SGLT2 Inhibitor; Type II Diabetes Mellitus
Study Population
This is a single centre study based at a tertiary level hospital with roughly 2000 heart failure hospital presentation on yearly basis. Cairns hospital provides an extensive range of service for more than 30 regional, rural and remote facilities across a geographical area of 142,900 square kilometres and caters for 250,000 populations. This has more than 20% of the population aged over 60 which is one third higher than national average. Roughly 14% of population is indigenous compared to 3.5% for Queensland as a whole.
Methods
An approval from FNQ human research ethics committee has been obtained for the exemption of full ethical review. We have obtained the data of patients presented with Myocardial infarction (MI) from September to December in 2015 and 2018 through case mix. The data screening was performed with reference of ICD code 121 in the given period. Chart review, medical admission notes, medication list and HbA1c were reviewed to identify the diabetic population. Use of SGLT2 inhibitors was meticulously documented in both the groups. Type I Diabetic was excluded in both cohorts. Variables of age, gender, ethnicity, and year presented, STEMI, Non STEMI, type II MI, eGFR, and HBA1C were noted. The major limiting factors identified were - glycated haemoglobin (HBA1C) <7 and renal function (eGFR <40). Trend analysis was performed to assess the change in practice for the targeted years.
Results
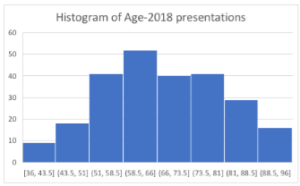
There were 247 hospital presentations in 2018 as compared to 202 in year 2015. 84(34%) patients in 2018 were diabetic with almost equal proportion of male (52%) and females (48%). 46 (54%) patients have identified themselves as Caucasians, 34(40%) as aboriginal and Torres street islander and 4(4.7%) belonged to others. In 2018 cohort the mean age of presentation was 66.72 ± 12.14, with median of 65, 25th quartile of 57.25 and 75th quartile of 76. 15(17%) patients were on SGLT2 inhibitor. HbA1C <7 %( 23%) and poor renal function (22% with eGFR <40) were the major limiting factors in commencing SGLT2 inhibitors (Figure 1 A & B).
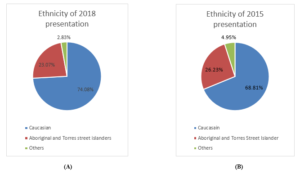
Figure 1 (A & B): Population distribution of 2015 and 2018 presentations showing similar trend.
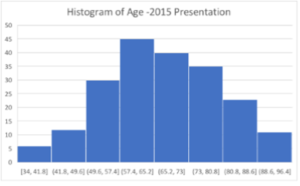
In 2015, out of 67(33%) diabetic patients, 34(50.74%) male and 33(49.25%) females were identified. 31(46%) patients identified themselves as Caucasians, 29(43%) as aboriginal and Torres street islander and 7(10%) as others. Mean age of presentation was 68.28 ± 12.14, with median of 68.5, 25th quartile of 61 and 75th quartile of 75. 3(4%) patients in 2015 cohort were on SGLT2 inhibitors. 17(25%) patients with HbA1C <7% and 12 patients with eGFR <40 were the major limiting factor for SGLT2 commencement (Figure 2 (A & B), Figure 3) (Table 1).
(A)
(B)
Figure 2 A & B: Histogram of 2015 and 2018 patients showing similar age distribution across the population.
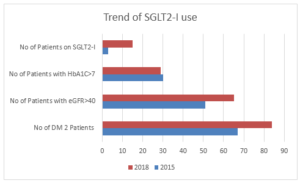
Figure 3: Trend of SGLT2 use – a clear indication of more frequent use in 2018 compared to 2015.
