Publication Information
ISSN: 2641-693X
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Cytology versus Biopsy Yield in Patients with Malignant Pleural Effusion at the Lagos University Teaching Hospital, Nigeria: A prospective review
Olugbemi AJ1*, Ogunleye EO1, Olusoji OO1, Ojo OO1, Sanni SB1, Olugbemi M2
1Cardiothoracic Surgery Unit, Department of Surgery, Lagos University Teaching Hospital
2General Surgery Unit, Department of Surgery, Lagos University Teaching Hospital.
Received Date: February 17, 2020; Accepted Date: February 29, 2020; Published Date: March 06, 2020
*Corresponding author: Olugbemi Aj, Small-Bore versus Large-Bore chest drain in the Management of Patients with Pleural Effusion at the Lagos University Teaching Hospital, Nigeria. Tel: +2348082550192, +2348055463013. Email: austinjostin@yahoo.com
Citation: Olugbemi AJ, Ogunleye EO, Olusoji OO, Ojo OO, Sanni SB, Olugbemi M (2020) Cytology versus Biopsy Yield in Patients with Malignant Pleural Effusion at the Lagos University Teaching Hospital, Nigeria: A prospective review. Int Jr Cardiac Sci and Res: IJCSAR-117.
Abstract
Background: The yield of cytology and percutaneous pleural biopsies has been researched into by many investigators and the findings have varied. The aim of this study was to determine the percentage yield of malignant cells from cytology and percutaneous needle pleural biopsies of malignant pleural effusions and to determine the common aetiologies of malignant pleural effusions presenting at the Lagos University Teaching Hospital (LUTH), Lagos. Nigeria.
Methods: A total of 55 consecutive patients with suspected malignant pleural effusion were recruited for this study within a 1 year period. Pleural aspirate was obtained from each patient and cytological analysis done. Percutaneous pleural biopsy was also done for each patient and histological analysis done. The diagnostic yields were then obtained for both cytology and percutaneous pleural biopsy and compared. The causes of these effusions were also noted in this study.
Results: The percentage yield of Cytology in patients with pleural effusion was 67.3%. Four (7.3%) results were inconclusive. However, the percentage yield of pleural biopsy was 56.5% (31 patients). The yield was better (81.8%) in patients that had both procedures done with either cytology or pleural biopsy being positive. For both Cytology and pleural biopsy, the yield was positive in 40% and negative in 12.7%. When compared with pleural biopsy yield, the cytology yield was not statistically significant (P-value – 0.252), however, the yield was higher. In this study, pleural effusion was caused by breast cancer in majority of the patients (31 patients– 56.4%). Gynaecological malignancies were responsible for another 25.5%. Together, breast and gynaecological malignancies made up 81.9% of the diagnoses. 1 patient (1.8%) had oesophageal cancer. Others included chest wall tumour (1.8%) and laryngeal tumour (1.8%).
Conclusion: The yield of cytology in the diagnosis of malignant pleural effusions was higher than that of percutaneous pleural biopsy, though, not statistically significant. However, evaluating these patients with percutaneous pleural biopsy as a diagnostic tool increased the chances of arriving at a diagnosis. Thus, percutaneous pleural biopsy is a useful tool in evaluating a patient with pleural effusion. Breast cancer is the leading cause of malignant pleural effusions in our environment.
Keywords: Cytology, Closed pleural biopsy, Malignant pleural effusion
Introduction
A malignant pleural effusion is a clinical condition in which cancer cells cause an abnormal amount of pleural fluid collection within the pleural space. Malignant pleural effusions are common clinical problems in patients with neoplastic disease. In one postmortem series, malignant effusions were found in 15% of patients who died with malignancies [1]. Malignant pleural effusion is also one of the leading causes of exudative effusions. Studies have demonstrated that 42 to 77% of exudative effusions are secondary to malignancy [2, 3]. About 40,000 cases of pleural effusions are attributable to cancer every year in the UK, and 175 000 in the USA [4]. Incidence of primary pleural malignant disease— mesothelioma—is rapidly rising in the UK, and is predicted to account for about 1% of all deaths in UK men born in the 1940’s [4, 5]. Cytological examination of pleural fluid for malignant cells establishes a positive diagnosis of malignancy in only 60% of carcinomatous effusions and 30% of effusions secondary to mesothelioma [6, 7]. Pleural biopsy to enable histological examination is needed for accurate diagnosis in the remainder. Pleural biopsy is therefore an important diagnostic method.
