Crizotinib as First Line Therapy in Advanced NSCLC with MET Amplification
Alexia Trevino-Quiroga1, Lea Shim2, Alejandro Calvo1*
1Department of Medical Oncology, Kettering Cancer Center, Kettering, Ohio, USA
2Departments of Graduate Medical Education, Grandview Medical Center, Dayton, Ohio, USA
Received Date: September 03, 2019; Accepted Date: September 10, 2019; Published Date: September 19, 2019
*Corresponding author: Alejandro Calvo, Department of Medical Oncology, Kettering Cancer Center, Kettering, Ohio, USA. Email: alejandro.calvo@ketteringhealth.org
Citation: Trevino-Quiroga A, Shim L, Calvo A, (2019) Crizotinib as first line therapy in advanced NSCLC with MET Amplification. Curr Res in Onco: CRIO-106.
Abstract
De novo mesenchymal-epithelial transition (MET) amplification is seen in 2-4 % of patients with non-small cell lung cancer (NSCLC) at diagnosis. MET amplification is also seen in 5-20 % of patients with endothelial growth factor receptor (EGFR)-mutated tumors that have acquired resistance to EGFR tyrosine kinase inhibitors (TKI) [1]. MET amplification is most commonly detected by fluorescence in-situ hybridization testing (FISH) or next generation sequencing (NGS) panels, unfortunately, current diagnostic molecular panels do not include screening for this particular molecular aberration in a routine manner [1, 4-6].
We present a patient with advanced NSCLC associated to high levels of MET amplification that was treated with first-line crizotinib and experienced a dramatic and swift response to targeted therapy. Guidelines for treatment of patients with MET abnormalities are ambiguous, mostly recommending the use of targeted therapy as second line or beyond. This unique case report, along with other publications, support the value of testing for MET amplification along with other standard tumor biomarkers at the time of diagnosis in patients with advanced-stage non-squamous NSCLC. This select group of patients could largely benefit from the use of first-line MET-targeted therapy. This approach would allow the use of a more tolerable long-term therapy upfront and moving more toxic and less effective therapies to later lines.
Introduction
Lung cancer is the second most common type of cancer diagnosed in both females and males in the United States. The incidence of lung cancer in 2019 is estimated to be over 228,000 cases. Lung cancer is currently the leading cause of cancer-related death with a mortality of approximately 142,000 deaths per year [2]. The most common type of lung cancer is NSCLC, seen in 85% of patients. NSCLC can be further classified into the following histologic subtypes, adenocarcinoma (40%), squamous cell (20%), and large cell lung cancer (3%) [3].
Patients diagnosed with advanced non-squamous NSCLC should undergo molecular testing for oncogenic drivers which may help determine a more effective targeted therapeutic approach [1, 4, 5]. These molecular abnormalities can be detected through different techniques such as FISH, NGS or polymerase chain reaction (PCR) [1]. Current commercial diagnostic panels attempt to identify the presence of EGFR mutations, anaplastic lymphoma kinase (ALK) and c-ROS oncogene 1 (ROS-1) rearrangements along with the degree of expression of programmed death ligand 1 (PD-L1).However, certain mutations such as MET that are not routinely included in initial molecular testing [1, 4, 5] may play an important role as a predictive and prognostic factor.
Detection of the presence of oncogenic drivers in advanced lung cancer has allowed the use of first-line targeted treatment resulting in an improved overall survival (OS), response rate (RR), progression-free survival (PFS) and paradoxically a decreased side effect profile [1, 8, 9]. Guidelines from the College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and Association of Molecular Pathologists (AMP) recommend routine screening for EGFR and ALK for all patients with adenocarcinoma [1]. The 2018 update by the CAP, AMP and IASLC state that “MET testing is not indicated as a routine stand-alone assay outside the context of clinical trial. It is appropriate to test for MET as part of larger testing panels performed either initially or when routine EGFR, ALK, and ROS1 testing are negative” [6].
MET amplification is known to be an oncogenic-addictive driver abnormality, meaning that cancer cells that harbor it depend on its presence to survive [8, 13, 14]. Such driver aberrations can be seen as promising biomarkers for selected targeted therapies which could result in improved efficacy along with decreased systemic toxicity compared to established but less specific first-line treatments such as chemotherapy and/or immunotherapy [1,7,15].