| Baseline characteristic of the patients | ||
| Characteristic | 2018 (247 patients) | 2015 (202 patients) |
|
Age |
Mean 66.72 ± 12.14 | Mean 68.28 ± 12.14 |
| Median 65 | Median 68.5 | |
| 1st Quartile 57.25 | 1st Quartile 61 | |
| 3rd Quartile 76 | 3rd Quartile 75 | |
|
Gender |
Male 145 (58.70%) | Male 127 (62.87%) |
| Female 102 (41.29%) | Female 75 (37.12%) | |
|
Ethnicity |
Caucasian 183 (74.08%) | Caucasian 139 (68.81%) |
| ATSI 57 (23.07%) | ATSI 53 (26.23%) | |
| Others 7 (2.83%) | Others 10 (4.95%) | |
| STEMI | 46 (18.62%) | 43 (21.28%) |
| Non STEMI and Type II MI | 201 (81.37%) | 159 (78.71%) |
| No of DM2 Patients | 84 (34%) | 67 (33.16%) |
| eGFR in Diabetes patients | ||
| >40 | 65 (77.38%) | 51 (76.11%) |
| <40 | 19 (22.61%) | 16 (23.88%) |
| HBA1C in Diabetes patients | ||
| >7 | 29 (34.52%) | 30 (35.71%) |
| <7 | 28 (33.33%) | 18 (21.42%) |
| HBA1C Not valid due to anaemia | 8 (9.52%) | 2 (2.38%) |
| No recent HBA1C | 19 (22.16%) | 14 (16.66%) |
| No of Pts on SGLT2-I | 15 (17.85%) | 3 (4.47%) |
Discussion
SGLT2 inhibitors have shown the significant reduction in overall cardiovascular mortality and rate of hospital admissions. These effects seem to be independent of their glucose lowering action and several hypothesis has been proposed for their cardioprotective mechanism. The EMPAREG outcome, CANVAS and DECLARE -TIMI 58 has shown the reduced hospital admissions and overall mortality in heart failure patients. Outcomes of DAPA HF trial has reemphasized the therapeutic role of SGLT2-I beyond diabetes given its effect in both Diabetes and non-diabetes population.
In EMPAREG outcome trial participants treated with Empagliflozin has 14% reduction in 3 point MACE (Cardiac death, nonfatal myocardial infarction, nonfatal Stroke). There was a 35% reduction in hospital admission within short intervals suggesting a role of acute effect on cardiorenal and hemodynamic system or direct cardiovascular effect. Similar benefits on 3 point MACE were noted in a subgroup of eGFR down to 30ml.min-1.1.73m-2. EMPAREG-OUTCOME has also reported a 39% reduction in the composite renal endpoint, new onset or worsening nephropathy (defined as macroalbuminuria, serum Cr doubling time, eGFR <45 ml.min-1.1.73m-2, initiation of renal replacement therapy or renal related death.) [7].
In CANVAS program the primary endpoint, 3 point MACE was significantly reduced and the risk of hospitalization for heart failure was reduced by 33%. There was a significant reduction in progression of albuminuria by 27% and composite outcome (sustained 40% reduction in eGFR, need of renal replacement therapy or death from renal causes) was reduced by 40% [8].
In DECLARE-TIMI 58 trial Dapagliflozin met the criteria of noninferiority to placebo with respect to MACE (Cardiovascular death, Myocardial infarction, Ischemic stroke) but it did result in lower rate of cardiovascular death or hospitalization for heart failure. There was 13% reduction in renal event in Dapagliflozin group as compared to placebo [9].
In recently published DAPA HF trial the role of SGLT2-I has been found irrespective of the presence or absence of Diabetes. Among patients with heart failure and a reduced ejection fraction the worsening heart failure or death from the cardiac cause was lower among the Dapagliflozin group than the placebo group regardless of the presence or absence of Diabetes. There was 18% reduction in cardiovascular death in Dapagliflozin group as compared to placebo (HR .82; 95% CI, .69 to .98) and 17% reduction in overall death from any cause (HR .83; 95% CI, .71 to .97) [10].
Mechanism of Action
Despite extensive explanatory analysis the exact mechanism of SGLT2-I remains unclear [11]. The likely mechanism of its cardioprotective effect is multidimensional which involves its effect on arterial stiffness [12, 13], cardiac function and cardiac oxygen demand (lack of sympathetic activation) [12], Cardiorenal effect, reduction in albuminuria and uric acid, glycaemic control, body weight, central obesity and blood pressure reduction.
Diabetes patients have increased sympathetic activity due to malfunction of baroreceptors and impediment of negative feedback. This chronic sympathetic drive leads to increased heart rate and afterload providing the trigger for precipitation of heart failure. SGLT2-I have noted to reduce the sympathetic drive by reducing the blood pressure and heart rate and also improve the pressure-diuresis curve leading to reduction in after load [14]. In a Japanese double blind trial of Luseogliflozin there was significant drop in heart rate noted over the 12 week period in the treatment group [15].
SGLT2 receptors are located in the proximal tubules where they reabsorb filtered glucose [16]. Its inhibition leads to glucosuria and an insulin independent reduction in HbA1C along with weight loss leading to a negative caloric balance [17]. This also leads to an Uricosuria via GLUT9 receptors which add to cardioprotective effects as increased uric acids are associated with adverse cardiovascular outcomes [18]. Its effect of natriuresis induction can largely explain the better management of heart failure leading to reduced heart failure hospital admissions [17, 19] however the diuretic effect of SGLT2-I cannot be explained just on the basis of osmotic diuresis as urinary and Na excretion returns to the baseline after a short period however glucose secretion continues to increase [20]. The effect on HbA1C reduction is more clearly related to prevention of micro vascular complication than macro vascular [21, 22].