Malignant pleural effusions are confirmed by finding cancerous cells in pleural fluid or in pleural tissue by percutaneous needle biopsy or at thoracoscopy, thoracotomy, or autopsy. In some patients with established malignancies who have pleural effusions, malignant cells cannot be demonstrated in either pleural fluid or pleural tissue and most likely are not present at the time of the diagnostic procedure. Sahn labelled such effusions “paramalignant” because they are associated with and caused by the malignancies but do not result from pleural invasion by tumor [8]. Paramalignant effusions can be caused by a direct local effect of the tumor, by systemic manifestations of the malignancy, or as a consequence of therapy. Impaired lymphatic drainage of the pleural space is an important mechanism responsible for the formation of both paramalignant and malignant pleural effusions.
The diagnostic yield is dependent on such factors as extent of disease and the nature of the primary malignancy. Studies have shown a large variation in diagnostic yields ranging from 62 to 90% [9, 10, 11, 12, 13].
Thoracentesis can be performed on almost any patient with a pleural effusion. There are no absolute contraindications.[14] Relative contraindications include a bleeding diathesis, systemic anticoagulation, a small volume of pleural fluid, mechanical ventilation, inability of the patient to cooperate, and cutaneous disease such as herpes zoster infection at the needle entry site.[15]
Diagnostic thoracentesis is performed to determine the specific cause of a pleural effusion. Since pathognomonic findings are often absent, efforts have focused on using various characteristics of pleural fluid to guide the subsequent diagnostic approach. [14, 16] Studies of pleural fluid characteristics in patients with diseases of known etiology have been used to develop criteria for separating effusions into transudates and exudates, each of which has a distinct differential diagnosis.
In malignant effusions, closed pleural biopsies are less sensitive than pleural fluid cytology. These blind percutaneous biopsies of the costal (parietal) pleura report a diagnostic yield of 40 to 75% [12, 13, 17, 18, 19]. If abnormalities of the pleura are identified on Computed Tomography (CT), as in mesothelioma, a CT-guided biopsy is recommended [20]. The relatively low yield of blind pleural biopsy is due to several factors, including early stage of disease with minimal pleural involvement, distribution of tumor in areas not sampled during blind biopsy, and operator inexperience [21]. However, studies have shown that 7 to 12% of patients with malignant effusions may be diagnosed by pleural biopsy when fluid cytology is negative [17],[13].
Contraindications to pleural biopsy include bleeding diathesis, anticoagulation, chest wall infection, and lack of patient cooperation. Important complications include pneumothorax, hemothorax, and vasovagal reactions. Post biopsy pneumothoraces are frequently due to air entry from the needle during the procedure and often do not require intervention. A rapid clinical deterioration or increased postprocedure effusion, should alert the clinician to possible hemothorax [22].
Ukadike and Ezekiel [23] retrospectively studied all consecutive cases of pleural biopsies done for indeterminate cause of pleural effusion in the University of Benin Teaching Hospital from December 2008 to May 2010. Blind pleural biopsy was carried out using the Abram’s Pleural Biopsy Needle. A total of 16 cases were retrieved, with a mean age of 46.8 ± 15.2 years and age range of 25-72years, with a male: female ratio of 3:5. All the pleural effusion were exudates. In their conclusion, blind pleural biopsy still sub serves as a useful tool in the evaluation of indeterminate cases of pleural effusion where its use can be taught and safely practiced.
Marel et al [24], in a prospective consecutive case series from 1986 to 1990, using one hundred seventy-one adults between ages 18 and 70 years with a pleural effusion found that 45% of the patients had malignant effusions, 19% had paramalignant effusions, and 36% had benign diseases, the pleural fluid cytologic study was the best for establishing a diagnosis. They concluded that patients with an undiagnosed pleural effusion should be evaluated in an individualized stepwise manner. If malignancy was strongly considered, the initial three steps should be relatively noninvasive and include clinical evaluation and cytologic study.
