Publication Information
ISSN 2691-8803
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Continence Repair Through Coordination Dynamics Therapy
Giselher Schalow*
(Non-Government-Organized-Medical-Research)
Received Date: August 20, 2022; Accepted Date: August 26, 2022; Published Date: September 01, 2022;
*Corresponding author: Giselher Schalow, NGOMR: Non-Government-Organized-Medical-Research. Email: g_schalow@hotmail.com
Citation: Schalow G (2022) Continence Repair Through Coordination Dynamics Therapy. Adv Pub Health Com Trop Med: APCTM-163.
DOI: 10.37722/APHCTM.2022501
Chapter 1
Continence Repair and Diuresis Induction through Coordination Dynamics Therapy
Summary
Urinary bladder dysfunction and incontinence are a big problem in spinal cord injury and brain injury/malformation. With the development of the discipline human repair-neurophysiology in general and a new recording technique in specific, the single-nerve fiber action potential recording method, the functioning of the sacral micturition center is analyzed under physiologic and pathologic conditions at the neuron level and a movement-based learning therapy developed to repair urinary bladder and kidney functions. In Chapter 1 continence is repaired in 8 out of 10 patients through a newly developed movement-based learning method, called coordination dynamics therapy. In Chapter 2 urinary bladder functions are analyzed at the single-neuron level by comparing bladder functioning in the rather healthy case (in brain-dead humans) and following spinal cord injury.
It is shown that incontinence is firstly due to bladder wall function impairments so that stretch, tension and flow receptors in the bladder wall fire already in the empty bladder and mimic a rather full bladder, even though being empty, and secondly due to pathologic neural network organization of the sacral and pontine micturition centers. The impaired neural network organization of the somatic (external bladder sphincter) and vegetative (detrusor) nervous systems and their coordination can be repaired through neural network learning, that means through movement-based learning. It is emphasized that animal research is no substitute for human research and that the infrastructure for human research is not existing as if quality of human life is not of interest to the world-wide society.
Keywords: Bladder receptors; Coordination dynamics therapy; Human repair-neurophysiology; Incontinence; Kidney repair; Neuro-urology; Neural network organization; Sacral micturition center; single-nerve fiber action potentials; System theory of pattern formation
Introduction
Urinary bladder and kidney repair through Coordination dynamics therapy
Based on human repair-neurophysiology [1, 2], a movement-based learning therapy was developed though neural network learning [3], called Coordination Dynamics Therapy (CDT), with which it is possible to improve or repair central nervous system (CNS) functioning after stroke [4], traumatic brain injury [5, 6], spinal cord injury [7-13] (Figure 1, Nefeli), cerebellar injury/atrophy [14, 15] (Figure 1, Sophie), cerebral palsy [16], hypoxic brain injury [17], in Parkinson’s disease [18], spina bifida (myelomeningocele) [19] and scoliosis [20]. Speech had been induced and improved in a patient with severe cerebral palsy [1]. A permanent coma patient could be brought out-of-coma and relearned to speak and move [21, 26] and cancer grows could be inhibited through CDT [22, 23] by improving cardio-vascular performance [1, 21] and building of natural killer cells [24]. Urinary bladder functions [1] could be cured in cerebral palsy [1] and spinal cord injury [7, 12, 13]. There is indication that general health can be improved via CDT to live longer with a better quality of life [25] and euthanasia can be avoided in organ donation [26]. Basal ganglia injury was also repaired [27] and spinal muscular atrophy stopped [28].
Emphasis was given so far of this human repair-neurophysiology project on the report of the repair of movements. But by learning transfer from movements (Method), also vegetative and cognitive functions could be repaired/improved. In this article it is mainly concentrated on the repair of urinary bladder functions. Since ascending bladder infections can ruin kidney functions, also kidney function improvement is tackled through neural network learning.
Figure 1 shows two typical patients with incontinence. In the spinal cord injury patient Nefeli, the urinary bladder function repair needed approximately 3 years of CDT and in the cerebral palsy girl Sophie approximately 3 months. In both patients CDT was suboptimal and after 5 years of CDT movement functions were only partly repaired.

Figure 1: The spinal cord injury patient Nefeli relearned to walk and became continent again (A-D) [7,13]. The cerebral palsy girl Sophie with atrophied cerebellum and pons could not stand, walk, run (E, F) or jump and was incontinent. She learned to walk, run (G, H) and jump, became continent and her higher mental functions improved [15].
Recording of single-nerve fiber action potentials (Electrophysiology)
Principle of recording single afferent and efferent nerve fiber action potentials
The progress in repairing the human sacral micturition center started with a new recording technique, the single-nerve fiber action potential recording method [29, 30].
The development of the single-nerve fiber action potential recording method was possible because of the unique anatomical landscape of the human spinal canal. Because of the Ascensus of the spinal cord, the lumbosacral nerve roots are very long and form the cauda equina (Figure 3). Since the caudal sacral nerve roots are very thin and nerve roots are only ensheathed by a thin layer of cells (Figure 6), they are ideal for recording single-nerve fiber action potentials (APs) from undissected nerve roots. Since humans have no tail, continence (mainly S2 to S5) and sexual functions are mainly located in the conus medullaris only. Those functions are therefore represented in the lower sacral nerve roots and they are not intermingled with tail functions as they are for example in rats, cats and dogs.
A schematic recording layout is shown in Figure 2 to record single-nerve fiber extracellular APs from undissected nerve roots (in this case from two fibers) with two pairs of platinum wire electrodes (electrode pair distance 10 mm; electrode distance in each pair 4 mm). The APs were pre-amplified (x1000), filtered (RC-filter, passing frequency 100 Hz – 10 kHz) and displayed on a digital storage oscilloscope, and also stored using a PCM-processor and a video recorder. APs from the afferent and the efferent fibers can clearly be distinguished since with the electrode arrangements, the main phase (second phase) from the afferent fiber is upwards and that of the efferent fiber downwards (Figure 2a). E.g., the AP of a skin afferent fiber reaches a pair of electrodes first as negative and then as positive. According to the electrode setting used, the main phase is upwards. An AP of a motoneuron, coming from the opposite direction, would reach the electrodes in the order positive-negative. The potential changes are therefore opposite and the main triphasic AP will point downwards. An AP in an afferent fiber reaches first the caudal electrode pair and then the rostral pair, whereas an AP of the efferent fiber reaches first the rostral electrode pair and then the caudal one. The conduction times are therefore also opposite. The reversing of the inputs to both preamplifiers does not change the ability to differentiate between afferent and efferent APs (Figure 2b).
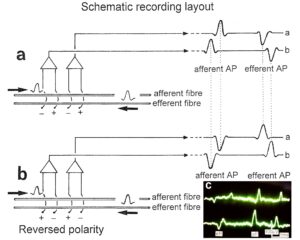
Figure 2: Schematic layout for recording single-nerve fiber action potentials (APs) from an afferent and an efferent nerve fiber (a). The reversing of the inputs to both preamplifiers does not change the ability to differentiate between afferent and efferent Aps (b). Real recording (c).
Recording of single-nerve fiber action potentials from nerve roots and splitting of the multiunit recording into natural impulse pattern of several single afferent and efferent fibers
A real recording arrangement during an operation is shown in Figure 3A, B. To obtain natural impulse patterns simultaneously from several single afferent and efferent nerve fibers to analyze CNS functioning, the summed impulse traffic of several afferent and efferent fibers of a nerve root has to be split into the impulse patterns of single fibers (Figure 4). The splitting is achieved by recognizing the APs from certain single fibers on the basis of wave form comparisons on the two recording traces and the conduction time which an AP needs to travel from one electrode pair to the other one (10mm) and selecting these APs out. The summed impulse traffic of the recording in Figure 4 is split into the impulse patterns of 5 single afferent and efferent nerve fibers. In the thin lower sacral nerve roots, there are afferent and efferent fibers in ventral and dorsal roots.
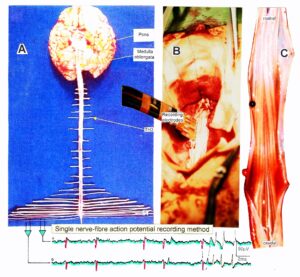
Figure 3: Anatomical layout for the recording of single-nerve fiber action potentials to analyze the self-organization of neuronal networks of the human CNS under physiologic and pathophysiologic conditions. A,B,C. By recording with two pairs of platinum wire electrodes (B) from sacral nerve roots (cauda equina, C) containing between 200 and 500 myelinated nerve fibers, records were obtained in which single nerve-fiber action potentials (APs) were identified from motoneurons (main AP phase downwards) and afferents (main AP phase upwards).
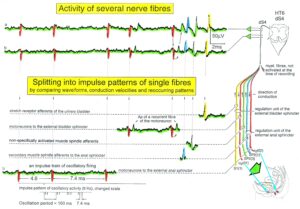
Figure 4: Schematic layout of the classification scheme for the human peripheral nervous system. By recording with two pairs of platinum wire electrodes from a nerve root containing approx. 500 myelinated nerve fibers, a recording is obtained in which 3 action potentials (APs) from 3 motoneurons (main AP phase downwards) can be seen. By measuring the conduction times and with the known electrode pair distance (10 mm) a conduction velocity distribution histogram is constructed in which the nerve fiber groups are characterized by ranges of conduction velocity values and peaks in asymmetrical distributions. After recording, the root was removed, fixated, embedded and stained, light microscope cross-sections were prepared and used to measure the mean diameter and the myelin sheath thickness (d). Distributions of nerve fiber diameters were constructed for four different ranges of myelin sheath thickness. Nerve fiber groups were characterized by the peak values of asymmetrical distributions. By correlating the peak values of the velocity distributions with those of the diameter distributions obtained from the same root, a classification scheme was constructed of the human peripheral nervous system. Brain-dead human HT6.
The splitting of skin afferent activity upon touch or pin-prick into the natural impulse patterns of different single touch and receptors is shown in Figure 5. Such messages inform the central nervous system (CNS) about changes in the periphery. Similar natural impulse patterns inform the CNS about changes in the urinary bladder or anal canal such as bladder filling or bladder or anal canal catheter pulling (Figure 7B).
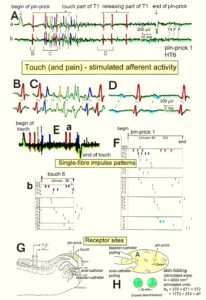
Figure 5: Touch (and pain)-stimulated afferent activity. Touch and pain activity stimulated by pin-pricking (A) and touching (Ea) at S5 or Co dermatomes and recorded extracellularly from a dorsal coccygeal root (brain-dead human HT6). T1, T2, T3, T4, P = mark action potentials (APs) from single touch and pain fibers. Subscripts 1, 2, 3 mark single fibers. A. Whole sweep following pin-prick 1 shown at a slow time base. The large upward artifact on trace ‘a’ marks electronically the beginning of the pin-prick. The large downward artifact on trace ‘a’ marks the end of the pin-prick. Note that 2 intervals of high activity of large APs occur, one after the beginning of the pin-prick with 1 AP in front, and a second before the end of the pin-prick; potentials with small amplitude follow potentials of large amplitude. Time intervals B, C and D are shown in a time-expanded form in Figures B, C and D. B, C, D. Time expanded sweep pieces of A. Identified APs are indicated. Note that the APs from the T11 touch unit can be safely identified by the waveforms in B, C, D. Eb, F. AP occurrence patterns of single touch and pain fibers following short touch 6 and pin-prick 1. No pain afferents are stimulated upon touch 6. Upon pin-prick 1, the single-fiber AP activity of the different touch and pain groups is identified by the AP waveforms on traces ‘a’ and ‘b’, and by the conduction times. The single touch afferents of the T1 group are marked with subscripts. One active secondary muscle spindle afferent fiber (SP2) could always be identified in F. Note that for pin-prick 1, touch and pain afferents are stimulated whereas for touch 6 only touch afferents. G. Recording and stimulation arrangement for simultaneous recording of several single touch and pain units. A = area stimulated by skin folding, drawn in H in more detail. T11, T16 = suggested touch points of the T11 and T16-units. H. Drawing of the very approximate skin area stimulated by skin folding. T11-6 = suggested focal T1 touch points. Two-point discrimination indicated for the sake of comparison. NA = number of stimulated units in the dorsal coccygeal root. Skin tractions evoked by anal and bladder-catheter pulling are indicated by the large open arrows.
As we can measure the natural impulse patterns, generated by certain single receptors in the periphery, which run into the spinal cord (CNS) (afferents) and those patterns which leave the cord (efferents) in ensembles of single fibers simultaneously (Figure 6), it becomes possible to analyze the integrative properties of the largely unchanged CNS of brain-dead humans (HTs) at a cellular level. This also means that the change in function, caused by a CNS injury, can be identified.
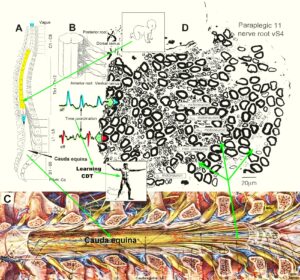
Figure 6: Anatomy to record single-nerve fiber action potentials. A. Ascensus of the human spinal cord gives rise to long nerve root in the lumbar and sacral range. B. The nerve roots in the cervical range are short. C. Picture of the opened spinal canal with the cauda equina nerve roots, ganglions and spinal nerves. D. Real ventral S4 nerve root cross section with single-nerve fiber action potentials of afferent (aff) and efferent (eff) nerve fibers and their time coordination. Principle sizes of different nerve fiber cross sections are indicated.
Classification of peripheral human nerve fibers (Electrophysiology combined with morphometry)
Classification of human peripheral nerve fibers by the group conduction velocity and the group nerve fiber diameter
For the analysis of CNS functioning and neural network learning for repair, we must first identify the kind of nerve fibers from which the recordings are taken. A classification scheme of human peripheral nerve fibers is needed.
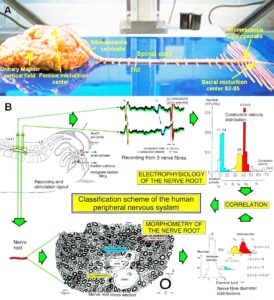
Figure 7: A. The human CNS with the sacral and pontine micturition centers. B. Development of a classification scheme for human peripheral nerve fibers. Conduction velocities (V) and nerve fiber diameters (Æ) of afferent and efferent nerve fiber groups in normal humans and in patients with a traumatic spinal cord injury for 0.5 to 6 years. The splitting of the a1-motoneurons into the 3 subgroups, a11, a12, a13, has not yet been confirmed.
Conduction velocities of single nerve fibers were therefore calculated from the conduction distance (electrode pair distance = 10 mm) and the conduction times (the time difference of a certain AP between the traces from two pairs of wire electrodes). Velocity distributions of afferent and efferent fibers were constructed, and distribution peaks were correlated to certain nerve fiber groups. The nerve roots from brain dead and surgical patients could be removed, fixated, embedded and stained. Mean nerve fiber diameters could be measured, and nerve fiber diameter distributions constructed for different myelin sheath thicknesses (morphometry). By correlating the identified conduction velocity peaks with nerve fiber diameter peaks (Figure 7), a classification scheme for the human Peripheral Nervous System (PNS) was constructed, in which individual groups of nerve fibers are characterized by group conduction velocities and group nerve fiber diameters (Figure 8) [31, 32]. This classification scheme is still incomplete and holds only for nerve fibers thicker than approx. 3.5µm. The classification schemes for animals do not apply to humans. Conduction velocities in rats, cats and dogs (max » 120 m/s), for example, are much higher than those in humans (max » 70 m/s).
It will thus become possible to record natural impulse patterns simultaneously from identified single afferent and efferent nerve fibers and analyze self-organizing mechanisms of the human CNS under physiologic and pathologic conditions, especially from the human sacral micturition center.