SGLT2 receptors are not expressed in human cardiac tissue, and its direct effect on cardiac myocytes is unclear [23]. Studies in rat model with Empagliflozin have shown the direct effect on heart muscles by reducing the Na-H exchange with a decrease in intracellular calcium and increased in mitochondrial calcium [24]. Mitochondrial calcium level is a regulator of ATP synthesis pathway and antioxidant pathway. High Mitochondrial calcium levels prevents the sudden death and heart failure in porcine models [25], on the contrary increased intracellular Na and Ca levels have been associated with increased cardiovascular deaths and heart failure [26].
Metabolic substrate shift to ketogenesis has also been highlighted as a possible cardioprotective mechanism of SGLT2-I. Its inhibition leads to direct increased effect on glucagon secretion and reduced insulin secretion, promoting production of ketones [27]. Ketones are considered as cardiac friendly substrate been taken up by cardiac myocytes quickly leading to better cardiac contractility and efficiency. This is in association with increased haematocrit count leading to increased oxygen delivery [28]. The increase in haematocrit is not due to hemoconcentration but rather an independent effect on erythropoietin stimulation. With the low glucose concentration after SGLT2 inhibition in proximal tubule, oxygen consumption of tubular cells decreases leading to improvement in local hypoxia. This leads to improvement in erythropoiesis by fibroblast cells of proximal tubule. This effect is very similar to B blocker effect on cardiac muscles, explaining the remodeling of renal cells by reducing the workload of tubulointerstitial tissues in Diabetes patients [29].
Possible Side Effects
Frequent genitourinary infections, glycosuria induced polyurea leading to volume depletion and acute kidney injury, postural hypotension secondary to reduced preload and euglycemic ketoacidosis has been cautioned as possible side effects [30]. Increased risk of fractures and amputation has only be reported in CANVAS program trial with the use of Canagliflozin but not with other agents [31]. The increased Parathyroid hormone levels with FGF23 increase have been associated with increased risk of Fractures secondary to Canagliflozin [32]. Reassuringly it’s not found with other agents though its cautious use in frail individuals is advised.
Acute kidney injury has been feared as possible side effects with this group of medications though the rate of incidence in both EMPREG outcome and CANVAS program for AKI was very low, its further reassuring as majority of patients in this trial was on concomitant use of RAAS blockers [7]. For safety purposes SGLT2-I should be withheld as a part sick day plan with NSAIDS or Radio contrast administration [33].
Conclusion
This QA activity found, increased use of SGLT2 inhibitors in diabetics with ischemic cardiomyopathy as evident by its use in 17% patients in 2018 compared to 4% in 2015. However, we also identified 10 patients in 2018 with HbA1C >7% and normal renal function who were not on this medication. This warrants a better clinical awareness in practicing physicians. Interestingly there was a large number of patients with HbA1c <7% which was a limiting factor in starting this medication as per our current federal funding guidelines. Given strong evidence of cardioprotective effect of this medication it remains a relevant question that whether we should start using SGLT2 inhibitors as a 1st line medication in diabetic with high cardiac risk profile.
Our literature review highlights the novel mechanism of therapeutics of SGLT2 -I and its role beyond the glycaemic control. It concluded the major salient features of recent trial and summarize their findings meticulously. With the proposed upcoming trials like VERTIS and CREDENCE it will be interesting to see the outcomes of SGLT2-I in nondiabetic kidney disease as well as nondiabetic heart failure.
Disclosure
As per out data base search of PUBMED and Google scholar this is the 1st retrograde study to analyse the trend of use of SGLT2-I in Ischemic cardiomyopathy patients in Australia. The Author has no conflict of interest regarding the publication of paper.
Abbreviations
ADHERE:
AP Acute Decompensated Heart Failure Registry International Asia Pacific
EHFS II:
EuroHeart Failure Survey II
FNQ:
Far North Queensland
ICD:
International statistical classification of disease and related health problems
HbA1C:
Glycated Hemoglobin
MI:
Myocardial infarction
STEMI:
ST Elevated Myocardial Infarction
eGFR:
Estimated glomerular filtrate rate
MACE:
Major adverse Cardiac event
Na-H:
Sodium- Hydrogen
FGF23:
Fibroblast Growth Factor 23
AKI:
Acute Kidney injury
RAAS:
Rennin Angiotensin Aldosterone System
References
- Chan YK, Tuttle C, Ball J, Teng TK, Ahamed Y, et al. (2016) Current and projected burden of heart failure in the Australian adult population: a substantive but still ill-defined major health issue. BMC Health Serv Res 16: 501.