James et al [25], assessed the diagnostic yield and safety of closed pleural biopsy in patients with pleural effusion. 48 consecutive cases of pleural effusion were evaluated with complete pleural fluid biochemical and microbiological analysis, cytology, routine bacterial and mycobacterial cultures. In all these 48 cases, closed pleural biopsy was done with tru-cut biopsy needle and the samples were assayed for histopathology and mycobacterial culture. The results showed that the main causes of pleural effusion were Tuberculosis in 21 (43.8%) cases, Malignancy in 14 (29.2%), paramalignant effusion in six (12.5%), empyema in three (6.3%), transudative effusion in three (6.3%) and parapneumonic effusion in one (1.9%) case. Diagnostic yield of closed pleural biopsy was 62.2% in cases of all exudative pleural effusion, 76.2% in cases of tuberculous pleural effusion and 85.7% in malignant pleural effusion. There was no incidence of post biopsy pneumothorax or hemothorax, thereby affirming the safety of pleural biopsy procedure. They concluded that closed pleural biopsy provided the highest diagnostic yield in cases of pleural tuberculosis and malignancy, the two most important causes of exudative pleural effusion. In view of low cost, easy availability and very low complication rates, it is a veritable diagnostic tool in the hands of a trained pulmonary physician.
In another study, Ong et al [26] assessed the diagnostic yield of pleural fluid cytologic examination in patients with suspected malignant pleural effusions.. They retrospectively reviewed the results of pleural fluid cytologic examination performed in 103 patients who presented with suspected malignant pleural effusions. Initial pleural fluid cytology was positive for malignancy in 48.5% of patients. The yield of this diagnostic procedure was improved with repeated pleural fluid cytologic specimens and when combined with a percutaneous pleural biopsy. They thus concluded that pleural fluid cytologic examination is a useful initial step in the diagnostic work-up of patients with suspected malignant pleural effusions. The diagnostic yield of such examination is improved with repeated pleural fluid cytologic specimens and when combined with a percutaneous pleural biopsy. Clinical presentation and pleural fluid characteristics were inadequate to differentiate malignant fromparamalignant effusions.
In a study by Salyer et al [27], a comparison was made of the efficacy of pleural needle biopsy and pleural-fluid cytopathology in the diagnoasis of pleural tumor in a group of 271 patients. A malignant tumour involving the pleura was present in 95 cases. Needle biopsy alone provided a diagnosis of tumour in 53 instances (55.8%), and cytopathologic preparations were diagnostic in 69 patients (72.6%). A diagnosis was established on either the biopsy or cytopathology, or both, in 86 cases (90 percent). These results indicate the value of using both biopsy and fluid cytology in the evaluation of pleural effusion, which often is due to involvement of the pleura by malignant neoplasm.
The yield of cytology and pleural biopsy in patients with malignant pleural effusions has thus been debatable. Most of the published works favour a better yield by cytology [10, 11, 12] against biopsy [12, 13, 18]. However, some researchers are now proposing a combination of the two procedures for better diagnostic yield [26]. This work therefore seeks to determine the diagnostic yield of these two procedures in patients with malignant pleural effusion at the Lagos University Teaching Hospital, Nigeria. A satisfactory search of local and international literature revealed little if anything published on this subject from the West African sub-region. It seeks to answer the question of whether both procedures should be combined in patients with malignant pleural effusion. It also seeks to bridge the identified knowledge gap on this subject in Nigeria and West Africa as there is a paucity of published work on the subject. The objective is to compare the rate of malignant cell yield from cytology with that obtained from pleural biopsy, in malignant pleural effusions.
Materials and Methodology
Study design
The study was a prospective hospital – based study involving all patients (including children) who presented at the Lagos University Teaching Hospital (LUTH) with pleural effusion. The study spanned 12 months (Feb 2013 – Jan 2014). The study included all patients with pleural effusion whose history, physical examination and investigations including radiographs, computerized tomography scans, ultrasound scans, biopsies (other than pleural) e.t.c, were suggestive of malignancy. The following categories of patients were excluded:
- Those with pleural effusion secondary to heart failure
- Those with pleural effusion secondary to pulmonary tuberculosis
- Those with pleural effusion secondary to trauma
- Those with pleural effusion secondary to surgical intervention
- Those with pleural effusion secondary to diseases other than malignancy.