Figure 8: Classification scheme for human peripheral nerve fibers. Conduction velocities (V) and nerve fiber diameters (Æ) of afferent and efferent nerve fiber groups in normal humans and in patients with a traumatic spinal cord lesion for 0.5 to 6 years. The splitting of the a1-motoneurons into the 3 subgroups, a11, a12, a13, has not yet been confirmed.
As we can measure simultaneously the natural impulse patterns, generated by certain identified single receptors in the periphery induced by touch, pin-prick, anal and bladder catheter pulling and bladder filling, which simultaneously run into the spinal cord (CNS) and those patterns which leave the cord (Figure 6), it becomes possible to analyze the integrative properties of the largely unchanged CNS of brain-dead humans (HTs) and the injured CNS in an operation of spinal cord injury patients at the cellular level and compare them.
The neuron microenvironment influences neural network functioning
As we will see below, the proliferation of stem cells depends on the microenvironment. Also the function of nerve cells is related to the microenvironment. The classification of human nerve fibers by a group conduction velocity and a group nerve fiber diameter is therefore only a first step towards a very exact classification. More exactness in the morphology of nerve fiber and nerve cell groups seem to exist with respect to classes. The myelin sheath thickness of nerve fibers roughly increases with the diameter. But the correlations indicate that there may be different populations of nerve fibers with their own correlation between myelin sheath thickness and diameter. From Figure 9 it can be seen, that certain nerve fiber groups lie in a certain myelin sheath thickness range. The motoneurons for example lie in the myelin thickness range between 1.8 and 2.2μm (Figure 9Ba). From the point of view of the morphology, nerve fiber groups could still be further exactly classified.
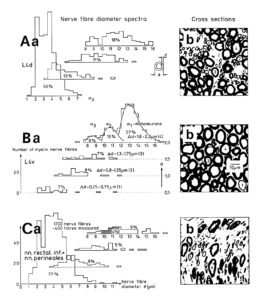
Figure 9: Nerve fiber diameter distribution histograms classified by 4 classes of myelin sheath thicknesses as indicated in Ba. % Indicates percentage of fibers in classes or subgroups. b. Corresponding characteristic cross sections. A few fibers are numbered by their myelin sheath thickness range they belong to. Dimension scale for A, B, and C is drawn in Bb. 8% shrinkage correction. For the definition of fiber diameter Ø and myelin sheath thickness d see insertion in Aa. – A. Nerve fiber diameter spectrum of a 4th dorsal lumbar root of a 47-year-old female human cadaver removed 2 to 5 hours after death, 660 fibers were measured. B. Spectra of a 4th ventral lumbar root (same case as in A). 320 fibers were measured. In the myelin sheath thickness range 1.8 ≤ d < 2.3μm the distribution curves of the 3 α-motoneuron classes are drawn into the histogram. C. Spectra of the nervi rectales inferiors and perineales. Note, the majority of fibers (77%) has a very thin myelin sheath (0.3 ≤ d < 0.8μm) with a relatively large number of thick fibers. In the histogram of very thick myelin sheaths (1.8 ≤ d < 2.3μm) the diameter range of α2-motoneurons, to which sphincter motoneurons belong, is crosshatched (4% of the fiber).

Figure 10: A. Schematic urinary bladder innervation [83]. The assignment parasympathetic may have to be replaced by sympathetic. B. Sweep piece of recording; conduction times and corresponding conduction velocities are indicated. Root temperature at recording, 35.5°C. C, D. Conduction velocity distributions of afferents (B) and efferents (C) obtained for a time interval of 3.6s with no additional stimulation. SP2 = secondary muscle spindle afferents, S1 = stretch receptor afferents of bladder, ST = tension receptor afferents, M = mucosal afferents, S2 = afferents responding to fluid movement; a1 = a1-motoneurons (FF), a2 = a2-motoneurons (FR), a3 = a3-motoneurons (S), gß = gß-motoneurons, g1 = g1-fusimotors (dynamic), g21 = g21-fusimotors (static), g22 = g22-fusimotors (static), par = preganglionic parasympathetic motoneurons. CAP comp. = group conduction velocities obtained from the components of compound action potentials (CAPs). Vesic. stimul. = group conduction velocities of bladder afferents obtained upon electrical intravesical stimulation (see Figs. 65, 66). Calibration relation indicates the same peak group conduction velocity of secondary spindle afferents and a2-motoneurons (cross-hatched). Velocity histogram classes £ and < (half closed (left) interval).
Also, the conduction velocities do not increase linearly with the diameter. The temperature dependence of the conduction velocity is different for different nerve fiber groups. But as can be seen from Figure 10, there exists at least one calibration relation (between the secondary muscle spindle afferents and the a2-motoneurons) to identify the conduction velocity groups in the velocity distributions.
In the learning process of neuronal networks for repair, the environmental cells are also included in the changes. It is not just the weights of synapses, which are modulated. The question remains why is there already such exactness in the size of neurons, their surrounding cells and the conduction velocities with respect to grouping. For further details see [1].
Self-organization of neuronal networks of the human CNS (Electrophysiology)
Self-organization of premotor spinal network oscillators
Typical firing patterns of motoneurons can be observed when motoneurons are activated with increasing strength of adequate afferent input. With low afferent input, the motoneurons fire occasionally. With increasing input, they fire intermittently in an oscillatory manner and then continuously in an oscillatory manner (Figure 11). The demonstration that neurons of the CNS, in this case motoneurons, can fire with both in an oscillatory manner and non-oscillatory manner is very important for the understanding of the functioning of the human CNS. To describe the functioning of the CNS merely by reflex pathways and loops or coupling of rigid oscillators (of cellular or network origin) is in contradiction to empirical human data, namely that premotor spinal oscillators self-organize as was concluded from measurements of simultaneous natural impulse patterns of afferent and efferent fibers (Figure 11).
With respect to continence, Figure 11 shows an important recording of urinary bladder and rectum functions from the brain-dead human HT6 at the single-neuron level, which will be used below as the healthy comparison when recording from a patient with bladder dysfunction (Figure 68). It is recorded from the premotor spinal a2-oscillator O1 (Figure 11A), which innervates the external urinary bladder sphincter (skeletal muscle) and the a2-motoneurons O2, which innervates the external anal sphincter (Figure 11F,H,I). The external anal sphincter motoneuron O2 fires oscillatory because the anal reflex is stimulated by the anal catheter. The bladder sphincter motoneuron O1 is firing with increasing activity from occasionally to oscillatory when filling the bladder retrogradely. The external bladder sphincter motoneuron starts to fire from 500ml filling onwards to secure continence. The external anal sphincter O2 is nearly not affected by the bladder filling. Simultaneously it is recorded from the bladder afferents, namely the urinary bladder stretch (S1), tension (ST) and flow (S2) receptors (Figure 11E,G). With bladder filling the stretch, tension and flow receptors increase their activity (Figure 11E). Figure 11B indicates schematically that there are phase and frequency relations between the stretch receptor S1(1) and the motoneuron O1 action potentials and between the secondary muscle spindle SP2(2) and the motoneuron O2. Both afferents contribute to the drive of the motoneurons because of the existing phase relations, one is activated by the bladder filling (S1(1)) and the other one (SP2(2)) by the stretch of the anal sphincter caused by the placed anal catheter. Figure 11C shows some real interspike intervals of bladder stretch receptor afferences and the oscillation period of the a2-oscillator O1.