- de Simone G, Devereux RB, Chinali M, Lee ET, Galloway JM, et al. (2010) Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart Study. J Hypertens 28: 353-360.
- Atherton JJ, Hayward CS, Wan Ahmad WA, Kwok B, Jorge J, et al. (2012) Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International-Asia Pacific). J Card Fail 18: 82-88.
- Adingupu DD, Gopel SO, Gronros J, Behrendt M, Sotak M, et al. (2019) SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob(-/-) mice. Cardiovasc Diabetol 18:16.
- Packer M (2019) Lessons learned from the DAPA-HF trial concerning the mechanisms of benefit of SGLT2 inhibitors on heart failure events in the context of other large-scale trials nearing completion. Cardiovasc Diabetol 18: 129.
- Kosiborod MN, Jhund P, Docherty KF, Diez M, Petrie MC, et al. (2020) Effects of Dapagliflozin on Symptoms, Function and Quality of Life in Patients with Heart Failure and Reduced Ejection Fraction: Results from the DAPA-HF Trial. Circulation 141: 90-99.
- Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, et al. (2016) Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. European heart journal 37: 1526-1534.
- Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, et al. (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine 377: 644-657.
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, et al. (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 380: 347-357.
- McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, et al. (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. New England Journal of Medicine 381: 1995-2008.
- Zelniker TA, Braunwald E (2018) Cardiac and Renal Effects of Sodium-Glucose Co-Transporter 2 Inhibitors in Diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol 72: 1845-1855.
- Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, et al. (2014) The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovascular diabetology 13: 28.
- Cardoso CR, Ferreira MT, Leite NC, Salles GF (2013) Prognostic impact of aortic stiffness in high-risk type 2 diabetic patients: the Rio de Janeiro Type 2 Diabetes Cohort Study. Diabetes care 36: 3772-3778.
- Sano M (2017) Hemodynamic Effects of Sodium-Glucose Cotransporter 2 Inhibitors. J Clin Med Res 9: 457-460.
- Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, et al. (2014) Dose-finding study of luseogliflozin in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, double-blind, placebo-controlled, phase II study. Current medical research and opinion 30: 1231-1244.
- Liu JJ, Lee T, DeFronzo RA (2012) Why do SGLT2 inhibitors inhibit only 30–50% of renal glucose reabsorption in humans? Diabetes 61: 2199-2204.
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ (2016) Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 134: 752-772.
- Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZ (2014) Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. American Journal of Physiology-Renal Physiology 308: F77-F83.
- Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, et al. (2014) Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587-597.
- Sano M (2017) Hemodynamic effects of sodium-glucose cotransporter 2 inhibitors. Journal of clinical medicine research 9: 457.
- Scheen AJ (2014) Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet 53: 213-225.
- Devineni D, Polidori D (2015) Clinical Pharmacokinetic, Pharmacodynamic, and Drug-Drug Interaction Profile of Canagliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor. ClinPharmacokinet 54: 1027-1041.
- Lambert R, Srodulski S, Peng X, Margulies KB, Despa F, et al. (2015) Intracellular Na+ Concentration ([Na+] i) Is Elevated in Diabetic Hearts Due to Enhanced Na+–Glucose Cotransport. Journal of the American Heart Association 4: e002183.
- Baartscheer A, Schumacher CA, Wüst RC, Fiolet JW, Stienen GJ, et al. (2017) Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 60: 568-573.
- Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, et al. (2014) Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circulation research 115: 44-54.
- Erhardt LR (1999) GUARD During Ischemia Against Necrosis (GUARDIAN) trial in acute coronary syndromes. The American journal of cardiology 83: 23-25.
- Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, et al. (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nature medicine 21: 512.
- Martens P, Mathieu C, Verbrugge FH (2017) Promise of SGLT2 inhibitors in heart failure: diabetes and beyond. Current treatment options in cardiovascular medicine 19: 23.
- Gilbert RE (2016) SGLT2 inhibitors: β blockers for the kidney? The Lancet Diabetes & Endocrinology 4: 814.
- Bakris GL, Fonseca VA, Sharma K, Wright EM (2009) Renal sodium–glucose transport: role in diabetes mellitus and potential clinical implications. Kidney international 75: 1272-1277.
- Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, et al. (2017) Rationale, design and baseline characteristics of the CANagliflozincardioVascular Assessment Study–Renal (CANVAS‐R): A randomized, placebo‐controlled trial. Diabetes, Obesity and Metabolism 19: 387-393.
- Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E (2017) Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes research and clinical practice 130: 53-60.
- Cherney DZ, Udell JA (2016) Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists: with great power comes great responsibility. Circulation 134: 1915-1917.