Ethical approval
Approval was obtained from the Research and Ethics Committee of the Lagos University Teaching Hospital before the commencement of the study. Informed consent was also obtained from the patients or parents (for children) before being administered with the questionnaires. Approval was obtained from the managing consultant before enrolling the patients on the scheme.
Study population
The study was conducted on patients including children with pleural effusion, presenting at the Accident and Emergency centre (A/E), Children Emergency Room (CHER), referrals from various wards in the hospital and Surgical Out-patient Clinics, at the Lagos University Teaching Hospital (LUTH), located within Surulere Local Government Area (LGA). Lagos state. Nigeria.
Data collection
Patient who met the inclusion criteria in the stipulated locations and circumstances, was administered with the Questionnaire after informed consent was obtained. Data collected included: biodata, history, physical examination, investigations, diagnosis, treatment and outcome.
Thoracentesis: Procedure
This was done at the bed side of the patient. The patient was placed in a sitting position, debilitated and bedridden patients made as upright as possible by elevating the back of the bed or placed in the lateral decubitus position with the side of the effusion down.
The presence and level of pleural effusion was first confirmed by percussing the chest for stony dullness and/or chest radiograph. An appropriate area of the chest wall was then cleaned with sterile methylated spirit-soaked gauze. After the instillation of a local anesthetic (2% Lidocaine with Adrenaline) into the skin and subcutaneous tissue, an 18- or 20-gauge needle was introduced close to the superior border of the rib in order to avoid the intercostal vessels and nerve, and then advanced as the injection of local anaesthetic continued intermittently as necessary into the parietal pleura. The needle was then advanced further into the effusion and 100mls aspirated and used within the hour for cytological analysis, for the presence of malignant cells. Firm pressure was then applied to the puncture site until any bleeding ceased. Pleural aspirate was analyzed within two hours of receiving the specimen.
All specimens were sent to the same experienced Pathologist for analysis.
Closed Pleural Biopsy: Procedure
This was also done at the bed side of the patient. Abrams’ needle (Figure 1) was used to obtain pleural biopsy specimens. The needle has an outer cannula with trocar point and cutting window, which can be closed with a turning action of the inner tube and an inner stylet. The patient was placed in a sitting position.
The presence and level of pleural effusion was first ascertained by percussing the chest for stony dullness and/or chest radiograph. A single dose of 1gram of intravenous ceftriaxone was administered for prophylaxis. The entry site was located posteriorly below the superior aspect of the percussible dull area. The area was then cleaned with dilute Savlon, and then with methylated spirit. After the instillation of a local anesthetic (2% Lidocaine with Adrenaline) into the skin and subcutaneous tissue, a small incision (approximately 3-5mm) was made, and the Abram’s needle passed through the parietal pleura, at which time a popping sensation was noted. The device was then withdrawn slightly until the pleura was engaged by the notch. The specimen was then severed by advancing the cutting cannula. 7-10 samples were obtained and put into a universal bottle containing Formalin. A firm dressing was then applied to the area. This specimen was then sent to the Laboratory for histological analysis, accompanied by a properly filled histology request form. All specimens were sent to 1(one) experienced pathologist for analysis. Parenteral analgesics were also administered to the patient after the procedure (Figure 01).
 Figure 01: Abram’s needle
Figure 01: Abram’s needle
Data analysis
Collected data was collated and analyzed using the Statistical Package for Social Science (SPSS) version 21 (SPSS© Chicago, Ill). Results were analyzed using Tables, charts and diagrams. Tests of significance was used where necessary and unless otherwise stated, a p-value of <0.05 was considered as significant.
Results
Fifty five patients who met the inclusion criteria were used for this study. The results and findings are depicted in the following tables and pictograms. The mode of the age ranges was 36-65 years, – 26 patients (47.3%) (Table I). Mean age range was 36-65years. No patient fell within the age group less than 10years of age. Cumulatively, 39 patients (70.9%) were aged between 21 and 65 years (Table I). Only 4 patients (7.3%) were aged more than 65 years, Table 1.