Figure 11: Self-organization of premotor spinal a2-oscillator O1, which innervates the external urinary bladder sphincter (skeletal muscle). Brain-dead human HT6; recording from a dorsal S4 nerve root. A. Recordings from a2-motoneurons O1 and O2, firing in the oscillatory mode with impulse trains of 2 (upper recording) and 3 (lower recording) action potentials (APs). The durations of the oscillation periods were 110 (O1) and 164ms (O2). The interspike intervals of the impulse trains were 5.9ms (O1) and 4.6 and 7.4ms (O2). Motoneuron O1 conducted at 36 m/s; its recurrent fiber conducted at 21 m/s. The measurement layout is shown schematically. The inserts show the oscillatory firing modes; they have not been drawn to scale. B. Impulse patterns of oscillatory firing a2-motoneuron O2 innervating the external anal sphincter, in relation to the muscle spindle afferent activity SP2(1 to 3), activated by the stretch of the anal sphincter by the anal catheter, and impulse patterns of oscillatory firing a2-motoneuron O1 innervating the external urethral sphincter, in relation to the stretch receptor afferent activity (S1(1)) of the urinary bladder, activated by 750 ml bladder filling. Phase relations between APs of SP2(2) and O2 and between APs of S1(1) and O1 are indicated by the small arrows. C. Three series of successive interspike intervals of the stretch receptor afferent fibers S1(1) and S1(2) activated by retrograde bladder filling. The oscillation period of oscillatory firing motoneuron O1, activated only by bladder filling is shown. D. The firing in the occasional spike mode, the transient and the constant oscillatory firing mode of a2-motoneuron O1 in response to filling of the bladder. In the ‘activity pattern’ column changing durations of oscillation periods are given. The oscillation frequencies in the brackets give the frequencies at the moment of oscillation for the transient oscillatory mode. Downward deflections are schematized APs. Interspike intervals of the close APs » 6.0ms (A). E. Activity levels of stretch (S1) and tension (ST) and flow receptor afferents (S2) (E) and of sphincter a2-motoneuron O1 (F) in response to retrograde filling of the bladder. The activity values of the S1, ST and S2 afferents are taken from histograms like the one in G. Filling of the bladder was stopped once between 600 and 650 ml. F. The small dotted lines represent mean activity (APs/s) and oscillation frequency (impulse trains/s) of a2-motoneuron O1 if bladder filling were not stopped in between. Note that the mean activity increases continuously with the filling of the bladder from 550 to 650 ml, even though motoneuron O1 started to fire in the oscillatory mode from 620 ml on (D). Note further that the oscillatory firing motoneuron O2 (frequency of firing with impulse trains is shown) is nearly not affected by the filling of the bladder and by the start of the oscillatory firing of motoneuron O1. G. Conduction velocity frequency distribution histogram of stretch, tension and flow receptor afferent activity at 750 ml. The activities of afferents S1, ST and S2 are quantified by counting the afferent conduction velocities under the peaks (open plus hatched part), with the conduction velocity limits given in the insert. The counts (27, 33, 59) are given below the peak labeled S1, ST and S2 and plotted into E for the afferent activity at 750 ml. H. Schematic drawing of the anatomical arrangement of the afferents and the motoneuron O1. I. Arrangements of external anal sphincter, innervated by the α2-motoneuron O2.
As will be shown below, these self-organized premotor spinal network oscillators, of which the motoneuron is most likely a part, are sub-neural networks which coordinate their functioning. When this coordinated communication becomes impaired due to insufficient inhibition, they synchronize their firing with the consequence of pathologic tremor occurring in patients with Parkinson’s disease [18].
In what follows, I shall concentrate mainly on the oscillatory firing of motoneurons [33, 34], which takes place for high activation. In this high activation mode these self-organized network oscillators can also be used as a reference basis when defining phase relations and thus phase and frequency coordination can be measured among neuron firing. For high and rather constant afferent input it was found that a-motoneurons fire repeatedly with impulse trains according to their type (Figure 12). The a1-motoneurons (FF) fire rhythmically at around 10 Hz (range 8 to 20) with an impulse train consisting of 1 AP; a2-motoneurons (FR) fire at approx. 6 to 9 Hz with 2 to 5 APs per impulse train, and a3-motoneurons (S) fire with a frequency in the range of 1 Hz and with long impulse trains consisting of up to 40 APs (and more). The rhythmic firing patterns of a-motoneurons are probably generated by local neuronal networks of the spinal cord since oscillatory firing can be recorded from motoneurons of the disconnected spinal cord. Probably the motoneuron is a part of the spinal network oscillator. The oscillation period (T) is roughly related to the number of action potentials (APs) per impulse train (nAP), and this can be expressed by the formula: T = 70ms + 30ms · nAP. A typical premotor a2-oscillator fires with 3 APs every 160ms (T = 70ms + 30ms · 3 = 160ms), and can change its firing pattern to 2 APs every 130ms for less activation or to 4 APs every 190ms for higher activation.
The a1-oscillators respond very dynamically, but have few oscillator network properties. Their firing is absolutely correlated to the firing of primary spindle afferent fibers (Figures 65 and 66 of [2]). The a2-oscillators respond less dynamically, have strong oscillatory properties and self-organize by the adequate afferent input patterns from several kinds of receptors including secondary muscle spindle and urinary bladder afferents. The behavior of a3-motoneurons is more static and their input is polymodal. The dynamics of responding to inputs (Figures 68 of [2]) increases from a3 to a2 to a1-oscillator in accordance with the dynamics of the 3 muscle fiber types the a-motoneurons innervate. The slow (S), medium fast (FR) (fast fatigue-resistant) and fast contracting muscle fibers (FF) (fast fatigable) have their own corresponding premotor networks in the spinal cord, namely that in which the a1, a2 and a3-networks are integrated in (Figure 12).
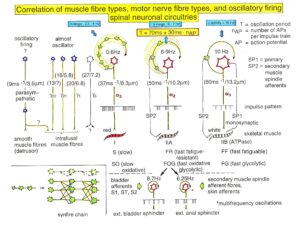
Figure 12: Correlation of muscle fiber types, motor nerve fiber types, and oscillatory firing spinal neuronal networks, based on histochemical, morphological and physiological properties. This figure provides a simplified correlation between muscle fiber, motoneuron and sacral oscillator types. No additional subtypes have been included. The existence of a1-motoneuron (FF) oscillators firing at 10 Hz has been predicted and they have been identified in paraplegics (unpublished observation). a = motoneuron, g1, g2 = dynamic and static fusimotors, parasympathetic = parasympathetic preganglionic motoneuron. S1, ST, S2 = stretch, tension and flow receptor afferents.
Now it is tried to measure organization principles of the human CNS. It will be shown that neurons and sub-neuronal networks coordinate their firing up to a few milliseconds. If this phase and frequency [35, 36] coordination becomes impaired, organization patterns of the CNS become impaired, instable or are even lost. Every functional or structural modulation of the neuronal networks changes this phase and frequency coordination among neuron firing. Learning is related to the exactness and complexity of the many coordination’s among single neuron firings or sub-neuronal networks. One strategy for repair is to improve the by injury impaired phase and frequency coordination among neuron firing by movement-based learning. The coordinated movements have to activate the CNS integrative, so that as many phase and frequency coordination’s as possible are trained simultaneously to improve the exactness and complexity of CNS self-organization. By exercising very coordinated movements on the special coordination dynamics therapy (CDT) device (Figures 27, 28), the CNS learns from the device via the movement induced afferent input to improve its coordinated firing of neurons and sub-neural networks.
Phase and frequency coordination
Relative phase and frequency coordination between the APs of the oscillatory firing a2-motoneuron O2 and the secondary muscle spindle afferent fiber SP2(1) can directly be seen in the original recordings in Figure 13B, C. The firing of the oscillator and the sweep pieces which are shown time-expanded are indicated at the summary trace “D”. Figure 13B, C shows the AP-impulse train of oscillator O2 in connection with one of its driving spindle afferent AP. Because of the duration of the phase relation of around zero milliseconds between the firing of the driving SP2(1)-fiber (firing mostly every 80ms) and the impulse train of the oscillatory firing motoneuron O2 (T(O2) ≈ 160ms), the SP2(1)-fiber AP (every second AP) appeared at a similar time as the impulse train. Because the AP of the spindle afferent fiber had a characteristic waveform, it was easy to extract its impulse pattern from the summed impulse traffic of this S4 dorsal root. During touch-induced skin afferent activity as in Figure 5, the activities of the motoneuron and the spindle afferent fiber were covered by the skin afferent activity. After the cessation of the skin afferent activity the afferent and efferent APs were found again at their expected time positions of the regular firings. The phase coordination between the firings of the oscillatory firing motoneuron O2 and the secondary muscle spindle afferent fiber SP2(1) at the time when records B, C were taken, was 1.6ms (3ms – 1.4ms, Figure 13B, C). In Figure 13D, the relative frequency coordination between the firings of the SP2(1)-fiber and the impulse train of the oscillator is indicated. For the time period evaluated, the correlation between the firing of the motoneuron and the spindle afferent fiber was in the range of between 3 and 5ms (Figure 13D).
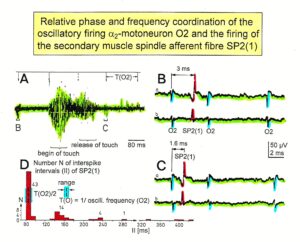
Figure 13: Time relation between the occurrence of the action potentials (APs) of oscillatory firing a2-motoneuron O2 and the firing of the secondary muscle spindle afferent fiber SP2(1). HT6. S4 dorsal root recording. A. Overall view of the used sweep piece; only trace “a” shown. Four oscillation cycle periods of motoneuron O2 are indicated (T(O2)). The APs of the impulse trains can be recognized only partly, because of the slow time base and poor digitalization. One impulse train (dashed arrow) is lost in the touch stimulated activity, which consists of a touch (large overall activity) and a release part (lower overall amplitude). B, C. Sweep pieces from A, time stretched. In B, motoneuron impulse train APs is marked O2, spindle afferent APs are marked SP2(1). Note that the APs of the spindle afferent fiber are not time-locked to the first AP of the impulse train of the rhythmically firing motoneuron (relative phase coordination). Digitalization 4 times better than in A, but still rather poor, as can be seen from the low amplitudes of the motoneuron APs on trace “b” in C. D. Occurrence of interspike intervals of the secondary muscle spindle afferent fiber SP2(1). The numbers give the amount of IIs in each distribution peak. The oscillation period of motoneuron O2 (and the range of variation) and the half period are indicated by short dashed lines. Note that the IIs of fiber SP2(1) are very similar to the oscillation period (or the half of it) of a2-motoneuron O2 (relative frequency coordination).
In Figure 14, considerations concerning the relative frequency coordination is extended to the activity of further afferent fibers and g-motoneurons of the same root. “G” of Figure 14 shows sweep pieces of the original recordings; A through F shows the interspike interval distributions of spindle afferents and g-motoneurons. It can be seen from the overlapping of the oscillator frequency T(O2) and T(O2)/2 distribution ranges and the interspike interval distributions of the afferents that, from the viewpoint of frequency coordination, fiber SP2(1) contributed strongly to the drive of oscillator O2, whereas there was a weaker contribution from other afferents, i.e., less overlapping between the distributions of the afferents and the range of the basic frequency or the first harmonic of the oscillator. Also, g-motoneurons showed only little frequency correlation at that time period.
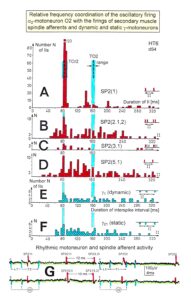
Figure 14: Interspike interval distributions of single endings of four secondary muscle spindle afferents (SP2) and two g-motoneurons, recorded simultaneously. In A, the oscillation period TO2 (impulse train length = 3 APs) with its range of simultaneously recorded oscillatory firing a2-motoneuron O2 (see G) is drawn for comparison; also, the halves of the oscillation period TO2/2 are indicated. Note that the interspike interval distributions of spindle afferents and g-motoneurons have shortest interspike interval, nearly identical to the half of the oscillation period (relative frequency coordination). The schematic impulse pattern in A to F shows the procedure for measuring the interspike intervals. Original records of the firing patterns of a2-motoneuron O2 and the secondary muscle spindle afferents SP2(1), SP2(2), SP2(3) and SP2(5) are shown in G. Brain-dead human HT6, dS4 root.
The fact that neurons fire in a relatively coordinated way of up to a few milliseconds is used for re-organizing the injured CNS by re-learning phase and frequency coordination between neuron firings when exercising movements coordinated with an exactness of up to a few milliseconds, using a special coordination dynamic therapy device, i.e., by instrumented supervised phase and frequency re-learning (Figure 89).
As will be shown below, phase and frequency coordination among α1-motoneuron firings can directly be seen in recordings of single-motor unit surface electromyography.
Relative phase and frequency coordination between the firings of a and g-motoneurons and secondary muscle spindle afferents recorded with the single-nerve fiber action potential recording method
With the single-nerve fiber action potential recording it was shown that the neurons of the human CNS organize themselves by phase and frequency coordination. Following injury, this organization principle is disrupted (see below). Figure 15 shows schematically a recording from a dorsal S4 root of a brain-dead human. Of the summed afferent and efferent impulse traffic, the natural impulse patterns of one a2-motoneuron, a dynamic (g1) and a static g-motoneuron (g21) and two to three secondary muscle spindle afferent fibers could be extracted (for classification see Figure 8). The natural stimulations performed were pin-pricking (pain) sacral dermatomes inside the continence automatism zone and urinary bladder catheter pulling [1,2] (Figures 7B, 79, 80). It can be seen from Figure 15 that the a2-motoneuron (a2(O2)) fired in an oscillatory manner with 2 to 3 impulses per impulse train and sometimes there was a break in the oscillatory firing. The impulse train for a1-motor units consisted of only one action potential. Phase coordination’s between the a2-motoneuron, the g-motoneurons and the secondary muscle spindle afferent fibers are indicated by different arrows and the dotted and dashed lines. It can be seen from Figure 15 that there were many coordination’s between the different neurons. The relative phase and frequency coordination seems to hold for all neurons and is an integrative mechanism for the self-organization of the neuronal networks of the human CNS.
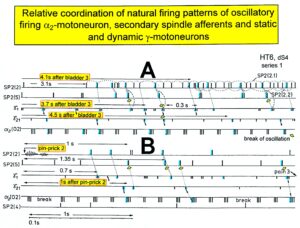
Figure 15: Phase and frequency coordination between the extracellular recorded action potentials of simultaneously recorded g-motoneurons (g1 and g21), secondary spindle afferent fibers (SP2(2), SP2(4), SP2(5)) and oscillatory firing a2-motoneuron O2 following bladder catheter pulling (bladder 3) (A) and pin-prick 2 (B). B was recorded before A. In A the impulse patterns of the 2 encoding sites SP2(2.1) and SP2(2.2) of the single parent fiber SP2(2) are indicated by the dotted curves. Times to the activity increases of g-motoneurons and secondary spindle afferents following stimulation are indicated. Similar time intervals of the occurrence of g-motoneuron APs and SP2(5) fiber APs (phase coordination) are indicated by the open arrows, and the similar time intervals of g-motoneuron APs and a-motoneuron APs are indicated by small arrows. Similar time intervals of the APs of fibers SP2(2) and SP2(5) are indicated by the double dotted lines, those of g1-APs and the SP2(2) fiber APs by a dotted line, and those of g1-APs and the SP2(2)-SP2(5) correlation by a curved dashed line. HT6; dS4-root.
Surface Electromyography to measure motor programs, oscillatory firing, and phase and frequency coordination among motor units through recording single-motor units
Another human electro-physiologic tool to measure natural impulse patterns of neurons is the surface electromyography (sEMG). With the same recording system used to record singe-nerve fiber APs, just replacing the wire electrodes with EMG surface electrodes, single-motor unit firing and motor programs can be recorded non-invasively. The sEMG recording arrangement is shown in Figure 16 for recording motor programs from an infant.
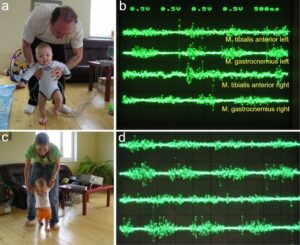
Figure 16: Surface EMG obtained from the healthy 5-months-old (a,b) and 8-months-old old “Jürgen” (c, d) during supported walking. a. Walking resembles automatic stepping, because of the strong lifting of the left knee. The toes of the right foot are plantar flexed, which is not physiologic. b. Surface EMG motor programs of left and right tibialis anterior and gastrocnemius muscles. Note that there is no antagonistic action between the tibialis anterior and gastrocnemius muscles. The right tibialis anterior muscle shows no motor program. c. The walking is more walking like and not so any more much automatic stepping like. d. Better motor programs then 3 months earlier (b). Still there exists no antagonistic action between the tibialis anterior and gastrocnemius muscles. The activation of the right tibialis anterior muscle is a bit better than 3 months ago (b).
When surface EMG is performed from a healthy person or child, coordinated motor programs can be recorded from the different muscles (Figure 16). The patterns of recruitment of motor units cannot be seen in such a motor program. Because the number of activated motor units is so high that single motor units cannot be followed. However, when only a few motor units can be activated in a certain muscle, then the pattern of activation of the motor units and the coordination between them can be seen. If the CNS of a patient with an incomplete spinal cord injury is functioning rather physiologically as a result of a long-lasting intensive coordination dynamics therapy, then an analysis of the generation of the motor program becomes possible based on single motor unit firing. Figure 17 shows such sEMG recording. Some phase and frequency coordination’s are indicated.
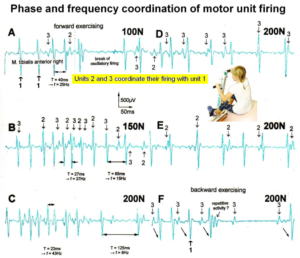
Figure 17: Phase and frequency coordination between oscillatory firing of 3 motor units (FF-type, motor units ‘2’ and ‘3’ are partly marked) during the generation of a motor program when exercising on the special coordination dynamics therapy device at loads increasing from 100 to 200N. Oscillation periods (T) and oscillation frequencies (f [Hz]) of oscillatory firing motor unit 1 (largest motor unit) are partly indicated. ‘C, F’ soleus electrodes shifted to gluteus muscles. In ‘F’, some coordination’s between motor unit ‘3’ and ‘1’ are marked.
Similar efferent impulse patterns obtained with the two electrophysiological methods single-nerve fiber action potential recording method and single-motor unit sEMG.
In Figure 18, the different frequency patterns of oscillatory firing of motoneurons are shown. Original records were taken with the single-nerve fiber action potential recording method from motoneuron axons and surface electromyography (sEMG) from single-motor units. a1-Motoneurons innervate FF-type muscle fibers and fire rhythmically with impulse trains consisting of 1 action potential in the order of 10Hz (Figure 12). a2-Motoneurons innervate FR-type muscle fibers and fire rhythmically with impulse trains consisting of 2 to 5 action potentials in the range of 4 to 7 Hz. The amplitude of the extracellular action potential of the a2-motoneurons (axon group diameter = 10.2µm, axon group conduction velocity = 50m/s) is on average smaller than that of the a1-motoneurons (axon group diameter = 13.1µm, axon group conduction velocity = 65m/s) (Figure 8), depending on the position of the axon in the nerve root with respect to the recording electrodes. FR-type motor unit potentials have much smaller amplitudes than the motor unit potentials of FF-type muscle fibers. The a3-motoneurons (axon group diameter = 8.3µm, axon group conduction velocity = 37m/s) innervate S-type muscle fibers and fire oscillatory at a frequency of around 1 Hz with long impulse trains (up to 50 action potentials per impulse train). The motor unit firing of single S-type muscle fiber motor units could not be safely identified by sEMG because their amplitudes are still smaller than those of FR-type motor units and are thus difficult to identify. The impulse patterns of oscillatory firing motoneurons obtained with sEMG are similar or the same as those obtained with the single-nerve fiber action potential recording method (Figure 18). This confirms the accuracy of the single-nerve fiber action potential recording method. Since sEMG is a non-invasive recording method, oscillatory firing can be recorded easily when using appropriate patients.
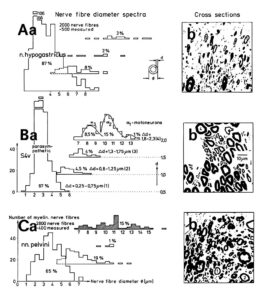
Figure 18: Oscillatory firing patterns of a1, a2, and a3-motoneurons recorded from motoneuron axons with the single-nerve fiber action potential recording method and measured by surface electromyography (sEMG) from FF, FR, and S-type motor units. The left panel shows original recordings, the middle panel the schematic patterns; the recording methods are indicated on the right side. The recordings were taken from patients with spinal cord injury and Parkinson’s disease and from brain-dead humans.
Integrative Physiology: System Theory of Pattern Formation
The System Theory of Pattern Formation for understanding Neuronal network organization and Learning
To understand the on-going changes of movement and other patterns in healthy humans and in patients with CNS injury, malformation and degeneration (aging), the System Theory of Pattern Formation is used. In a complex system like the human CNS, patterns are generated by a nervous system which seeks cooperative stability. Stability is what defines collective states. The system has the tendency to slip into the collective states to which it is attracted. When an infant crawls (Figure 19), its arms and legs are strongly attracted to the ‘pace’ and ‘trot’ gait patterns. The attraction is so strong that intermediate crawling patterns seemingly do not exist, as if the patterns are hard-wired. But with the help of the special CDT device (in the background of Figure 19) the CNS can generate intermediate coordination patterns. A patient with a CNS injury often crawls with intermediate arm and leg coordination patterns and has to re-learn the pace and trot gait coordination’s for CNS repair and shifts in this way the attractors for crawling to the pace and trot gait coordination’s. Attractive states and attractors of CNS organization can be pictured as a ball in a potential well or more generally in an attractor layout (Figures 21, 22). Changes in CNS functioning are characterized as continuous stabilization and destabilization, over time, of preferred attractor states.