So far, it is not yet established whether MET inhibitors, chemotherapy, and/or immunotherapy should be first-line therapy for patients with de novo MET amplification [9]. Established guidelines, however, do provide recommendations for targeted first-line regimens for more common NSCLC molecular aberrations such as EGFR and ALK [1, 2].Targeted approaches have shown better efficacy, longer duration of response and a more tolerable side effect profile, thus allowing for a prolonged treatment and minimal effect on quality of life[1, 12, 13].
Immune checkpoint inhibitors (ICI) have demonstrated survival benefits over chemotherapy in the initial treatment of patients with NSCLC in several randomized trials. Its use has revolutionized the treatment landscape of NSCLC. However, the role of these agents in NSCLC harboring driver mutations is poorly defined as a clear benefit has not been shown in these patients [15].
In summary, there is no established first-line targeted therapy for patients with de novo MET amplification [9]. Current guidelines only recommend therapy with either crizotinib or cabozantinib in patients with MET amplification who progress on chemotherapy or immunotherapy [1].
Case Report
A 72-year-old Caucasian male, former smoker with a 25 pack-year history of tobacco use and previous work exposure to asbestos, presented to the emergency room with a 4-week history of progressive, intractable right shoulder pain. He also related increasing dyspnea, and a 15 lb. weight loss in this timeframe. A CT scan of the chest revealed a 3.3 cm right upper lobe mass with chest wall invasion, right hilar and mediastinal adenopathy. PET/CT scan showed a hypermetabolic right upper lobe mass with an elevated standardized uptake value (SUV) of 9.1 along with lymphatic metastatic involvement of neck, right axilla, mediastinum, bilateral hilar, abdominal, and pelvic lymph nodes. In addition, PET-CT reported bilateral adrenal uptake predominantly on the left side, as well as bone uptake in the right clavicle and left glenoid process. Hypermetabolic lesions were seen in transverse and descending colon reflecting metastatic versus primary malignant lesions (Figure 5). Staging MRI of the brain with gadolinium was negative for CNS involvement.
CT-guided biopsy of the left adrenal mass was performed. Pathology revealed poorly differentiated adenocarcinoma (Figures 1-4) with immunohistochemistry (IHC) positive for CK7, TTF-1, and napsin A; negative for CK5/6, synaptophysin, chromogranin, and p63. Molecular studies via NGS reported high levels of de novo MET amplification with a copy number of 12, and high PD-L1 expression at 80%. Testing for the presence of EGFR, ALK, ROS-1 and BRAF was negative. He was diagnosed with stage IV, metastatic non-squamous NSCLC with high MET amplification and high PD-L1 expression; metastatic sites included extensive nodal involvement, as well as bone and adrenal lesions. Treatment recommendations from an institutional tumor board were varied, ranging from first-line immunotherapy, chemoimmunotherapy and targeted therapy. In light of his high de novo MET amplification; he was started on crizotinib 250 mg p.o. BID. He experienced a rapid response both clinically and radiologically. Interval PET/CT scan after 6 weeks of crizotinib therapy demonstrated marked improvement in size and fluoro-deoxyglucose (FDG) uptake in all lesions compared to the initial study (Figure 5).
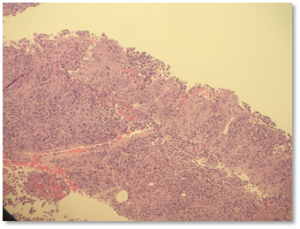
Figure 1: Core biopsy shows a poorly differentiated neoplasm characterized by solid sheets of pleomorphic cells with abundant eosinophilic cytoplasm.
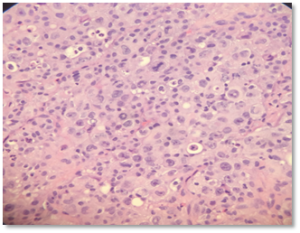
Figure 2: Higher magnification highlights irregular nuclear contours, prominent nucleoli, and increased mitotic activity with frequent apoptotic bodies and sparse inflammatory cells.
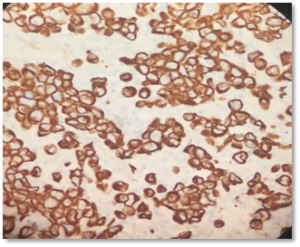
Figure 3: Immunohistochemistry demonstrates strong diffuse membranous/cytoplasmic staining with pancytokeratin AE1/AE3 and CK7.