Age (Years)
Frequency
Percentage (%)
<1
0
0
10-Jan
0
0
20-Nov
12
21.8
21-35
13
23.6
36-65
26
47.3
>65
4
7.3
Total
55
100
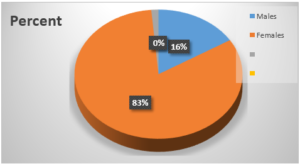 Figure 2: Sex distribution of patients
Figure 2: Sex distribution of patients
Ninety-eight percent (54) of the patients presented with difficulty in breathing (33 patients – 60% on exertion and 21 patients – 38.2% at rest), (Table II). 37 patients – 67.3% presented with cough, 17 patients – 30.9% with chest pain, 44 patients – 80% with anorexia and 51 patients – 92.7% with malaise, (Table II). Associated symptoms at presentation included weight loss, 47 patients – 88.7%; haemoptysis, 3 patients – 5.7%; abdominal distension, 34 patients – 64.2%; abdominal mass, 13 patients – 24.5%; breast mass, 23 patients – 43.4%, leg swelling, 18 patients – 34.0% and upper limb swelling, 5 patients – 5.8%. Table 2.
Symptoms
Frequency
Percentage (%)
Difficulty Breathing
54
98.20%
Cough
37
67.30%
Chest pain
17
30.90%
Anorexia
44
80.00%
Malaise
51
92.70%
(Total number of patient – 55)
The nature of pleural effusion in the patients is depicted in Table III below. Majority of the patients (27, 49.1%) had serosanguinous effluent. Only 1.8% had purulent effluent with serous and sanguinous effluent fairly equal. Table 3. Table 3: Distribution of nature of pleural effusion In this study, the percentage yield of cytology in patients with pleural effusion was 67.3% (Table IV). Four (7.3%) results were inconclusive. However, the percentage yield of pleural biopsy was 56.5% (31 patients) as shown in Table V. The yield was better (81.8%) in patients that had both procedures done with either cytology or pleural biopsy being positive. For both Cytology and pleural biopsy, the yield was positive in 40% and negative in 12.7%, (Figure 3). Though the yield was higher, when compared with pleural biopsy yield, the cytology yield was not statistically significant (P-value – 0.252). Total
Frequency
Percentage (%)
Serous
13
23.6
Serosanguinous
27
49.1
Sanguinous
14
25.5
Purulent
1
1.8
Total
55
100
Frequency
Percentage (%)
Negative
14
25.5
Positive
37
67.3
Inconclusive
4
7.3
Total
55
100
Frequency
Percentage (%)
Negative
15
27.3
Positive
31
56.4
Inconclusive
9
16.4
Total
55
100
Pleural Biopsy
Negative
Positive
Inconclusive
Cytology
Negative
7
6
1
14
Positive
7
23
7
37
Inconclusive
1
2
1
4
Total
15
31
9
55
P=0.252 (chi-square)
Positive
Negative
Inconclusive
Total
Cytology
57
14
4
55
Pleural Biopsy
31
15
9
55
Frequency
Percentage (%)
Negative
13
23.6
Positive
22
40
Inconclusive
13
23.6
None
7
12.7
Total
55
100
Frequency
Percentage (%)
Negative
7
12.7
Positive
45
81.8
inconclusive
3
5.5
Total
55
100
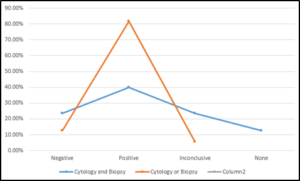
Figure 3: Comparison of ‘Cytology and Biopsy yield’ and ‘Cytology or Biopsy yield’
In this study, pleural effusion was caused by breast cancer in majority of the patients (31 patients– 56.4%), as illustrated in Table IX. Gynaecological malignancy was responsible for another 25.5%. Together, breast and gynaecological malignancies made up 81.9% of the diagnoses. 1 patient (1.8%) had oesophageal cancer. ‘Others’ included chest wall tumour and laryngeal tumour.
Frequency
Percentage %
Diagnosis
Breast cancer
31
56.4
Cervical cancer
5
9.1
Ovarian cancer
4
7.3
Endometrial cancer
5
9.1
Lung cancer
4
7.3
Mesothelioma
2
3.6
Mediastinal – lymphoma
1
1.8
Oesophageal cancer
1
1.8
Others
2
3.6
Total
55
100.0
Discussion
Most of the patients recruited for this study were females – 84%. This is in contrast to result obtained by Puncho et al [28] in which 42% of the patients were males, but similar to result obtained by Ukadike et al [23].