Figure 19: Trot gate crawling of a cerebral palsy girl in interpersonal coordination with the therapist. The crawling performance of the therapist is not optimal. The right arm is leading with respect to the left knee.
Figure 19 shows a cerebral palsy girl who tries to relearn the attractor state patterns pace and trot gait crawling. A therapist is crawling in interpersonal coordination to speed up the learning process. The visual input from the exact crawling of the therapist into the CNS of the girl improves the performance of the in this case the trot gait pattern. For this supervised learning the cerebral palsy girl needs not to concentrate to it. It is working automatically. This interpersonal coordination is something like when soldiers march together. Once they got the rhythm among each other, the marching coordination works automatically. It was even reported that soldiers could march together in interpersonal coordination when half sleeping.
This supervised teaching of the therapist, so that the patient learns faster needs a lot of concentration. The therapist has to copy the pattern of the patient and has to drag her then into a better performance. In doing so, the therapist sometimes is also losing the own movement pattern. With the concentration on the patient and adapting partly to the pattern of the patient, the stability of the own movement pattern is reducing strongly and easily lost. With adaptation to the patient, her potential well of her movement pattern became shallower and more deformed and the ball jumps then easily out of the well (Figures 21, 22).
To reduce for understanding the complexity of human neural networks of the many billions of neurons, order parameters or collective variables are introduced for the generation of certain movements. An equation of motion describes the coordination patterns dynamics. However, coordination patterns are not only determined by the task or biological function. Patterns adjust continuously to requirements from the environment (transmitted by impulse patterns from stimulated receptors in the periphery), memory, intention, and support given by a therapist. The specific requirements are captured by the concept of behavioral information and are made part of a vector field that attracts toward the required patterns. The coordination pattern dynamics, characterized by equations of motion of collective variables (the vector X), takes the general following form [37]:
dX/dt = Fintr(X) + ∑cinfFinf(X,t) (2)
where Fintr designates the intrinsic dynamics of the nervous system. These intrinsic dynamics capture the anatomical (neuronal network structure), physiological and pathological states of the CNS and its muscular-skeletal elements.
∑cinfFinf(X,t) represents the sum of external influences (Finf(X,t)) with their relative strength (cinf) pertaining to each influence. The so-called behavioral information Finf(X,t) includes cognitive states, emotional states, intentions, motivations, instructions, inter-personal coordination, movement support etc. During motor learning or while applying therapy to a patient these extrinsic influences become extremely important, because the intrinsic (pattern) dynamics can be changed with these extrinsic influences by altering the equation of motion. By modulating the behavioral information, the intrinsic dynamics of the neuronal networks can be influenced further, that is if CDT is no longer efficient in repairing the injured CNS, the therapy has to be updated. With respect to a healthy athlete, the movement performance can be improved by modulating the behavioral information by for example including in the training program the exercising on a special CDT device to improve CNS functioning.
If the behavioral information includes the exercising of extremely coordinated, integrative movements, like exercising on the special CDT device, the quality of CNS self-organization can be enhanced by improving the exactness of self-organization, namely the precision of phase and frequency coordination between neuron and neural assembly firings. By improving the precision of organization of the intrinsic dynamics, that is the specific variability of the injured networks, certain patterns do eventually re-appear in the case of repairing the injured CNS by movement-based learning.
Learning implications for treatment derived from the equations of motion of the collective variables (Formula 2)
From the repair by learning in the severely injured CNS we can learn about learning in the healthy CNS.
- Behavioral requirements Finf (like intention, support, and instruction) affect the whole coordination dynamics, including stability, rather than only certain coordination patterns. The change of the whole coordination pattern dynamics of the CNS by the behavioral information is one scientific basis for learning transfer between different patterns and stability changes of patterns (as for example the reduction of spasticity). The other scientific basis for learning transfer is followed from human neurophysiology, namely that nerve cells or neural sub-networks are involved in different neural network organizations [1].
- Intrinsic coordination tendencies captured by the intrinsic dynamics influence the performed pattern systematically because the degree to which intrinsic tendencies conflict or agree with the required patterns determines the variability of the performed coordination pattern.
- A reduction in stability of movements and other patterns when intrinsic and informational requirements conflict, may lead to loss of stability and abrupt change while behavioral information is changing smoothly.
- The intrinsic dynamics Fintr include vegetative and higher mental functions (these are also patterns of the coordination dynamics), which indicate that via exercising coordinated movements with support and/or instructions (Finf), urinary bladder function, intelligence and speech may be partly repaired or improved following CNS injury or malformation.
- When in an injured CNS with a certain set of behavioral information (∑cinfFinf) the intrinsic coordination dynamics (Fintr) can no longer be influenced during coordination dynamics therapy, then this set of behavioral information has to be changed (using different Finf), or balanced differently (using different cinf), to further improve CNS organization dynamics.
- However, the equations of motion of the coordination pattern dynamics (formula 2) provide no information about the specific behavioral information (Finf) and training intensity (cinf) with which the CNS can be efficiently repaired by learning in the patient. We need to have detailed knowledge of the human CNS on the single neuron and neural assembly level [1], as well as the knowledge at the integrative level, to find the specific behavioral information for the repair by learning of the human CNS.
A first novel step in coordination dynamics therapy is the inference derived from the formula 2 of the equation of motion. It suggests that the movement learning not only improves the performance of that particular movement, but also improves other non-trainable functions by transfer of learning. These functions include vegetative functions like urinary bladder control, speech (if the patient cannot speak) and higher mental functions.
Furthermore, we have a means by which the stability of physiological network states can be increased (e.g., movements, continence, continuous concentration in performing certain tasks, speech etc.) and simultaneously the stability of pathological network states, like spasticity, decreased. The coordination (pattern) dynamics therapy, partly based on the System Theory of Pattern Formation in combination with human neurophysiology (including neuro-urology), thus offers us an important theoretical basis and a practical tool to diagnose, quantify and repair/improve the functioning of the human nervous system at the macroscopic level. Through neural network learning we can reach for repair the whole CNS, including the sacral and pontine micturition centers (Figure 7A) and the plexuses outside the CNS (Figure 20).
Figure 21 shows that in the complexity of neural networks of the human body, including the plexuses, there is a unique location, the sacral nerve roots, where one can measure and analyze CNS functioning at the single-neuron level. With this obtained knowledge, nervous system functions can partly be repaired through CDT in different diseases.
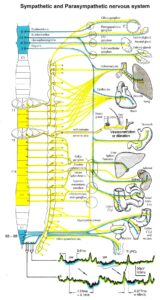
Figure 20: Schematic diagram of the sympathetic and parasympathetic nervous system. Yellow = sympathetic, blue = parasympathetic (it may be that the sacral parasympathetic division is also sympathetic). The recording of single-nerve fiber action potentials from preganglionic neurons (par) and a skin afferent fiber from a S5 sacral root is inserted.
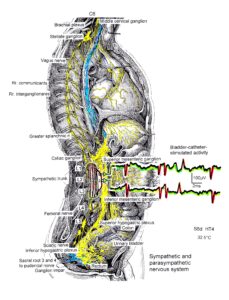
Figure 21: The picture illustrates the complexity of the autonomic nervous system. Connections of the different plexuses bypass the spinal cord and offer the structure for a functional repair of vegetative (and may be of somatic) functions like urinary bladder control, cardio-vascular performance and breathing. The recording of single-nerve fiber action potentials from human nerve roots, on the other hand, shows that in this complexity of human nervous system structures it is possible to record single-nerve fiber activity from several single identified neurons under rather natural conditions. Yellow = sympathetic, blue = parasympathetic.
Geographical landscape of attractors
The drawback of the equation of motion of the order parameters (formula 2) is that it is normally not possible to find a mathematical solution to it. But by defining a potential function and by picturing the attractive states and attractors by a ball in a potential well or rather by a ball moving in a geographical landscape of attractors (Figures 22, 23), we form a theoretical basis to understand and measure stability of certain coordinated movement patterns (i.e., the deepness of the potential well of an attractor) in patients with CNS injury who receive on-going therapy. By studying the pattern change of certain highly coordinated arm and leg movements, while a subject is exercising on a special coordination dynamics therapy device (Figure 28), pattern stability can be made visible and the mean stability per one minute can be measured by the arrhythmicity of exercising (see below). Such value, so-called coordination dynamics value, quantifies CNS functioning objectively, integrative, and non-invasively. The assessment of quality of CNS organization by pattern change is a second novel step in CDT.
To make the strategy of pattern formation, pattern stability, pattern assessment, and pattern picturing understandable, the procedure is demonstrated for the simple movement ‘jumping on springboard’, which is used during CDT, especially for the repair of the urinary bladder and training in the up-right weight-bearing posture (very important in patients with SCI, Figures 24 and 25).
Equation of motion, potential function and attractor layout for the movement ‘jumping on springboard’
For the special movement ‘jumping on springboard’ with no behavioral information (∑cinfFinf(X,t) = 0) the equations of motion (formula 2) take the form:
dφ/dt = fintr(φ)
Where φ is the relative phase between the two moving legs and is the only collective variable of this special movement.
The mathematical solution of dφ/dt = fintr(φ) in the Haken-Kelso-Bunz model [37, 38] (for the approximations being made) gives the equation of motion for jumping on a springboard for the space-time symmetry:
dφ/dt = – a(t)sinφ – 2b(t)sin2φ
The so-called potential function is defined by
dφ/dt = -∂V(φ,t)/∂φ
By integration we obtain the potential function for jumping on a springboard:
V(φ,t) = – a(t)cosφ – b(t)cos2φ
For an easy understanding, the potential function can be developed approximately as follows. With the space-time symmetry V(φ+2π) = V(φ) (time symmetry) and V(φ) = V(-φ) (space symmetry) and using the first two terms of the Fourier series with sines and cosines we obtain V(φ,t) = – a(t)cosφ – b(t)cos2φ by regarding that only cosines are invariant when φ is replace by – φ. The minus signs allow to interpret the function, V, as a landscape with attractor states for positive values of a and b [37, page 55].
The potential function V(φ,t) = – a(t)cosφ – b(t)cos2φ can be plotted for different φ and certain ratios of the parameters a and b and is shown in Figure 22.
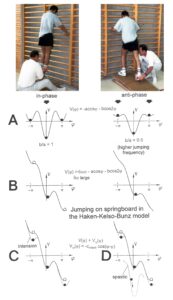
Figure 22: The jumping on springboard in in-phase and in anti-phase, analyzed by the Haken-Kelso-Bunz model in the framework of coordination dynamics. The stability of jumping patterns is represented by the potential wells (derived from the formulas) and a ball moving in the potential landscape. Dark ball = stable state (attractor state), white ball = unstable state. In ‘A’, the CNS injury is small (symmetry case); in ‘B’ and ‘C’ the injury is more severe with impaired symmetries. The Author is the therapist.
The potential function shows two attractor states, namely the jumping in in-phase (φ = 0) and the jumping in anti-phase (φ = ± π). Especially for higher frequencies (smaller b/a) the jumping in-phase has a higher stability (the potential well is deeper) than the jumping in anti-phase. Asymmetry (not tackled mathematically here) strongly changes the stabilities of the attractor states (depths of potential wells) (Figure 22).
The human CNS, seeking for cooperative stability, slips into the collective states to which it is attracted. For jumping on springboard these attractive states are the jumping in in-phase and in anti-phase. For crawling (Figure 19) (not creeping) the attractive states are the pace (in-phase) and trot gait coordination’s (anti-phase).
Since such a potential function can no longer be derived from more general movements, especially when the CNS is injured, malformed or degenerating, the temporal stability of different movement patterns for a characterization of CNS functioning has to be measured. This is partly possible by measuring the so-called coordination (pattern) dynamics (see below).
Including the variability of phase and frequency coordination among neuron firing into the equation of motion of the collective variables
Depending on the relationship between the initial coordination dynamics (so-called intrinsic dynamics, Fintr(X), depending strongly on the severance of the injury) and the patterns to be re-learned (termed behavioral information, ∑cinfFinf(X,t), which act as attractors of the coordination pattern dynamics toward the required patterns), qualitative changes in the attractor layout occur with learning, accompanied by qualitative evidence for loss (or change) of stability. The nature of change due to learning (e.g., abrupt versus gradual) arises from the cooperative and competitive interplay between the behavioral information (supported jumping or walking of the patient) and the intrinsic dynamics.
A completely different, additional nature of necessary learning is needed in the repair of CNS injury. The impaired phase and frequency coordination among neuron firing has to be repaired by re-learning for proper CNS self-organization. This perturbation of CNS self-organization produces deviations from the attractor states and changes the attractor layout because of altered hard-wiring due to injury. In a first approximation, this tremendously increased variability of phase and frequency coordination can be included into the equations of motion of the collective variables and gives further understanding of pattern change in patients with CNS injury as for example the switch from a movement pattern to a spastic pattern (Figure 23B).
In the Haken-Kelso-Bunz model, the jumping on springboard (Figure 22) can be described in terms of relative phase between the rhythmically moving legs. Without specific behavioral information the dynamical description is defined by a vector field (a differential equation) expressing the rate of change in relative phase, dφ/dt, as a function of the derivative of its potential, V(φ):
dφ/dt = – dV(φ)/dφ + (Qξt)1/2 (3)
where V(φ) = – acos(φ) – bcos(2φ) and (Qξt)1/2 is the phase and frequency variability of strength Q (where ξt is Gaussian white noise of unit variance). Zanone and Kelso [40] introduced noise in Equation 3 (from a logic point of view), because all real systems described by low-dimensional dynamics are coupled to many subsystems at a more microscopic level. One may view noise as a continuously applied perturbation that produces deviations from the attractor state. Such fluctuations are conceptionally important in dynamical modeling of phase transition or bifurcation phenomena and are essential in effecting transitions.
I included noise in Equation 3 (from the experimental point of view) because of the measured increased variability of phase and frequency coordination among the coordinated firing of neurons and neural assemblies in the human CNS. This at the neuron level measured fluctuation of phase and frequency coordination is giving rise to phase transitions or bifurcation phenomena and is essential in causing transitions among attractor states under physiologic (small fluctuation; Figure 86 (HT5 or normal Eigenfrequency distributions)) and pathologic conditions (large range of the Eigenfrequency in Figure 86; Para 2 distribution). The relative stability of an attractor states is, therefore, reflected by the depth of each potential well (I) and the strength Q of the variability of phase and frequency coordination (II), and the attraction of attractor states is reflected by the slope at each point of the potential curve.
The behavioral changes when jumping on springboard (Figure 22) are represented by the over-damped movement of a rolling ball in the potential landscape for the physiologic (Figure 23A, Q small = little fluctuation of phase and frequency coordination) and the pathologic case (Figure 23B,C, Q large = large variability). The increased fluctuation in the rather stable state, due to increased variability of phase and frequency coordination, will have greater probability of “kicking” the system out of attractor the basin (Figure 23B,C), especially in the asymmetric case.
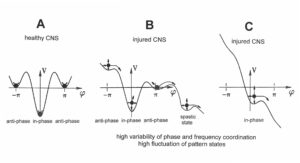
Figure 23: The potential, V(φ), of the coordination dynamics for jumping on springboard of a healthy (A) and injured CNS (B, C). The region around each local minimum acts like a well that weakly traps the system into a coordinated state. Behavioral changes are represented by the over-damped movement of a rolling ball in the potential “landscape”. High fluctuations (indicated by long arrows attached to the ball (network state)) in the stable state, due to high variability of phase and frequency coordination (in the injured case), will have a greater probability of “kicking” the system out of the basins of attraction (B, C) than for low fluctuations (short arrows) (A), due to small variability of phase and frequency coordination (in A). In B, only the in-phase jumping is stable, even though the fluctuation is high. In C there is only an attractor basin for the in-phase jumping, but the fluctuation is so high that there is a high probability that the system is kicked out of the basin of attraction. The patient can no longer jump in anti-phase and has difficulty with jumping in-phase. The stability of jumping depends on the motor program (deepness of basin of attraction) and the fluctuation of the pattern state (moving of the ball) caused by the increased variability of phase and frequency coordination due to the injury.
In the healthy CNS, the phase and frequency variability is small (short arrows) and the jumping in-phase and anti-phase is stable (Figure 23A). Following injury, the potential landscape is deformed and the fluctuation of the network states, generating jumping, is high (Figure 23B). The in-phase jumping is still stable in spite of the increased fluctuation, because the basin of attraction is deep. The jumping anti-phase became unstable because the basin of attraction is shallow and the increased fluctuation in the state has a greater probability of “kicking” the system out of the basin. A switch into a spastic state is also possible. In severe CNS injury or malformation, the patient cannot jump any more in anti-phase because of the missing of attractors for anti-phase jumping (Figure 22C). Support is needed for anti-phase jumping (Figure 22, upper right). The jumping in-phase is still possible but unstable (Figure 22, upper left).
Upon performing very exact coordinated movements, imposed by devices, the nervous system of the patient learns to reduce the variability of phase and frequency coordination and achieves in this way a small fluctuation of the network states again as shown in Figure 23A. The progress in treatment (learning) is that the in-phase jumping in Figure 23C and the anti-phase jumping in Figure 23B become stable (Figure 23A) again. Also, the potential landscape will change due to the reduction of the phase and frequency variability. The important consequence for treatment is that when exercising on special CDT devices and reducing in this way the variability of phase and frequency coordination, the patient can induce the formation of patterns again, without having trained them (learning transfer). Through improving the coordinated firing of neurons, a cerebral palsy child will become continent and my become able to speak or may develop social behaviors.
In conclusion, the impairment of phase and frequency coordination, measured at the neuron level in human (see below), can be included in the coordination dynamics at the collective variable level. The decrease of the variability of phase and frequency coordination (one kind of coordination repair) is an essential part of CNS development and repair by movement-based learning (neural network repair).
Geographical landscape of attractors
The drawback of the equation of motion of the order parameters (formula 2) is that it is normally not possible to find a mathematical solution to it. But by defining a potential function and by picturing the attractive states and attractors by a ball in a potential well or rather by a ball moving in a geographical landscape of attractors (Figures 22, 23), we form a theoretical basis to understand and measure stability of certain coordinated movement patterns (i.e., the deepness of the potential well of an attractor) in patients with CNS injury who receive on-going therapy.
CNS repair upon stability changes of physiologic and patho-physiologic patterns: improvement of geographical landscape of attractors
Before showing with human neurophysiology that the phase and frequency coordination becomes impaired following injury and that even in the healthy CNS the coordinated firing of neurons is sub-optimal, the integrative aspects of CNS learning should be followed up.
When jumping on springboard (Figures 24, 25) the pattern changes can be represented by the over damped movement of a rolling ball in the potential landscape for the physiologic (Figure 23A, little fluctuation of phase and frequency coordination) and the pathologic case (Figure 23B,C, large variability of phase and frequency coordination). In the healthy CNS, the phase and frequency variation is small (short arrows of the moving ball) and the jumping in in-phase and in anti-phase is stable (Figure 23A). Following injury, the potential landscape is deformed and the fluctuation of the network states, generating jumping, is high (Figure 23B). The in-phase jumping is still stable in spite of the increased fluctuation (larger fluctuation arrows), because the basin of attraction is deep. The jumping in anti-phase became unstable because the basin of attraction is shallow and the increased fluctuation in the state has a greater probability of “kicking” the system out of the basin. In CNS injury, a switch into a spastic state is also possible. In severe CNS injury or malformation, the patient can no longer jump in anti-phase because of the missing of attractors for anti-phase jumping (Figure 23C). The attractor layout is asymmetrical and deformed. Support is needed for anti-phase jumping. Jumping in-phase is still possible but unstable.
Upon performing very exact coordinated movements, imposed by devices, the nervous system of the patient learns to reduce the variability of phase and frequency coordination and achieves in this way a small fluctuation of the network states again as shown in Figure 23A. The progress in treatment (neural network learning) is that the in-phase jumping in Figure 23C and the anti-phase jumping in Figure 23B become stable (Figure 23A) again. Also, the potential landscape will change due to the reduction of the phase and frequency variability. The important consequence for learning/treatment is that when exercising on special devices and reducing in this way the variability of phase and frequency coordination, the patient can induce the formation of patterns again, without having trained them (learning transfer). But exercising only on the special device is not enough in itself to improve the attractor layout sufficiently. Regrettably, there is no miracle device. Many different movements have to be trained to repair the CNS.

Figure 24: In-phase jumping on springboard of a patient (Kadri) with a formally motoric complete cervical spinal cord injury (C5/6) supported by fixations and the Author. Within 3 years of CDT the urinary bladder functions were repaired.
Jumping on springboard (Figures 24, 25) and exercising on the special CDT device (Figures 27-29) are the most important movements to repair the urinary bladder by neural network learning. Figure 24 shows the supported jumping on springboard. The therapist (Author) is supporting the in-phase jumping to realize/feel the movement in the patient with a motoric complete cervical spinal cord injury (SCI) (C5/6) [12]. The hands are fixed because of only little hand grip power. The legs are supported that they cannot slip to the side and because of very little leg functions to manage the gravity there is further trunk support needed. The Author is actually supporting the jumping movement. Such jumping movement became possible following three years of coordination dynamics therapy (CDT) when some regeneration of the spinal cord took place.
Re-learning of up-right movements is very important for patients with SCI. With an Eigenfrequency of 1Hz of the springboard the a3-motoneuron (S) oscillators activating leg muscles of slow type (S) (Figure 12) are especially improved in its firing. The jumping on springboard is oscillator formation training. It is tried that the patient re-learns the jumping on springboard and changes the geographical landscape of attractors for jumping from the pathologic case (Figure 23C) to the physiologic case (Figure 23A). First the in-phase jumping can be achieved because the stability of the in-phase jumping is higher (Figure 23A) or frankly speaking, the in-phase jumping is the easier movement.