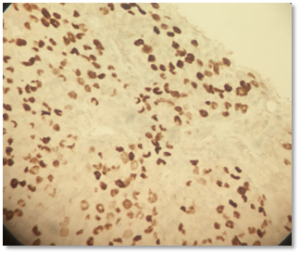
Figure 4: Immunohistochemistry with antibodies against thyroid transcription factor (TTF-1) shows moderate to strong nuclear staining in tumor cells.
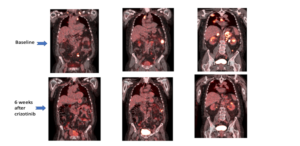
Figure 5: Representative PET/CT images taken at baseline (top) and 6weeks after starting crizotinib therapy (bottom).
Discussion
Tyrosine kinase inhibitors are the standard of care for patients who are found to have molecular alterations with a matched FDA-approved drug.
Recent published data demonstrate that molecular aberrations, other than EGFR and ALK, can be targeted yielding significant clinical benefit. That is the case for ROS-1, BRAF V600E, HER2 mutations, RET translocations and MET abnormalities. National Comprehensive Cancer Network guidelines for NSCLC do not fully discuss the management of patients with MET amplification, although other actionable drivers such as ALK, BRAF, and ROS-1 which are found in a similar percentage of NSCLC patients, have been outlined in these guidelines and are required as part of initial tissue screening [4-6].
Dysregulation of the MET pathway in lung cancer occurs through different mechanisms, including overexpression, amplification, rearrangement or exon 14 skipping mutations. The incidence of de novo MET amplification is variable and has been reported in 2 to 4 % of treatment-naive patients, whereas the frequency of MET amplification seen in patients with acquired resistance to EGFR TKI is around 20% [1, 10, 11]. There is evidence that the development of MET amplification in patients with EGFR mutant lung cancer is associated with EGFR-tyrosine kinase inhibitors resistance as well as a more aggressive clinical behavior [1, 6-11].
Determination of MET amplification can be obtained through FISH or NGS. FISH can measure copy number per cell or ratio of MET-to-centromeric portion of chromosome 7 (MET/CEP7). There are 3 levels of mutation using the ratio of MET/CEP7: low level > 1.8 to < 2.2; intermediate level > 2.2 to <5; and high level >5 [8]. As with FISH, copy number gains detected by NGS are reported as continuous variables, and cutoffs can vary significantly between assays [8]. In general, high MET amplification by NGS is defined as a MET copy number >5. Although the presence of MET amplification may indicate an overall worse prognosis, the presence of high levels of MET/CEP7 ratio or a high copy number gain, have shown to be predictive of high response rates to MET inhibition compared to intermediate or low amplification levels [1, 7, 8, 13, 14, 17].
Past phase III trials have researched the efficacy of MET-selective tyrosine kinase inhibitors (such as tivantinib) in patients with NSCLC; these were somewhat limited in that patients were not selected based on MET status. Since these studies did not show improvement in overall survival, clinicians became more hesitant towards viewing MET as a useful target for some time afterwards [7]. During the past decade, however, there has been a resurgence of interest in MET abnormalities due to discoveries regarding its association with a more aggressive cancer phenotype [1, 10, 11].
More recently, attention towards the multikinase MET TKIs (crizotinib, cabozantinib) in more specific MET-defined NSCLC populations has so far shown more promising results. [1, 7-9, 11, 17]. For instance, in a phase I study presented in 2014 at the American Society of Clinical Oncology meeting, 67% of patients with advanced NSCLC with high MET amplification treated with crizotinib showed disease stabilization or tumor response for a period that lasted between 6 months to 2.5 years [12].
The treatment of metastatic lung cancer has been radically changed in the last few years with the advent of immunotherapy.
The role of checkpoint inhibitors in the subset of patients with oncogenic addiction has not been defined, as they have been traditionally excluded from randomized trials using immunotherapy.
There is a paucity of evidence about the efficacy of immunotherapy in patients with MET amplification. Immunotarget, a retrospective study done in 551 patients with advanced NSCLC and at least one oncogenic driver mutation who received immunotherapy, showed a response rate of 12% in the MET (+) cohort and a low clinical activity across all other actionable abnormalities compared to wild-type NSCLC. Another publication in patients harboring MET exon 14 skipping mutations reported an objective response rate to immunotherapy of only 17%, even in the setting of high PD-L1 expression [16]. These findings support the concept that patients with known actionable molecular drivers, including MET, should have exhausted targeted therapies and chemotherapy before considering ICI regardless of PD-L1 expression [15].