The mode of the age ranges was 36-65, – 26 patients (47.3%). Ukadike [29] had similar results. No patient fell within the age group less than 10yrs of age. Elderly patients made up 7.3% of the patients. This figure was also not in keeping with the findings of Puncho et al [28].
Dyspnoea was the commonest symptom at presentation representing 98% of the patients (60% on exertion and 38.2% at rest) as also demonstrated by Taryle and colleagues [30]. This was as a result of the fact that most of the patients presented with moderate and massive pleural effusion. More so, some of the patients had lung parenchymal disease, especially patients with breast cancer. 67.3% presented with cough, 30.9% with chest pain, 80% with anorexia and 92.7% with malaise. Chernow and Sahn [31] and Weick et al [32] reported that up to 25% of patients with carcinoma or lymphoma of the pleura, respectively, may be relatively asymptomatic when the pleural effusion is initially discovered on a routine chest radiograph.
Associated symptoms at presentation included weight loss, 88.7%; haemoptysis, 5.7%; abdominal distension, 64.2%; abdominal mass, 24.5% (especially those who presented with suspected gynaecological malignancies); breast mass, 43.4% (in patients with breast cancer) and leg swelling, 18 – 34.0%. A total of 60% of patients had lymphadenopathy (25% axillary, 20% groin, 9.1% supraclavicular, 5.5% cervical). Chernow and Sahn[31] noted that signs of a pleural effusion are typically found on physical examination and cachexia and lymphadenopathy may be seen in cancer.
The nature of pleural effusion in the patients varied. 27 patients, (49.1%) had serosanguinous effluent. 13 patients (23.6%) had serous effluent, 14 (25%) sanguinous while 1 (1.8%) had purulent effluent. Similar result was obtained by Ukadike who found in a retrospective study that 56.2% were haemorrhagic and 31.2% were straw colored and 12.5% were pyogenic [23].
The percentage yield of cytology in patients with malignant pleural effusion was 67.3% as noted in this study. Four (7.3%) results were inconclusive, showing atypical cells. However, the percentage yield of pleural biopsy was 56.5% (31 patients) with 16.4% inconclusive, specimen containing muscle cells stroma and blood. When cytology and pleural biopsy were put together, the yield was higher (81.8%). Together, the diagnostic yield was positive in 40% and negative in 12.7% for both cytology and pleural biopsy. When compared with pleural biopsy yield, the cytology yield was not statistically significant (P-value – 0.252), however, the yield was higher as shown above.
James P and colleagues [25] in their study to evaluate the diagnostic yield of pleural biopsy, found that 85.7% of cases of malignant pleural effusions were positive. In another study by Ong et al, initial pleural fluid cytology was positive for malignancy in 48.5% of patients. The yield of this diagnostic procedure was improved with repeated pleural fluid cytologic specimens and when combined with a percutaneous pleural biopsy [33]. In a study by Salyer et al [27], a comparison was made of the efficacy of pleural needle biopsy and pleural-fluid cytopathology in the diagnoasis of pleural tumor in a group of 271 patients. A malignant tumour involving the pleura was present in 95 cases. Needle biopsy alone provided a diagnosis of tumour in 53 instances (55.8%), and cytopathologicpreparations were diagnostic in 69 patients (72.6%). A diagnosis was established on either the biopsy or cytopathology, or both, in 86 cases (90 percent).
In this study, pleural effusion was caused by breast cancer in majority of the patients (31patients – 56.4%). Gynaecological malignancies including cervical, ovarian and endometrial cancer were responsible for 25.5%. Lung cancer made up 7.3 %, mesothelioma 3.6%, lymphoma 1.8% and 1 patient (1.8%) had oesophageal cancer. ‘Others’ included chest wall tumour and laryngeal tumour accounting for 1.8% and 1.8% respectively. The distribution of the diagnoses and age range showed a predominance of malignancy among the 36-65years age range (56.3%). Four (7.2%) of the patients were older than 65years. No malignancy was noted in patients less than 11years old. There was no statistical significance between the diagnoses and age range.