Figure 25: Supported jumping on springboard of a girl (patient Nefeli of Figure 1) after tumor removal and damage of the lumbar spinal cord to repair the lumbar cord by learning. The Author supports the jumping in anti-phase. Urinary bladder repair needed through CDT needed more than 3 years.
In Figure 25, the girl Nefeli is trying to re-learn jumping in anti-phase after cancer treatment. Following extirpation of the tumor, located besides the lumbar spinal cord at the ganglion and foramen intervertebrale, the lumbar spinal cord got damaged and probably also the blood supply to the cord. The blood supply of the spinal cord [41] is critical in spinal cord injury and tumor operations. Leg functions and continence, located in the caudal spinal cord, got impaired in the girl. The relearning of mainly jumping, walking and exercising on the special CDT device repaired partly the functions of the lumbar spinal cord (including continence) and its blood supply.
Method
After the introduction to Coordination dynamics therapy (CDT), further details of the neural network learning through movement-based learning will be given. It was necessary to give some details of the ‘System Theory of Pattern Formation’ in connection with human neurophysiology to understand that CDT is a scientifically based method. In Part 2 details of the human neuro-uro-physiology of the sacral micturition center will be given.
Performed movements in CDT
The performed movements in CDT are creeping (Figure 26), crawling (Figure 19), up-righting, walking, running, jumping (Figures 24, 25), old-learned movements and other movements, if the patient can perform them with or without support. The exercising on a special CDT device (Figure 27) is most important. The always impaired phase and frequency of CNS functioning can be repaired and CNS functioning can be measured by a single value with ongoing therapy.
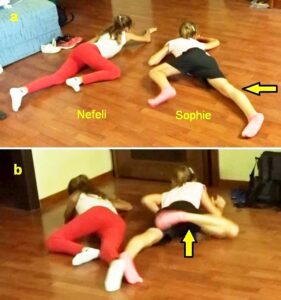
Figure 26: Sophie during creeping in interpersonal coordination (antiphase) with Nefeli (SCI). Sophie is overstretching (a) and overswinging the legs (b) in comparison to Nefeli. She had not fully learned to control the inertia and centrifugal forces of leg movement. She cannot stop leg movement on time. The spinocerebellum (vermis) had not been repaired sufficiently so far. In Sophie and Nefeli bladder functions were repaired.

Figure 27: Patient with SCI (Nefeli) during exercising on the special CDT device different movement patterns to improve phase and frequency coordination of neuron firing. In A and B also trunk rotation is trained. When turning in the standing position (F), the performance of the right foot is pathologic (plantar flexed).
Measuring CNS functioning by the arrhythmicity of exercising (coordination dynamics value)
The impaired phase and frequency coordination at the single neuron level, the assembly level and the macroscopic level can be measured macroscopically when the patient is exercising on a special coordination dynamic therapy device (Figure 28) on which arms and legs turn with a slightly different frequency (transmission 19 (arms) : 18 (legs)). The phase coordination between arms and legs is imposed by the device. The loss of phase and frequency coordination between arm and leg movements becomes visible and measurable by the arrhythmicity of turning. During a turning cycle the coordination between arms and legs changes between pace (P) and trot gait (K). According to the difficulty of the coordination, the turning frequency increases and decreases. This frequency variation (df/dt; f = frequency) can be recorded, quantified and displayed on a computer screen (Figure 28A) and is called coordination dynamics value. CNS functioning is therefore measured though pattern change (continuous pattern change from trot gait to pace gait) according to the System Theory of Pattern Formation.
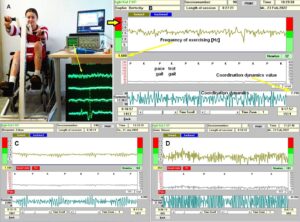
Figure 28: A. Layout for measuring coordination dynamics (arrhythmicity of exercising, (df/dt)) between arm and leg movements, displayed on the laptop, when exercising on a special CDT device. The recording of sEMG activity (displayed on the oscilloscope) from the tibialis anterior and other muscles is also shown. The inset shows a single motor unit action potential on the lowest trace. The recordings are taken from a patient (Kadri) with a motoric complete cervical spinal cord injury C5/6. B. Coordination dynamics measurements of the CP patient Sophie with repaired continence. C. In the brain-injured patient Benjamin, df/dt is higher between P and K but not between K and P. D. The sporty 10-year-old healthy Anna has problems for the difficult coordination’s between P an K and K and P. Also the healthy CNS needs CDT for improvement. The coordination dynamics value is the mean arrhythmicity value for 1min. P = pace gait, K = trot gait.
Brain-injured and healthy persons have problems with these difficult intermediate coordination’s between pace and trot gait, especially for higher loads (Newton) (Figure 28C, D), because the deep complexity of CNS organization is needed to generate these movement patterns. In reverse, if brain-injured patients learned to generate these difficult coordination’s, then their CNS improved its functioning in the deep complexity of CNS organization.
During the functional reorganization of the injured CNS of patients, the relative phase and frequency coordination among neuron firings has to be entrained as exactly as possible by the movement induced afferent impulse patterns from the receptors (learning through feedback information) to restore coordination in the range between 3 to 5 milliseconds (approximate lengths of postsynaptic potentials). The device has therefore to impose the exercising patient a coordination in the millisecond range for the different coordination’s of arm and leg movements between pace gait and trot gait. The easy pace and trot gait coordination’s, but not the difficult intermediate coordination’s, can often be performed by the patient easily. Therefore, the continuous change from the easy to the difficult coordination’s and backwards diagnoses the capability of the CNS to organize easy and difficult organizational states. If the movement states can be easily generated by the neuronal networks of the CNS, then the frequency variation of turning is small during the turning cycle, and if the movement state is difficult to be organized by the CNS, then the frequency variation is large (the coordination dynamics value is large).
Unique properties of special CDT devices
The special CDT device has three important properties.
First, the patient performs coordinated arm, leg and trunk movements when exercising on it. The training of integrative patterns takes care of that the pathologic organization cannot escape from repair by shifting to another part of the CNS and the whole CNS, including the injured parts, is reorganized so that other CNS parts can take function over through plasticity. Figure 29 shows for the motor cortical fields that nearly the whole brain is activated, if the patient is performing simultaneously speech therapy or if the patient is counting or speaking in coordination with the turning movement.
Second, neurons are coordination detectors (Figure 30). Because the mechanical coordination between arm handles and leg pedals is extremely exact, the generated time-coordinated afferent input endplate potentials onto a neuron in the neural networks (approximately 5ms long) overlap more. The excitation threshold of the neuron is reached earlier. In this way, the efficiency of organization is improved. In spinal cord injury, for example, the transmission over the injury site will increase. If the mechanical coordination between arm handles and leg pedals of the device is not extremely exact any more, then the turning is only a muscle training and not a training through which the nervous system can learn from the device to function better. Maintenance of the device and supervision by an educated therapist is necessary.
Third, the coordination between arm and leg movements changes from pace to trot gait, imposed by the device. The intermediate coordination patterns between pace and trot gait are difficult to generate for the CNS neural networks. If the patients CNS learns to generate these intermediate patterns, imposed by the device, then the neural networks have learned to function better (more precise) in the deep complexity of CNS organization. The patient’s nervous system learns by turning from the device, to function more physiologic through improving especially the phase and frequency coordination among neuron firings.
This phase and frequency coordination can be measured by single-motor unit surface electromyography non-invasively (Figure 17) and by the single-nerve fiber action potential recording method (Figures 3, 13-15) invasively.
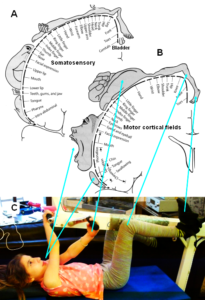
Figure 29: Relative sizes of cortical representations of different parts of the body which are activated when exercising on special CDT devices in coordination with instructions. Nearly the whole somatosensory (A) and motor cortical fields (B) are activated. When moving only the legs, as in case of a fitness bicycle, the activated areas are relatively small. Note, the cortical representation of the urinary bladder is close to the representation of the toes, and during jumping (Figure 25), the toes are activated. The patient Nefeli in ‘C’ suffered a spinal cord injury during a cancer removal by medical malpractice and had also the urinary bladder to be repaired. The crossing of the arms trains the corpus callosum. – This special CDT device for measuring and therapy (int.pat.) is produced by the firm: Giger Engineering, Martin Giger dipl.Ing.ETH/SIA, Herrenweg 1, 4500 Solothurn, Switzerland, www.g-medicals.ch.
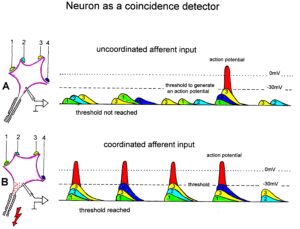
Figure 30: Neuron operating as a coincidence or coordination detector. A. Afferent input is reaching rather uncoordinated the cell soma. Only sometimes an action potential is generated, because the threshold of action potential generation is mostly not achieved. B. The action potentials in fibers 1 through 4 are reaching time-coordinated the dendrites or the cell soma. The postsynaptic potentials add up and the threshold is achieved at approximately –30mV, and action potentials are generated time-coordinated at the axon hillock. In the real CNS mostly, many more smaller postsynaptic potentials will contribute to the generation of an action potential and passive conduction from the dendrites to the cell soma has to be taken into account. Coordinated afferent input may thus induce or enhance (coordinated) communication between neuronal network parts following CNS injury.
In Figure 13 the coordinated firing between a motoneuron and spindle afferent fiber is recorded and measured. This spindle afferent fiber contributes to the drive of the motoneuron, because of a constant phase drive. In Figure 13B, C, the phase variation was 1.4ms (3-1.6ms). For a longer motoneuron drive, many spindle action potentials have to contribute; there have to be also frequency coordination’s. And this was really the case (Figure 13D). From Figure 31 it can be seen that from the point of frequency coordination, the SP2 (1) spindle afferent fiber was contributing most to the drive of the oscillatory firing motoneuron. The other spindle afferent fibers were contributing less. The recorded γ-motoneurons were only little correlated to the motoneuron firing. Figure 31 shows, from the point of frequency coordination, which spindle afferent fibers (SP2(6) till SP2(13)) and urinary bladder afferent fibers (S1 and S2) contributed to the drive of the oscillatory firing motoneurons innervating the external anal sphincter (TO2) and the external bladder sphincter (TO1).
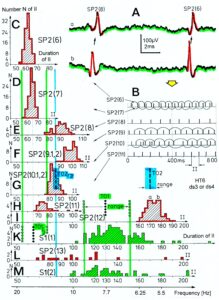
Figure 31: Measurements from brain-dead human HT6 from different spinal cord segments after retrograde bladder filling (700 to 800 ml), with the exception of “I,” which was obtained before filling. A. Sweep piece of a recording from a dorsal S3 or S2 root filament. It can be seen that the secondary muscle spindle afferent SP2(6) AP can be distinguished by the waveform on the two traces from the secondary spindle afferent fiber SP2(8) AP (different amplitude of the three phases of the triphasic APs). B. Simultaneously recorded impulse patterns of the six parent secondary spindle afferents SP2(6) through SP2(11) obtained from dS3 or dS2 root recordings. The impulse patterns of SP2(6) and SP2(7) fibers are not separated to show the similarity of the patterns. The impulse patterns of the parent spindle afferents SP2(9) and SP2(10) are split into patterns of the single endings (single ending activity partly connected by circle lines) with the assumption that single endings of parent secondary muscle spindle afferents should have interspike intervals of duration longer than 50 ms. C to H. Interspike interval distributions of six simultaneously recorded single secondary spindle afferent endings. F, G. Interspike interval distributions of parent fibers, which are the sums of the distributions from the two activated endings. I. Interspike interval distributions of a secondary spindle afferent fiber (SP2(12)) of a coccygeal root. K, L, M. Interspike interval distributions of single-fiber afferent activity from a lower sacral dorsal root. In L, most likely the activity from a secondary spindle afferent fiber is shown. In K and M, most likely the interspike intervals from afferents (S1(1) and S1(2)), innervating stretch receptors of the urinary bladder wall, are shown. In G, H and K, the durations of the oscillation periods (mean and range) of the oscillatory firing a2-motoneurons are indicated by thick dashed and dotted lines; the motoneurons innervate the external anal sphincter (TO2) and the external bladder sphincter (TO1). The sites of innervation of the oscillatory firing motoneurons are identified (and distinguished from each other) by anal reflex stimulation, bladder filling and catheter pulling. Note that the TO1 and TO2 ranges and their halves overlap with the interspike interval distributions of the secondary spindle and stretch receptor afferents (relative frequency coordination).
The innervation of the urinary bladder is very complex and only partly known with respect to the innervating nerves (Figure 32).
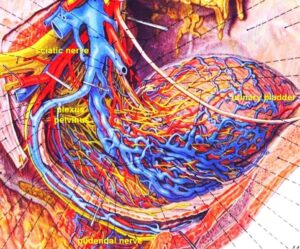
Figure 32: Anatomy of the innervation of the urinary bladder. The plexus pelvinus makes it very difficult to clarify further the innervation of the bladder; the Author tired it without success. Picture taken from Pernkopf (‘Topographische Anatomie des Menschen’, University Library Turku, Finland).
But when recording from sacral nerve roots with the single-nerve fiber action potential recording method, and using the classification scheme for human peripheral nerves (Figure 8), we can clearly identify continence functions and sites of receptors and motor units of continence muscles as Figures 11, 14 and 31 indicate. In Figure 33, the sites of the urinary bladder afferents and the external anal sphincter (TO2) and the external bladder sphincter (TO1) are indicated. For further details, see [35, 42].
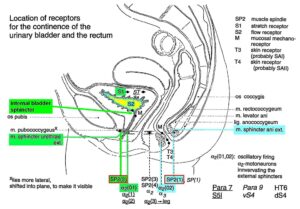
Figure 33: Schematic anatomy of the lower pelvis with the location of urinary bladder and rectum continence muscles and receptors from Figures 11, 13-15. The external bladder and anal sphincters are skeletal muscles. The indicated internal bladder sphincter is a part of the detrusor (bladder) and is a smooth muscle innervated by the sympathetic nervous system.
Fourth, the exercising on the special CDT device repairs especially urinary bladder functions. The mechanism is not fully clear. It is connected to the improvement of the phase and frequency organization of the neural networks of the sacral and pontine micturition centers (Figure 7A). Through learning transfer from movements, also the sympathetic and parasympathetic nervous system divisions are activated for better functioning and repair. Figure 20 shows the innervation of the different organs by the sympathetic and parasympathetic nervous system divisions. The site of recording single-nerve fiber action potentials is indicated.
But as this article should emphasize, the progress in continence repair is the human repair-neurophysiology and not a special device. Other devices could be developed on the basis of this human neurophysiologic research.
Repair strategies at the neuron membrane and genetic level
For the repair of urinary bladder functions/patterns it is likely that excitation-neurogenesis coupling [43-45] contributed, stimulated through CDT.
- Repair depends on learning and memory formation, mediated or supported by epigenetic mechanisms. Epigenetics is the interplay between genes and the environment resulting in phenotype and epigenetic landscape.
- Epigenetic mechanisms, like DNA methylation, are probably sensors for movement-based learning and memory formation and fine modulators of neurogenesis though CDT (Figure 34).
- The epigenome consists of non-coding RNA and chromatin, a proteinaceous matrix surrounding DNA. The dynamic interactions of post-translationally modified chromatin proteins, covalently modified cytosines inside DNA and non-coding RNA define the complex pattern of gene expression beyond the four bases of DNA.
Figure 34: Epigenetic regulation for repair by movement-based learning. CDT-induced stimulation of the pathways that regulate neural network repair is a proven therapeutic and preventive tool. Epigenetic mechanisms, stimulated by physiologic network activation, are likely key players within signaling networks, as DNA methylation, chromatin remodeling and small non-coding RNAs superfamilies’ are required for the fine-tuning and coordination of gene expression during neural network repair by learning.
- The hippocampus plays an essential role in learning and memory. In the hippocampus there exists a specialized form of neural plasticity, which is the generation of new functional neurons from stem cells occurring throughout life. Adult hippocampal neurogenesis contributes to learning and memory formation.
- New neurons are important for learning and memory formation (besides functional reorganization), i.e., for increasing the rate of repair, for the following reasons:
- The insertion of new neurons helps to store the memory of the same activity that led to the creation of the neuron.
- Activity-dependent neurogenesis enhances the learning of new memories and degradation and clearance of previously stored unwanted memories like spasticity, because the synapses, dendrites and axons can be devoted more fully to the newer memories. The old neurons with large and complex axon and dendritic trees are difficult to change. They can only be changed with sustained effort.
- New neurons seem to improve the accuracy of relearned patterns (from model study [43]). This means that new neurons help to improve phase and frequency coordination of neuron firing and pattern stability.
- The advantage of new neurons seems to be dramatically greater in networks that had been more active and had been required to store more memories [43]. The advantage of neurogenesis for memory storage in heavily active networks is that it provides an increased rate of repair if movement-based learning is administered aggressively and if different movements are trained.
- Specific natural network activity is required for multiple aspects of repair. Specific activity is essential for correct migration of interneurons and it also controls the development and repair of their axons and dendrites. During repair there is a specific requirement of network activity in shaping the cortical integration of specific neural subtypes. Newly build neurons are likely electrically active shortly after their birth and participate in the early network activity that contribute to circuit maturation during repair by CDT.
- Specific activity is required for migration and maturation at several stages of repair. A break in CDT may invalidate the whole chain of repair events. Specific interneuron subtypes require activity for migration and morphological maturation at two distinct stages of development [43]. Newly built neurons may even require specific activity for migration and maturation at several distinct stages of repair. During a break in CDT, the specific activity, required for neuron migration, maturation and network integration may not be supplied at one of these stages so that the chain of repair events is severed and the whole repair chain has to be started anew.
- Drug application may undermine repair. Altering the level of neuronal excitability within genetically targeted neurons from drug application, for example antiepileptic drugs may have profound consequences on multiple aspects of the repair of select types of neurons within a population of neurons, as well as their associated gene expression. The pain-killer ‘Contergan’, taken during pregnancy, changed gene expression and the babies were born without arms.
- Excitation-neurogenesis coupling [43]:
- Excitation increases or decreases neuron production directly by excitation-neurogenesis coupling.
- The excitation acts indirectly on the surrounding mature (hippocampal) cells through depolarization-induced release of growth factors.
- Adult neurogenesis is enhanced by excitatory stimuli and involves Ca2+ channels and NMDA receptors.
- The Ca2+ influx pathways are located on the proliferating stem/progenitor cells (NPCs), allowing them to directly sense and process excitatory stimuli. The Ca2+ signal in NPCs leads to rapid induction of a proneural gene expression pattern.
- Integrative coordinated movements have to be trained to allow functional reorganization and new nerve cell integration across very large distances. CDT has to activate injured and uninjured networks to enhance physiologic CNS functioning and learning transfer.
- Conclusion for optimal therapy according to the present stage of knowledge. If there is similarity between development and repair, animal (mice) data also hold in humans and the principles of neurogenesis of the hippocampus also hold in other parts of the brain, albeit to a much lesser extent, then the patient has to be trained at his limits (1) to induce substantial building of new nerve cells [46]. The treatment has to be continuously administered (2) to support all stages of repair at the progenitor level as migration, maturation and integration. The networks requiring repair have to be activated specifically (3) to generate repair-friendly, micro-environmental properties in the networks. No drugs should be administered that change neuron excitability (4). The exercises have to include coordinated arm, leg and trunk movements (if possible) to improve the impaired phase and frequency coordination for CNS self-organization (5). The performed movements have to be as integrative as possible to reconnect distant brain parts and to induce learning transfer.
It is movement-based learning and learning transfer that achieves repair
It is learning and not simply training that elicits the survival effects of new neurons in the hippocampus. Learning appears to promote the survival of newborn neurons in cognitively unimpaired aged rats [47]. Learning elicits different influences on neural precursors at different developmental stages. The regulation of subgranular zone neurogenesis by hippocampus-dependent learning is complicated and can be affected by factors such as the age of the newborn neurons, the stage of learning and specific learning protocols [48].
When the human patient is exercising on the special CDT device, he should not just turn, but should try to turn more smoothly. He should try to reduce the arrhythmicity of exercising. The patient should try to improve the performance of movements by learning. During learning, it is essential to concentrate and to be aware of what needs to be corrected. When the patient is well-practiced at exercising smoothly, the skill can be accomplished without conscious effort, much like in walking, swimming, cycling or skiing. Simply exercising will also improve CNS functioning. However, the rate of learning is significantly lower. This is the learning of automatic movements, in which the process is subconscious. A problem in some patients is that the cognitive functions are that much impaired that they cannot understand that they have to learn to improve their nervous system functioning. The hope in such cases is that the simple training improves their cognitive functions to a point that they can understand that they have to improve the performance of the trained movements to improve their CNS functioning. Older patients are intelligent and can understand that they have to perform movement-based learning. But according to their experience, they are not trusting the research and treatment systems any more.
With respect to the repair of continence functions, it is the learning transfer from the movements (and instructions) which repairs the bladder [49] because it was unsuccessful to train bladder functions through filling and emptying the bladder.
Reduction of spasticity
When performing movements like walking, running, crawling, or exercising on a special CDT device which imposes highly coordinated movements on the patient, the coordination dynamics can be changed in the way that the stability of spastic states decreases and the stability of the movement states increases. Such changes of coordination pattern dynamics can be pictured again by means of an attractor layout. An attractor is pictured as a potential well (attractor valley) into which a rolling ball is attracted. The position of the ball represents the momentary state of the system. Figure 35 shows schematically such an attractor layout with the two attractor’s spasticity and coordinated movement. When exercise is commenced (A), the spastic state is very stable (the attractor valley is deep) and the state of the system is attracted towards the attractor state spasticity. With exercise, the attractor layout is changing in the short-term memory in the way that the attractor spasticity is getting shallower and the attractor physiologic movement is getting deeper (B). Because of fluctuation due to variability of phase and frequency coordination, the position of the ball, which represents the momentary state of the system, is switching between the attractor states spasticity and movement. Spasticity and movements are present simultaneously in the patient. With further exercise, the attractor movement becomes deeper (more stable) than the attractor spasticity. The patient can now perform the movements with little or no spasticity. The transient reduction of spasticity in the short-term memory, achieved by many hundreds of coordinated movements, can last anything from a few minutes up to several hours, as is indicated in Figure 35 by the two long arrows. The shorter backward arrow (from right to left) indicates that spasticity has slightly reduced in the long-term memory. The coordination dynamics have changed. Repeated exercising will further reduce the stability of spasticity and increase the stability of the coordinated movement and will further change intrinsic coordination tendencies in the long-term memory.
The same holds for a spastic external bladder sphincter. Important is that repair through movement-based learning is leading always in direction of physiologic patterns.
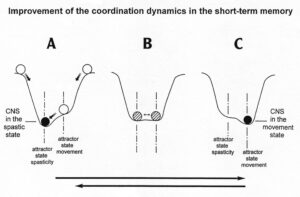
Figure 35: Therapy-induced spasticity reduction in the short-term memory. The position of the ball represents the state of the system and the potential well, the attractor. The ball is attracted to the stable Figure position in the deepness of the whole, called attractor state. The attractor layout, consisting of two attractive states of different stability, is changing upon exercising very coordinated rhythmic movements. Black ball = stable state, open ball = very unstable state, hatched ball = spasticity and movement co-exist. Variability of phase and frequency coordination not indicated.
In Figure 36 a girl is exercising on the special CDT device to reduce the side effects of the cancer treatment, which were in this case the damage of the lower spinal cord. By training on the special CDT device, she is improving the impaired phase and frequency coordination to enhance pattern stability via improving (reducing) the variability of phase and frequency coordination. Because of the damage of the lumbar spinal cord, she got the pathologic pattern extensor spasticity, similar as in traumatic spinal cord injury. When she trains the coordinated movements, she has to avoid the pathologic pattern not to deepen the potential well (the stability) of the extensor spasticity. The girl is therefore exercising in a rather flexed position to be far away from the extension. If she would train more in a stretched position, she would slip partly into the extensor spasticity. In the picture of system theory of pattern formation (Figure 35), the ball representing the state of the system would partly be in the attractor ‘extensor spasticity’.
The picture of a ball in a potential well will be used below for understanding urinary bladder repair (Figure 35), when the bladder functioning pattern (the ball) changes/improves (the ball changes its position in the attractor layout).