Available evidence outlines a lack of efficacy when using immunotherapy in patients with driver mutations like EGFR and ALK abnormalities even after TKI failure, that phenomenon seems to be similar in patients with MET amplification [15]. It would therefore make sense to handle MET amplification first with targeted therapy and then advance to broader treatment approaches of chemotherapy and/or immunotherapy when necessary, such as with disease progression or patient intolerance to targeted therapy.
Strategies being evaluated to enhance ICI activity in the setting of oncogenic addiction include the use of ICI and TKI together as well as combination of chemotherapy, anti-vascular endothelial growth factor (anti-VEGF) and ICI.
Commercial laboratories offering NSCLC molecular testing should include MET assessment and report the level of MET amplification in the context of already known parameters, clearly identifying the group with high MET amplification as they will benefit the most from targeted therapy compared to the group with low amplification levels.
Randomized data about how to approach this population of NSCLC patients with molecular abnormalities occurring at low frequencies will be available only after large-scale molecular screening is implemented to allow for accrual to clinical trials.
In this particular case study, a patient with newly diagnosed advanced NSCLC was found to have a high level of de novo MET amplification. Without published standard recommendations, various treatment options were considered. The decision to pursue targeted therapy proved to be advantageous, the patient showed a remarkable response to first-line crizotinib within a short period of time.
References
- Lecia VS, Joel WN (2019) Personalized, genotype-directed therapy for advanced non-small cell lung cancer. Up to Date. Version 74.0 Topic 16538.
- Siegel RL, Miller KD, Jemal A (2019) Cancer Statistics, 2019. Cancer Journal for Clinicians 69: 7-34.
- Brambilla E, Travis WD (2014) Lung cancer. World Cancer Report. World Health Organization 353-359.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer. May2019.NCCN Evidence Blocks: Testing. Version 5.2019.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer. August 2019.Version 6.2019
- College of American Pathologists, International Association for the Study of Lung Cancer, Association for Molecular Pathology. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors. College of American Pathologists.2018.
- Mendenhall MA, Goldman JW (2015) MET-Mutated NSCLC with Major Response on Crizotinib. Journal of Thoracic Oncology 10: e33-e34.
- Drilon A, Cappuzzo F, Ou SI, Camidge DR (2017) Targeting MET in Lung Cancer: Will Expectations Finally Be MET? Journal of Thoracic Oncology 12:15-24.
- Zheng X, Zhang G, Li P, Zhang M, Yan X, et al. (2019) Mutation tracking of a patient with EGFR-mutant lung cancer harboring de novo MET amplification: Successful treatment with gefitinib and crizotinib. Lung Cancer 129: 72-74.
- Bean J, Brennan C, Shih JY, Riely G, Viale A, et al. (2007) MET amplification occurs with or without T790M mutations in mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proceedings of the National Academy of Sciences of United States of America 105: 20932-20936.
- Baldacci S, Kherrouche Z, Cockenpot V, Stoven L, Copin MC, et al. (2018) MET amplification increases the metastatic spread of EGFR-mutated NSCLC. Lung Cancer 125: 57-62.
- Camidge RD, Ou SHI, Shapiro G, Otterson GA, Villaruz LC, et al. (2014) Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 32: 8001-8001.
- Yap TA, Popat S (2017) Targeting MET Exon 14 Skipping Alterations: Has Lung Cancer MET Its Match? Journal of Thoracic Oncology 12: 12-14.
- Caparica R, Yen CT, Coudry R, Ou SI, Varella-Garcia M, et al. (2017) Responses to Crizotinib Can Occur in High-Level MET-Amplified Non-Small Cell Lung Cancer Independent of MET Exon 14 Alterations. Journal of Thoracic Oncology 12: 141-143.
- Mazières J, Drilon A, Lusque A, Mhanna L, Cortot AB, et al. (2019) Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Annals of Oncology 30: 1321-1327.
- Sabari JK, Leonardi GC, Shu CA, Umeton R, Montecalvo J, et al. (2018) PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Annals of Oncology 29: 2085-2091.
- Chen RL, Zhao J, Zhang XC, Lou NN, Chen HJ, et al. (2018) Crizotinib in patients with MET-amplified non-small cell lung cancer (NSCLC): Updated safety and efficacy findings from a phase 1 trial. American Society of Clinical Oncology-Poster Session 18: 1171.