Ogunleye et al [34] in a review of 372 patients noted M:F ratio was 1:1 approximately. The combined mean age was 37.8 ± 0.92 years at 95% confidence interval. Malignant effusions constituted majority of sample size and the right side was consistently affected more often than the left side. They concluded that advanced malignancies were the commonest causes of symptomatic pleural effusions and that within the group breast carcinoma exerted much weight over and above other malignancies. Malignant causes of pleural collection accounted for 212 (57.0%) of all cases. Within this group, breast carcinoma constituted 46.7% of the malignant cases. This was followed bybronchogenic and ovarian carcinomas which were 20 (5.3%) and 10 (2.7%) cases respectively.
In the study by Ong et al [33]., The underlying malignancies in the patients were: bronchogenic carcinoma (51.5%), breast carcinoma (29.1%), hepatocellular carcinoma (1.9%), carcinoma of the stomach (1.9%), malignant mesothelioma, nasopharyngeal carcinoma, renal cell carcinoma, carcinoma of the oesophagus, lymphoma, carcinoma of the colon (1% each), unknown (9.7%).
Conclusion
The percentage yield of cytology in patients with pleural effusion was 67.3%. However, the percentage yield of pleural biopsy was 56.5%. This may be due to the fact that percutaneous pleural biopsy is a blind procedure, and diseased areas in the pleura may be missed. The yield was better (81.8%) in patients that had both procedures done with either cytology or pleural biopsy being positive. When compared with pleural biopsy yield, the cytology yield was not statistically significant. In patients with pleural effusion, many centers don’t do blind percutaneous pleural biopsy because they feel the yield is low, except for those who have the resources and equipment for image guided pleural biopsy. This is however not readily available in resource poor countries like ours. This study has shown therefore, that blind percutaneous pleural biopsy continues to be a relevant tool in diagnosing malignant pleural effusions. We thus recommend both Cytology and pleural biopsy in patient’s suspected malignant pleural effusion.
Reference:
- Rodriguez-Panadero F, Borderas Naranjo F, Lopez-Mejias J (1989) “Pleural metastatic tumours and effusions: frequency and pathogenic mechanisms in a post-mortem series,” Eur Respir J 2: 366-369.
- Marel M, Zrustova M, Stasny B, Light RW (1993) “The incidence of pleural effusion in a well-defined region: epidemiologic study in central Bohemia,” Chest 104: 1486-1489.
- Valdes L, Alvarez D, Valle JM, Pose A, San Jose E (1996) “The etiology of pleural effusions in an area with high incidence of tuberculosis,” Chest 109: 158-162.
- J Wiggins (2001) British Thoracic Society Standards of Care Committee, “Statement on malignant mesothelioma in the United Kingdom,” Thorax 56: 250-265.
- Peto J, Hodgson JT, Matthews FE, Jones JR (1995) “Continuing increase in mesothelioma mortality in Britain,” Lancet 345: 535-539.
- Renshaw AA, Dean BR, Antman KH, Sugarbaker DJ, Cibas ES (1997) “The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma,” Chest 111: 106-109.
- Peto J, Decarli A, La Vecchia C, Levi F, Negri E (1999) “The European mesothelioma epidemic,” Br J Cancer 79: 666-672.
- Sahn SA (1985) “Malignant pleural effusions,” Clin Chest Med 6: 113.
- Johnston WW (1985) “The malignant pleural effusion: a review of cytopathological diagnoses of 584 specimens from 472 consecutive patients,” Cancer 56: 905-909.
- Hsu C (1987) “Cytologic detection of malignancy in pleural effusion: a review of 5,255 samples from 3,811 patients,” Diag Cytopathol 3: 8-12.
- Molengraft FL, Vooijs GP (1988) “The interval between the diagnosis of malignancy and the development of effusions, with reference to the role of cytologic diagnosis,” Acta Cytol 32: 183-187.
- Starr RL, Sherman ME (1991) “The value of multiple preparations in the diagnosis of malignant pleural effusions,” Acta Cytol 35: 533-537.
- Loddenkemper R, Grosser H, Gabler A, Mai J, Preussler H, et al. (1983) “Prospective evaluation of biopsy methods in the diagnosis of malignant pleural effusions: intrapatient comparison between pleural fluid cytology, blind needle biopsy and thoracoscopy,” Am Rev Respir Dis 127: 114.