Figure 36. A girl, following cancer treatment, is exercising on the special CDT device. Same girl as in Figure 1 (Nefeli). She is exercising in a rather flexed position of the legs to avoid the pathologic pattern extensor spasticity of the legs.
Quantifying CNS functioning by measuring pattern stability upon pattern change when exercising on the special CDT device
Experimentally, the underlying dynamics of coordinated movements can be found in the temporal stability of coordination patterns and can be assessed through pattern change. A change of the coordinated movement patterns is generated, when a subject is exercising on the special CDT and recording device (Figures 27,29), where the coordination between arms and legs, imposed by the device, changes continuously between pace (P) and trot gait (K) and backwards. The stability of the intrinsic coordination tendencies is measured by the deviations and differential stability during the performance of these rhythmic movements (Figure 36F). When the differential stability of the movement pattern is high, the arrhythmicity of exercising is small and when the stability is low the arrhythmicity of exercising in that pattern is high. From the coordination dynamics trace in Figure 36F it can be seen that in the healthy case the arrhythmicity is low for the pace and trot gait coordination’s and is high for the intermediate coordination’s between pace and trot gate. The pace and trot gait coordination’s between arms and leg movements have a high stability and the intermediate coordination pattern have a low stability.
The plotting of the differential stability over time of the frequency of exercising generates an attractor layout for this special movement (Figure 36G) and the mean stability per minute can be measured by the arrhythmia of exercising (df/dt:f, f = frequency; or dν/dt, ν = angular velocity). Such differential stability value per minute, the so-called coordination dynamics value, quantifies CNS functioning objectively, integrative and non-invasively. The assessment of quality of CNS organization by pattern change is a third novel step in CDT.

Figure 36: Forward-backward movement symmetry impairment in a patient with severe cerebellum injury for exercising on the special coordination dynamics therapy device (A-C). Coordination dynamics figures of a healthy person are shown for comparison in D and F. In G the attractor layout is constructed for the healthy case. The attractors are the pace and trot gait movement patterns. The state of the system (the white ball) is attracted to the ground of the potential well. Being in the attractor state, the ball is pictured black. In E the attractors of a brain injured patient are not at the pace and trot gait coordination’s and at different coordination’s for forward and backward exercising.
Summary of the Method
Because of the Ascensus of the human spinal cord, the lower sacral nerve roots are thin and long (Figure 7A) and afferents and efferents are mixed in lower sacral nerve roots. They are ideal to record simultaneously with two pairs of wire electrodes the impulse traffic running in and out of the sacral micturition center. To identify the nerve fibers in which the impulse traffic is conducted, a classification scheme for human peripheral nerve fibers was developed, the only existing one for human. The nerve fibers are classified by a group conduction velocity and a group nerve fiber diameter (Figures 7B and 8). It was found that the neural networks organize themself by phase and frequency coordination among neurons and neural subnetworks. With every injury, degeneration or malformation, this phase and frequency coordination becomes impaired and has to be repaired. This can be achieved when patients exercise on a special coordination dynamics therapy device (Figures 27, 29). The nervous system learns from the device to function more physiologically, including the sacral and the pontine micturition centers (Figure 7A). With the repair of the phase and frequency coordination, some patterns including continence patterns re-appear. In severe CNS injury, other parts of the nervous system have to take function over by plasticity. Therefore, also other arm, leg and trunk movements have to be trained.
The patients train exactly coordinated arm and leg movements on the special device (Figures 27, 29) and the automatisms creeping (Figure 26), crawling (Figure 19), walking, running and jumping (Figures 24, 25) with or without support. For bladder repair the exercising on the special device and the jumping on springboard are most important. The jumping on springboard activates the pelvic floor muscles rhythmically, of which the external bladder and anal sphincters are a part. When exercising on a special device, the neural networks of the central nervous system are activated and repaired in the deep complexity of neural network organization. This is achieved because arm and leg movements change their coordination between the pace and trot gait patterns. Through this pattern change, according to the system theory of pattern formation, the quality of CNS functioning can be measured through pattern change by a single value (Figures 28, 36).
The movement-based learning process is optimal by training 20 hours per week. Through neural network learning mostly first the cardio-vascular performance is repaired, then the continence and then the motor functions. The higher mental functions are most difficult to repair through neural network learning.
Results
Summary: Urinary bladder function repair in a group of patients through Coordination dynamics therapy
In 7 spinal cord injury and 3 cerebral palsy patients, urinary bladder functions were improved. Incontinence was repaired in 5 spinal cord injury patients and all 3 cerebral palsy patients. The repair time depended on the severity of the brain or spinal cord injury and the intensity and duration of the treatment. In the cerebral palsy patients, the repair time was approximately 3 months. In a 50% cervical spinal cord injury, the repair time was approximately also 3 months. In a rather complete cervical spinal cord injury (95% injury), the repair time was 2.5 years. In a complete thoracic spinal cord injury (100% injury) the bladder was not repaired within 2.5 years. Additional stem cell therapy did not help. Obviously, in severe spinal cord injury, the bladder repair is very difficult, but important. The by movement-based learning stimulated nervous system has to use all available repair mechanisms in and outside the spinal cord, including genetics. Urinary bladder repair is the biggest problem in spinal cord injury. Ongoing urinary bladder infections ruin the bladder wall and stimulate bladder receptors to function pathologically, which is measured in detail by a comparison of the activity of stretch, tension and flow receptors in a paraplegic patient and a brain-dead human. Further, ascending infections may ruin kidney functions, which is analyzed in a medulla oblongata injury. Through coordination dynamics therapy the immune system is improved in its functioning through movement-induced building of additional K-cells and improvement of cardio-vascular performance. Movements are repaired simultaneously. With the improvement of cardio-vascular performance, pressure ulcers do not occur any more in spinal cord injury patients. Exercising on the special coordination dynamics therapy device induces diuresis, which may be of importance in some patients and can support drug-induced diuresis. In cerebral palsy, simultaneously the higher mental functions are improved through neural network learning.
Urinary bladder repair in spinal cord injury
The most important functions to be repaired in spinal cord injury (SCI) are firstly the bladder repair, followed by the sexual function. The walking is on place 3 followed by spasticity and scoliosis.
Spinal cord repair depends on the severance of the injury. In the patient with a 50% SCI (Sten) the bladder was fully repaired through 2 months of CDT. In the patient Kadri with 95% SCI 2.5 years were needed. In a 99% injury the bladder was not repaired within 2.5 years. It is obvious from Figure 37 that possible repair depends strongly on the severance of the injury.
Because in 50% SCI the bladder is somehow functioning, animal researcher cut only 50% of the cord in animals to avoid urinary bladder problems, even though it is the most important function to be repaired in SCI.
Figure 37: The outcome of a SCI repair depends strongly on the severance of the injury. A-D. MRI’s of approximately 99%, 95% and 50% injury. C shows the 50% injury with titan fixation and B without. The severance of an injury can also be estimated with a fixation in place. G, H. In 50% injury, the patient can relearn walking, running and jumping. E, F. In 95% (and 99%) the patient cannot re-learn free walking and jumping.
In an almost complete cervical SCI, the caudal spinal cord is disconnected from the cerebral cortex and brain stem. With respect to urinary bladder functioning, the sacral micturition center is disconnected from the pontine micturition center (Figure 7A). Following the spinal shock some reflexes, such as the stepping automatism and urinary bladder reflex (similar to those in infants), may re-appear, especially when stimulated. Pathologic reflexes or automatisms also appear as for example extensor spasticity or spastic bladder. With very limited regeneration upon CDT, motor and vegetative functions partly re-appear in a cephalo-caudal direction and movements become controlled first proximally and then distally; spasticity reduces. This cephalo-caudal and proximal to distal scheme only partly holds, because the injured CNS uses all existing repair and adaptation mechanisms through movement-based learning, including plexus connections outside the spinal cord. But with respect to the improvement of trunk stability and breathing the repair in cephalo-caudal direction seems to hold.
To understand bladder repair through neural network learning and regeneration, the repair will be analysed in the two patients Kadri (Figure 37F) and Nefeli (Figure 1).
Urinary bladder repair in 95% spinal cord injury
A 17-year-old female patient (Kadri) suffered a severe cervical SCI in a car accident. No motor functions remained below the injury level of C5/6 and the patient had impaired feelings. From the MR images the author estimated that approximately 5% of the spinal cord matter was spared (Figure 37B, C). When the spinal shock faded away, it became obvious that no motor functions remained below the injury level but spot wise sensitivity remained more or less all over the lower body. Two months after the accident CDT was started. Upon 2.5 years of CDT the sensitivity improved and some motor functions returned below the injury level, indicating that some regeneration of the spinal cord had occurred. Urinary bladder functions were repaired. Details of the repair are given below (Figure 38). The connectivity over the injury site, according to the magnetic resonance imaging (MRI), may have increased to 8%.
Generally, a urinary bladder repair is very important in rather complete C5/6 SCI. The tetras are not able to perform intermittent bladder catheterization by themselves, because of the mainly lost finger functions due to the lost finger-function-motoneurons in C5/6 spinal cord segments. Through urinary bladder repair, the patients get their private sphere back.
The time course of the improvement of urinary bladder functions upon CDT
It is reported about the stages of bladder repair through 2 years of therapy. The changes of functions of the detrusor (bladder) and the external and internal bladder sphincters are extracted from a detailed anamnesis and are pictured by an evolution of the attractor layout (in similarity to motor function of Figure 23) with the re-learning of bladder functions (Figure 38).
- After the operation (fixation and alignment of the broken cervical spine) a lying catheter was installed in the patient. The patient was suffering continuous infections and fever.
It is understood that the bacteria are ‘creeping up’ the lying catheter into the bladder (especially in female, because of the anatomy of the urethra) to give rise to ongoing infections in spite of antibiotic therapy. Before World War II (time of no antibiotics), patients were dying on such infections. It is the benefit of Sir Guttmann from Breslau [50] who stopped or reduced these infections by introducing sterile intermittent catheterization. The introduction of antibiotic therapy helped further.
- One month later (at a time when the spinal shock weaned) a suprapubic catheter was installed and no more infections occurred. But the bladder did not show any physiologic functions. The patient had no feeling of bladder fullness, no desire to void and did not feel when the fluid was leaving the bladder. The catheter was used for emptying (when opened) and storing (when closed) the urine. Since no fluid was leaving the bladder through the sphincters, the external striated sphincter was spastic (continuous contraction) and the internal sphincter (smooth muscle), as a part of the detrusor, was probably not working.
Physiologically, the internal sphincter (smooth muscle, slowly acting, probably a part of the detrusor) is keeping the continence for low and medium bladder pressure. For high bladder pressure and sudden bladder pressure increases (as for example during coughing), the rather fast-external sphincter (innervated by α2-motoneurons and consisting of fast fatigue resistant muscle fibers (FR), Figure 12) is contracting to secure continence. If the striated external sphincter does not work properly, patients suffer the so called ‘stress incontinence’.
- Seven to eight months after the accident (end of 2005), the fluid was leaving the bladder by itself after a small storage phase. This means that therapy had reduced the spasticity of the external sphincter. The patient was now incontinent. So far, the spastic external sphincter had mainly stopped the fluid from leaving the bladder by its spasticity. The internal sphincter started to work a bit to allow a small storage phase. When the fluid was leaving the bladder, there was first no feeling of fluid movement. Later on, the patient had some feeling of fluid movement. Probably flow receptor afferents (S2) (Figure 33) started to work. Three months later the suprapubic catheter was removed. The patient started to use diapers.
- 20 months after the accident (beginning of 2007, upon 18 months of therapy) the patient felt bladder fullness and the desire to void. Probably stretch (S1) and tension receptor connections (ST) (Figure 33) started to work again. The vegetative symptoms of bladder fullness information (sweating and sudden heard rate increases, probably transmitted by plexus connections) were replaced by bladder fullness feeling and the desire to void.
- The patient became able to press the fluid out of the bladder. To get all fluid out, the reflex bladder had to be activated a bit, by tapping, touching or massaging the skin above the urinary bladder, which is the reflex skin area for the bladder reflex (Zones of Head). Sometimes body positioning was used to influence the bladder reflex. With these maneuvers the desire to void reappeared and the patient could empty the bladder further.
Often patients (to whom no CDT is administered) are training the bladder reflex for emptying. The reflex is stimulated by tapping the skin above the bladder. But if, for example, the external sphincter is spastic (as in this patient), it may not be possible to generate a good functioning reflex bladder. The neural networks of the sacral micturition center are working too pathologic.
- After the appearance of the desire to void, the patient became able to hold the fluid for 30s till 1min. Sometimes she could keep the continence better and sometimes not so good. This means that the external bladder sphincter (which can be controlled volitionally) started slowly to work, but irregularly. The feeling of bladder emptying became similar to those before the SCI.
- 24 months after the accident (spring 2007), the bladder started to function rather physiologically again. After a storage phase the fluid came out on volition. The detrusor started to work fully. But if the patient was pressing too much at the end of bladder emptying to get all fluid out (to reduce the residual urine, not to get bladder infections), the external sphincter contracted. The external sphincter co-contracted with the detrusor. Detrusor-sphincteric dyssynergia of the urinary bladder occurred (Figure 38E). But when she then activated the reflex bladder by tapping or touching the reflex bladder area, the desire to void reappeared and she could empty the bladder fully. The residual urine was not measured.
At this stage of bladder repair two patterns existed: the synergy of the bladder, in which the detrusor and external sphincter contracted antagonistically, and the dyssynergia of the bladder, in which the detrusor and the external sphincter co-contracted (Figure 38E). The synergy pattern was for emptying the bladder and the dyssynergia pattern was the pathologic pattern. The pathologic co-contraction of the external sphincter with the detrusor occurred more easily when there was less fluid in the bladder and the patient had to press more (inducing stress to the CNS).
Physiologically both bladder emptying patterns do exist. The synergy pattern is for emptying the bladder and the dyssynergia function is for stopping the micturition. But both patterns are under volitional control.
- Upon two years of CDT (26 months after the accident) the patient was full continent again and could empty the bladder on volition. The time interval from the first feeling of the desire to void to the situation that the fluid was coming out by itself (including 4 times of occurrence of the desire to void) was one hour. The patient did not use diapers any more. The patient had never used drugs which are supposed to improve urinary bladder functions. The repair of urinary bladder functions was achieved by the re-learning of urinary bladder functions including transfer of learning from the movements jumping on springboard, treadmill walking (Figure 37EF) and exercising on the special CDT device. The strong improvement of urinary bladder functions occurred, when the coordination dynamics values strongly increased, indicating a bit of regeneration.
For patients with incomplete spinal cord injuries, it is very important how long they can hold the urine from the first desire to void till to the moment when the fluid comes spontaneously. Can they safely reach the WC or not? The improvement of bladder functioning can also be judged by how long the patient can hold the fluid following the first desire to void. In this case it was 1 hour after 2 years of CDT.
The feeling of the lower abdomen, which was poor after the accident, improved also strongly at the time of nearly full bladder repair. The patient felt again the lower abdomen very good (inside and outside as the patient reported) and felt also again the working of the abdominal muscles. At that time, also the finger functions got a tiny bit better and her supported treadmill walking improved (Figure 37F). During walking on treadmill, and during other movements, the patient got goose-pimples all over the body. It seems that an overall improvement of vegetative and somatic functions occurred at the time of full bladder repair.
Attractor layout changes during urinary bladder repair
Within the framework of System Theory of Pattern Formation, the repair of the urinary bladder functions can be understood and pictured by the changes in the attractor layout.
One month after the injury, when the spinal shock faded away (Figure 38A, only the pathologic bladder pattern ‘spasticity of the external sphincter’ was present (Figure 38B). Six to 10 months later, the spasticity of the external sphincter reduced and a small storage phase of the bladder re-appeared as a first sign of bladder repair (Figure 38C). 20 months after the operation, the reflex bladder pattern organized itself with bladder fullness feeling and the desire to void (Figure 38D). 24 months after the accident, the attractor layout showed two attractors, the bladder synergy (the detrusor action inhibits the external sphincter) and the dyssynergia (co-contraction of detrusor and external sphincter) (Figure 38E). The state of the system (pictured by the ball) switched easily from the attractor synergy to the attractor dyssynergia. 26 months after the accident, the stability of the attractor synergy had increased and the stability of the attractor dyssynergia had decreased (Figure 38F). On volition (intention), the micturition could be induced and stopped as in healthy individuals.
Conclusion
Upon 2.5 years of CDT urinary bladder functions could be cured in severe (95%) cervical SCI. Since the injury was motoric complete and the cord was ‘free’ in the spinal canal (Figure 37BC, the cord does not touch the vertebrae), some regeneration in the human spinal cord should have occurred.
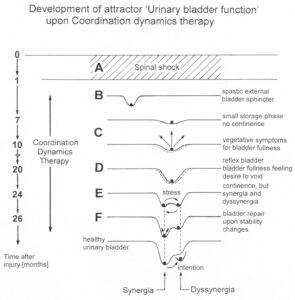
Figure 38: Evolution of the attractor layout of bladder functioning induced by learning transfer from movements to bladder functions upon CDT. The region around each local minimum of the potential landscape acts like a well that weekly traps the system into a coordinated state. Black balls correspond to stable minima of the potential. With learning, the pattern ‘spasticity of the external bladder sphincter’ vanishes and the patterns for bladder functioning (‘synergy’ and dyssynergia’) appear anew and gain their physiologic stability (physiologic deepness of each basin of attraction). The corresponding attractor layout for physiologic bladder functioning is given. Fluctuation of pattern state (the black ball) (C), and their decrease (F), due to the impairment of phase and frequency coordination of neuron firing, is pictured in ‘C’ and ‘F’ by long and short arrows. Dotted and dashed lines indicate the re-occurrence of bladder sensation. Note that more than two years of optimal continuous CDT were needed for bladder repair.
Integrative functions and central pattern generators for urinary bladder repair
It was shown in this patient Kadri with a 95% SCI that urinary bladder functions could be cured upon 2.5 years of CDT. Three important steps were achieved. First, the patient got the bladder under volitional control again. Second, a physiologic attractor layout for bladder patterns could be generated; and third, the dyssynergia of the urinary bladder could be cured by increasing the stability of the synergy pattern and decreasing the stability of the dyssynergia pattern. The stability changes of the two bladder functioning patterns can be understood within the framework of system theory of pattern formation and human neurophysiology [1-3] but not in the framework of central pattern generators (CPG’s). This knowledge is used below for the bladder repair of the 9-year-old Nefeli with an SCI of approximately 70% at the level of Th10. In Nefeli a transient regeneration of the spinal cord could be achieved and quantified by segment-indicating muscles (Figure 40).
Bladder repair in Spinal cord injury – case Nefeli
Summary of the repair of a 70% spinal cord injury at the level of Th10 (9-year-old girl Nefeli)
In the 5.5-years-old Nefeli a neuroblastoma was found to grow from the Th10 ganglion. With the surgery to remove the cancer she suffered a SCI at Th10/11 levels by medical malpractice. An 8-months-rehabilitation in Switzerland brought only little progress. Most of the repair was probably due to spontaneous recovery. When Nefeli started school, she was incontinent and could not walk. An assistance helped her to manage at school (Figure 1B).
At an age of 9 Nefeli started CDT with the Author. Spasmolytic drug and urinary bladder medications were stopped. Following four weeks of aggressive CDT, supported walking and jumping improved. The crawling became better. Following three years of CDT, Nefeli became mainly continent and she learned to walk (Figure 1C, D) with some balance problems. She learned to creep, crawl and jump. Even a bit of running became possible. At an age of 12.5 years, following 3.5 years of CDT, Nefeli learned to ride a normal two-wheel bicycle (Figure 39). Some details of bladder repair will be given.