- Sahn SA (1988) “The pleura,” Am Rev Respir Dis 138: 184-234.
- Light RW, Macgregor I, Luchsinger PC, Ball WC (1972) “Pleural effusions: the diagnostic separation of transudates and exudates,” Ann Intern Med 77: 507-513.
- Burgher LW, Jones FL Jr, Patterson JR, Selecky PA (1989) “Guidelines for thoracentesis and needle biopsy of the pleura,” Am J Respir Dis 140: 257.
- Prakash UBS, Reiman HM (1985) “Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusions: analysis of 414 cases,” Mayo Clin Proc 60: 158-164.
- Poe RH, Israel RH, Utell MJ, Hall WJ, Greenblatt DW, et al. (1984) “Sensitivity, specificity, and predictive values of closed pleural biopsy,” Arch Intern Med 144: 325-328.
- Escudero BC, Garcia CM, Cuesta CB, Molinos ML, Rodriguez RS, et al. (1990) “Cytological and bacteriologic analysis of fluid and pleural biopsy specimens with Cope’s needle,” Arch Intern Med 150: 1190-1194.
- Beauchamp HD, Kundra NK, Aranson R, Chong F, MacDonnell KF (1992) “The role of closed pleural needle biopsy in the diagnosis of malignant mesothelioma of the pleura,” Chest 102: 1110-1112.
- Canto A, Rivis J, Saumench J, Morera R, Moya J (1983) “Points to consider when choosing a biopsy method in cases of pleurisy of unknown origin,” Chest 84: 176-179.
- Sahn SA (1991) “Thoracentesis and pleural biopsy In: Shelhamer J, Pizzo PA, Parillo JE, Masur H, editors. Respiratory disease in the immunosuppressed host. Philadelphia: J.B,” Lippincott, pp. 129.
- Ukadike OS, Ezekiel U (2010) “Pleural biopsy for indeterminate cases of pleural effusion,” Annals of Biomedical Sciences.
- Marel M, Stastny B, Melínová L, Svandová E, Light RW (1995) “Diagnosis of pleural effusions. Experience with clinical studies, 1986 to 1990,” Chest 107: 1598-603.
- James P, Gupta R, Christopher DJ, Balamugesh T (2010) “Evaluation of the diagnostic yield and safety of closed pleural biopsy in the diagnosis of pleural effusion.” Indian J Tuberc 57: 19-24.
- Ong KC, Indumathi V, Poh WT, Ong YY (2000) “The diagnostic yield of pleural fluid cytology in malignant pleural effusions,” Singapore Med J 41: 19-23.
- Salyer WR, Eggkston C, Erozan YS (1975) “Efficacy of Pleural Needle Biopsy and Pleural Fluid Cytopathology in the Diagnosis of Malignant Neoplasm Involving the Pleura,” CHEST 67: 536-539.
- Puncho Gurung, Mark R, Goldblatt DO, John T, et al. (2009) Huggins MD, “PLEURAL FLUID CHARACTERISTICS OF PARAMALIGNANT EFFUSION,” CHEST 136: 44S-c-45S.
- Ukadike OS, Ezekiel U (2010) “Pleural biopsy for indeterminate cases of pleural effusion,” Annals of Biomedical Sciences, no. 1596-6569.
- Taryle DA, Lakshminarayan S, Sahn SA (1976) “Pleural mesotheliomas. An analysis of 18 cases and review of the literature,” Medicine (Baltimore) 55:153-162.
- Chernow B, Sahn SA (1977) “Carcinomatous involvement of the pleura: an analysis of 96 patients,” Am J Med 63: 695-702.
- Weick JK, Kiely JM, Harrison EG (1973) “Pleural effusion in lymphoma. Cancer,” 31: 848-853.
- Ong KC, Indumathi V, Poh WT, O. YY (2000) “The diagnostic yield of pleural fluid cytology in malignant pleural effusions.,” Singapore Med J 41: 19-23.
- Ogunleye E, Thomas E, Olusoji O (2013) “Aetiology and Demographic Attributes of Common Pleural Collections in an African Population,” Surgical Science 4: 332-338.