Figure 39: The SCI patient Nefeli just after learning to ride a normal bicycle at an age of 12.5 years (A). She manages also to ride curves (slalom) (B). She still has problems to keep the feet on the pedals, but when she slips from pedals, she has no problems to keep the balance (C). Note that she has to concentrate very much to keep the feet on the pedals (A), seven years after the SCI injury.
Speed of regeneration of the human spinal cord in children
Figure 40 shows the segment-indicating muscles and partly cutaneous sensory distributions of the L4, L5, S1, S2/3 and S4/5 nerve roots or spinal cord segments.
Figure 40: Segment-indicating muscles of the L4, L5, S1, S2/S3 and S4/S5 spinal cord segments for measuring the level of spinal cord regeneration. A. Relation between spinal cord and vertebra segments. B. The spinal cord segment L4 indicating muscle is for example the quadriceps. The extensor halluces longus is characteristic for the L5 segment. C. The plantar muscles of the foot represent S1 to S3 spinal cord segments. D. The vesical and anal sphincters are activated from the S4/S5 spinal cord segments. The skeletal muscles of the leg are innervated by α1, α2 and α3-motoneurons, but the external bladder and anal sphincters are innervated only by α2-motoneurons.
It was measured in Nefeli at what times the different segment-indicating muscles started to work again in cephalo-caudal direction. At the second of February, Nefeli was able to lift substantially the knees and dorsal flex a bit the feet but could not activate the big toes separately or abduct the small toes. The quadriceps femoris and the tibialis anterior could therefore be activated again. A part of spinal cord regeneration had reached the L4 level (Figure 40). At the 12th of February she could activate the extensor halluces longus muscle, which activates the big toe for dorsal flexion and represents the L5 spinal segment. At the 18th of February she could first time in the morning after sleep abduct and adduct the small toes which represent the S1-S3 segments. The fastest regenerating fibers had reached the motoneurons of the S1-S3 segments for muscle activation. The external bladder and anal sphincters (S4/S5) could not be activated substantially so far. The sphincter muscles (somatic) are not innervated by fast conducting a1-motoneurons, but only by the thinner and probably slower regenerating a2-motoneurons (Figure 8).
The distance between the rootlets vL4 and vS2 (Figure 41B) is 20mm in adults. The growing of the nerve fibers from the segment vL4 to vS2 spinal segments needed 13 days. The time for building functional synapses is the same for all muscles indicating segments. But Nefeli was smaller (130cm) than adults, the cord distance is reduced therefore to 20mm • 130/180 = 14mm. The regeneration needed 13 days for the 14mm that means approximately 1mm/day. In the peripheral nervous system, the fastest regenerating fibers need 1 to 2 mm per day for regeneration [1].
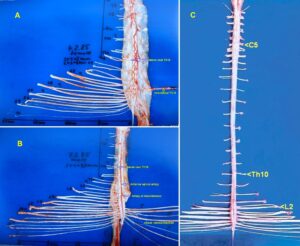
Figure 41: Human spinal cord from dorsal (A) and ventral (B, C). Intumescentia cervicalis and lumbosacralis are visible in C. The caudal ventral roots are thinner than the dorsal roots. The passage of the artery spinalis magna (Artery of Adamkiewicz) and the anterior spinal artery are indicated. The C5, Th10 and L2 roots and the intercostal nerve Th12 are indicated. Dissection by the Author.
Now the time is calculated which the nerve fibers probably needed to grow down from the injury site Th10 to the S2 segment. The cord distance from vTh10 to vS2 (Figure 41B) is approximately 90mm • 130/180 = 65mm. With 1mm/day regeneration speed we obtain 65 days for growing. Assumed that the building of functional synapses needed approximately 35 days, the regeneration down the spinal cord till to the foot muscles and the building of functional synapses needed 100 days. The administered CDT time was ten and a half months that means approximately 320 days. Therefore, before the regenerating fibers grew down the human spinal cord, 220 days were needed. That means, approximately two thirds of the regeneration time were needed to start the regeneration in the spinal cord below the injury level.
Assumed that tract fibers regenerated from above the injury site, the time needed for crossing the 2 to 10mm large gap (Figure 36 of [7]) was 200 days, which is quite a long time. In rat the longest observed growing distances are a few mm up to 20 mm. In human we expect much shorter possible growing distances, because in the PNS, in nerve sutures, the nerves have to be adapted for enabling nerve fibers grows into the distal nerve part. A substantial regeneration of tract fibers is therefore unlikely.
If we assume that relay neurons were built from stem cells at the injury site and neural networks were built in the gap from which the spinal cord regeneration started, then this neural network building process needed 200 days. But where are the neural network stem/progenitor cells are migrated from? Are some stem cells in the spinal cord or did they migrated from other places or were they transported in blood vessels?
A neural network building is likely, because Nefeli could contract the muscles only slowly. Further, the building of neural networks for spinal cord re-connection will take more time if the gap is larger. By comparing the SCI site of Nefeli (Figure 36 left of [7]) with that of the 17-year-old patient Kadri with a cervical SCI, it can be seen that Kadri’s SCI gap was much larger (longer) (Figures 37B, C) and a bit of regeneration in the cephalo-caudal direction needed three to five years.
To understand the cause of repair in humans is of importance because it may have consequences for the treatment to be administered. If neural networks are built at the SCI injury site, then the building of these networks has to be enhanced by training those movements of which the activated neurons lie in and around the injury site. To activate and stimulate neural network growing at the Th10/11 injury site, Nefeli was training with the hula hoop, trained trunk rotation on the special CDT device and trained several rotational movements of the Tiktaalik [7]. Stem/progenitor cells do only proliferate if the membranes are depolarized [43]. That means, the neurons around the injury site have to be activated by certain movements. Further, the tract fibers for all functions below the injury level have to be activated to make them growing into the neural networks at the injury site and stimulate the relay neurons to grow into degenerated tract fibers below the injury site.
The regeneration considered is very complex and only the fastest growing fibers reaching caudal spinal cord segments were measured so far. Many more fibers and thinner/slower regenerating fibers have to grow down to the caudal spinal cord and bring more motoneurons (motor units) and interneurons under supra-spinal control to generate more functional muscle power.
The improvement of the sensory innervation has not been analyzed so far. To get more feeling in the soles of the feet for better walking and balance performance, sensory fibers, entering the lumbar-sacral spinal cord, have to regenerate in the caudal-cephalon direction across the injury site.
Conclusion, even though much more knowledge about the regeneration of the human spinal cord is needed, this documented regeneration of the human spinal cord is a history progress. It is the first time, documented with sufficient diagnostic, that the human spinal cord partly regenerated by movement-based learning. Through 320 days of therapy, nerve fibers grew from the Th10 spinal segment down to the S2 segment. With a growing velocity of 1mm/day, the 65mm of the cord were covered in 65 days. Assuming 35 days for the building of functional synapses, 100 days were needed for growing caudally. This means that that 220 days of the 320 were needed to start the regeneration process at the Th10 level. The critical area of the regeneration process is located at the injury site and needs special considerations with respect to treatment.
Also, the plasticity of the brain is strongly involved in SCI repair. Whatever neurons are built anew and fibers are growing caudally or rostrally in the cord, the brain has to make the neural network changes functionally correct.
Repair of sexual functions
In the sensory-motor cortex the toe functions are sited close to genital and bladder functions (Figure 29). The repair of toe functions may help therefore with the functional repair of bladder and sexual functions. Most spinal cord injuries occur in man between 20 and 25 years and their biggest problem is the disturbed sexual function even though the repair of the bladder functions is more important. The conventional management of the sexual function in SCI is given in [51, 52]. But a causal therapy of the disturbed sexual function is the repair of the nervous system. The Author is telling the male patients with a SCI that if the urinary bladder functions are repaired by aggressive CDT, then also the caudal sexual function will improve because both functions are located in the S2 to S5 segments (Figure 20) and there is learning transfer from movements to vegetative functions including sexual functions. Also, in the sensory-motor cortex (Figures 29) the sexual and bladder functions are close to leg and foot functions, making a learning transfer from foot function repair to bladder and sexual function repair easier. With the improvement of leg, foot and toe functions by movement-based learning as for example jumping on springboard in antiphase, also the adjacent neural networks, that means sexual and bladder functions will improve.
Bladder repair within 6 months
The urinary bladder of Nefeli was not functioning physiologically. She had no spared sacral tract fibers to the bladder (in German: keine sacrale Aussparung). But she had no dyssynergia of the urinary bladder (co-contraction of the detrusor muscle and the external bladder sphincter), which made it easier to improve bladder functioning. Exercising on the special CDT device and the jumping on springboard strongly activates and trains urinary bladder and bowel functions. Because of the rhythmic and dynamic load changes, exerted onto the pelvic floor during jumping, the external bladder and anal sphincters (somatic muscles), as a part of the pelvic floor, are rhythmically and dynamically activated and relaxed, and passively stretched for movement-based learning. The inner sphincter (smooth muscle) is probably trained and activated by the pressure changes in the detrusor muscle.
At school Nefeli was using diapers. When going to the WC for disabled, a helper was assisting. At home during CDT, she was not using diapers. Often, she got wet and the trousers had to be changed. The time from the urge/desire to void and reaching safely the WC improved. Figure 42 shows the improvement of urinary bladder functioning by the times she reached the WC safely and could keep the fluid. The times to hold the fluid longer improved but varied quite much. Once she was even be able to hold the fluid for 20min. Also, the emptying of the colon and rectum (defecation) became more regular.
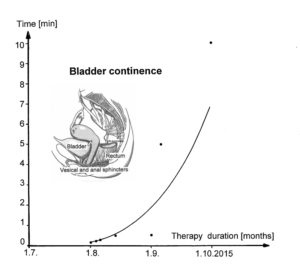
Figure 42: Improvement of urinary bladder functioning quantified by the time from the urge/desire to void until micturition.
When training regularly, Nefeli had no dyssynergia of the bladder. That means, when the detrusor (smooth muscle) contracted and the inner sphincter (smooth muscles) opened, the external sphincter (striated (somatic) muscle) relaxed and the fluid could leave the bladder via the urethra (Figure 33). But when she was performing the program unregularly (not according to the program), it happened sometimes that she got very spastic in the legs and pelvis. Then also the external bladder sphincter got spastic and she could not void even though she had the desire to void. By exercising a bit on the special CDT device and jumping on springboard, the external bladder sphincter relaxed and she could empty the bladder physiologically again.
This shows that even though the bladder functioning improved within 6 months, still a physiologic storage and emptying was not established in the long-term memory. The repairs of the vegetative nervous system and especially those activating bladder functions (in coordination with the somatic nervous system division) need time. SCI treatment for children should be able to repair the urinary bladder so that they have a private sphere in five or ten years.
Repair of rectum und colon functions within 12 months
Emptying the rectum is similar to emptying the urinary bladder [1]. Filling of the rectum activates stretch, tension, mucosal and under certain circumstances pain receptors in the rectal wall (and may be some skin receptors around the anus), which transmit impulse patterns by way of the inferior hypogastric plexus and sacral roots to segments S2 through S5 of the sacral spinal cord, where probably a defecation center is located in similarity to the micturition center in the sacral spinal cord. The sacral defecation center communicates probably with a pontine defecation center in the reticular formation and the cerebral cortex.
Rectal peristalsis is induced by parasympathetic activation from the defecation center, which also induces relaxation of the internal sphincter in similarity to micturition. The sympathetic nervous system inhibits peristalsis. The external sphincter consists of striated muscle fibers of FR type, innervated by α2-motoneurons (Figures 8, 12), and is under volitional control. The fast fatigue resistant muscle fibers secure that the sphincter can be contracted quickly and the closure can be hold for longer times. Rectal emptying is mainly accomplished voluntary by abdominal pressing.
The stretch, tension and mucosal receptors of the rectum are similar to those of the bladder and their activity was recorded when stretching the external anal sphincter by pulling the anal catheter and changing the catheter thickness from thin to thick (Figure 7B).
The Author had given research emphasis to the repair of the urinary bladder functions because of the bladder infections and the risk of ascending infections. Kidneys cannot be transplanted to those patients because of the necessary suppression of the immune system after transplantation. It was difficult to administer movement therapy to Nefeli when she had pain. Pain is a general problem in SCI. Even though CDT inhibits most likely cancer growing [22, 23], the differential diagnoses of pain include besides infections of kidneys and ovaries the growing of metastasis in the bowel.
Transection of the spinal cord above the lumbosacral center for defecation leads to fecal retention. Overfilling of hollow viscus is perceived as pain. In incomplete SCI, the pain can be felt. There are SCI patients which are afraid of a repair of the sensitivity because of the possibility of getting untreatable pain.
Since the middle and left abdomen was sensitive to touch and pain, Nefeli could feel the pain in the Heads zones for bladder and rectum/colon. Nefeli felt pain in the Heads zone for the bladder with bladder/urethra problems and she nearly every day felt pain in the Heads zone for the rectum/colon in the morning and after eating. When exercising for approximately 30min on the special CDT device the pain mostly waned. Her bowel/colon/rectum pain most likely occurred due to the impaired peristalsis, caused by the impaired function of the parasympathetic nervous system, due to the SCI. The exercising on the special CDT device activated the peristalsis of the bowel by activating the parasympathetic division and the pain disappeared. The exercising on the special CDT device therefore does not only activate in the short-term memory the bladder for micturition but also the colon/rectum for defecation.
Improvement of urinary bladder functioning within 3 years
The vegetative functions of Nefeli improved further. After three years of CDT, she had no bowel pain anymore, the urinary bladder infections did not occur anymore and the storage volume of the bladder had increased. When CDT was started, she had a urinary bladder storage volume of 50ml, then 70ml and after three years of CDT the storage volume had reached 100ml. Even once a storage volume of 140ml was measured. Her normal healthy urinary bladder storage volume would be around 200ml. It seems therefore that her sacral and pontine micturition centers were still not fully repaired. Nefeli could mostly keep the fluid from the first desire to void to the second desire, which increased the storage volume. Still the detrusor seemed not to have the full power to empty the bladder, because once Nefeli could empty the bladder with 70ml (only partly) and two minutes later again with 70ml (fully).
It will be shown in Figures 73 and 74 (Chapter 2, below) how the detrusor is activated in the rather healthy case, in the brain-dead human and following SCI (Para 9). Nefeli’s bladder functioning was probably somehow between both cases. The detrusor activation was probably still a bit undulating (working unregularly), that means the detrusor was not getting the full-strength activation from the micturition centers and could not fully relax between the voiding’s. More CDT is needed for a further repair of the micturition centers.
Concluding remarks with respect to animal research
The regeneration/repair in Nefeli [7] emphasizes the importance of continuous treatment on the long-term, especially in children during development. It would be interesting to see how animal researchers could simulate such repair over several years in rat or mice. In the patient Nefeli, the administered treatment lasted so far 3.5 years, in the former coma patient Manolis 7 years [21] and in brain injury patient Benjamin over 20 years. It needs to be known what repair can be achieved in what time period with an efficient therapy applied aggressively and continuously.
Kidney repair in medulla oblongata injury – case Rafaela
Summary: Incomplete cervical spinal cord and medulla oblongata injury repair
The 8-year-old Rafaela suffered a spinal cord and brain stem injury from the thoracic segments Th1/2 up to the medulla oblongata in a trampoline accident. Due to edema and/or lack of blood supply, she lost consciousness and the breathing arrested. In spite of medical malpractice, she survived. One year after the accident she was treated in a rehabilitation center in Switzerland for four months with no or little progress. The time period of spontaneous recovery from an incomplete spinal cord injury is approximately one year. When Rafaela was sent to the Swiss rehabilitation center, the spontaneous recovery was already over and could not be ‘sold’ as rehabilitation success.
When coordination dynamics therapy (CDT) was started, five years after the accident, she could just exercise by herself on the special CDT device in the recumbent position in which help muscles can be used. She could walk one or two steps alone before losing balance. She could not exercise in the sitting position. She was still incontinent. She could speak but quietly. But she could write and use the iPhone with the right hand.
After three months of CDT, she could stand up from the sofa by herself with a few trials and could exercise on the special CDT device in the sitting position (Figure 43). The continence had improved substantially, including kidney function. The functions of trunk, arms and hands and the positioning of the shoulder had improved. She could perform sky-waking in the standing position. Her mental state got much better as could be judged from the expression of her face. She could laugh again (Figure 43D).

Figure 43: Motor function improvement through CDT within 3 months of the patient Rafaela following cervical spinal cord and medulla oblongata injury when jumping on a trampoline.
Case report till CDT was started five years after the accident
When jumping on a trampoline, the eight-year-old Rafaela probably fell on the back and hit with her neck the metal support surrounding. She got up and went to toilette and vomited. When coming back she felt pain in the back and got dizzy. Her sister put her on a chair. The last what Rafaela said before losing consciousness was that she could not move the arms and hands any more. What really happened during the accident is unclear because Rafaela was alone and lost the memory around the accident (retrograde amnesia). After losing consciousness she was brought to a regional hospital. Four hours later she was sent to a university clinic. Her breathing became unregularly and she was connected to a respirator. In the university clinic she was put to bed and the stuff waited and waited for her recovery from the coma! Being in coma for two days without recovery an MRI (Figure 44A) was made. As can be seen from the MRI, Rafaela had suffered in the meantime an incomplete injury of the spinal cord extending from the thoracic segments Th1/2 rostral till to the medulla oblongata. All the cervical segments were damaged probably by pressure, caused by the edema and/or lack of blood supply caused by the pressure. When comparing this first MRI (Figure 44A) with the one made two weeks later (Figure 44B), it seems that the first MRI shows the cord with some edema.
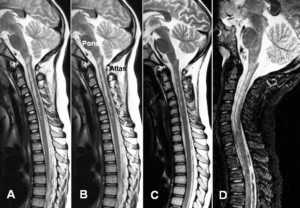
Figure 44: MRI’s made two days (A), two weeks (B), two (C) and 6 months (D) after the accident.
Comparing the first two MRI’s with an anatomical picture of the medulla oblongata and a part of the MRI (Figure 45), it seems that the injury included also a damage of the caudal part of the reticular formation and the nuclei of the vagus (X) and accessory nerve (XI). The caudal reticular formation is the nuclear area for inspiration and expiration. This conclusion is supported by the clinics. Rafaela, being in the coma, had problems with breathing and was connected to a respirator. Five years later, Rafaela was operated because she had stones in the kidneys and a big stone in the bladder, indicating that, most likely, also the vagus nerve/nucleus was damaged, because the vagus nerve regulates the secretion of the kidneys. From Figure 46 it can be seen that the shoulders of Rafaela are too much curved forwardly. Obviously, the accessory nerve/nucleus, innervating the sternocleidomastoid and the trapezius muscles (Figure 52), were also damaged.
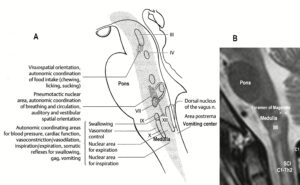
Figure 45: Injury of the medulla (MI) and the spinal cord (SCI, C1-Th2) of the eight-year-old patient Rafaela. By comparing the MRI (B) with an anatomical picture (A) and a clinical picture, it turned out that at least the nuclear area for inspiration/expiration, the nuclei of the vagus nerve (X), the phrenic nerves (C3-5) and the accessory nerve (XI, C2-4) were injured.
When the coma regressed in Rafaela, 10 days after the accident, she had no motor functions below the injury level. The physicians told the mother that Rafaela will stay like this for the rest of her life, misunderstanding that Rafaela was now in the phase of a spinal shock following the SCI. When after approximately three weeks the spinal shock regressed, some functions returned in her body spontaneously. Two months later the respirator was not needed any more. As the MRI of two months after the accident shows (Figure 44C), the breathing center of the reticular formation seemed to have partly repaired itself spontaneously. When in a dog the breathing center was damaged, it repaired itself within a few hours [53, 54].
After 3.5 months Rafaela was sent home. She could not walk, had little power in the arms (Figure 46), was incontinent and could not speak. Her higher mental functions were not impaired, even though a small hypoxic brain injury cannot be excluded. One year after the accident she was treated in a rehabilitation center in Switzerland, where the Author was previously the head of the research department (Figure 47), with no or little progress. The time period of spontaneous recovery from an incomplete spinal cord injury is approximately one year. When Rafaela was sent to the Swiss rehabilitation center, the spontaneous recovery period was already over and no progress was achieved with the inefficient treatment there. The mother of Rafaela was not informed about CDT, even though plenty of theoretical and practical medical research had been published (Figure 47, [55]) in the time period, when the Author was the head of the research department of that Swiss rehabilitation center.
Based on the thinking and knowledge that it is impossible to partly repair the human CNS, an orthopedic surgeon from USA made some muscle transpositions (indicated in Figure 53) in Rafaela to improve the remained arm and leg functions. Such operations complicate CNS repair by movement-based learning and are in most cases contraindicated.

Figure 46: The patient Rafaela (with mother and sister) with an incomplete spinal cord injury extending from the thoracic Th1/2 segments rostrally to the medulla oblongata (including all cervical segments). Note the bending of the shoulder and the hanging of the arms, due to the injury. The expression of her face characterizes her poor psychological state.

Figure 47: An important publication during the time period when the Author (GS) was the head of the research department of that Swiss rehabilitation center. This publication connects theory with praxis (31 case reports).
When Rafaela came for consultation to the Author five years after the accident, informed by the father of the patient Nefeli about CDT, she was psychologically in a very poor condition as can be seen from the expression of her face (Figure 46). She could not walk and was afraid of trying out the special CDT device. She could not persuade to try out the sky-walker. She could stand when leaning against the wall (Figure 48A) and she was able to write with the right hand and she liked it as can be seen from her face (Figure 48B). With the mother she managed physically at school and she learned well. But school knowledge is not helping much in later life, if she is fully dependent on her mother. Independence is the first big repair goal to be achieved. The mother tried to exercise her (passively) on the special CDT device in the sitting position which was not very successful but helped a bit. When Rafaela got her own sponsored special CDT device for training in the lying and sitting position, it turned out that in the lying position, when help muscles can be used for exercising, Rafaela could exercise by herself (Figure 49) and her self-confidence improved strongly and she wanted now to fight for a better future. This was the beginning of CDT.

Figure 48: When leaning against the wall the patient Rafaela could stand (A). When writing and sitting in a wheelchair (B), the patient looked happy. In the right hand was some power, sufficient for writing and using a mobile phone and the left hand was spastic like in stroke patients.
From the point of view of injury severance, Rafaela had a good prognosis. She could become much better with respect to motor functions and she was good at school. The question remains whether optimal CDT can be administered to her on the long-term. A sponsor argued that Rafaela got worse in the last years when conventional therapy was administered to her.
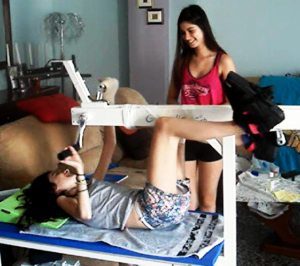
Figure 49: With the left hand fixed, the patient Rafaela with an incomplete spinal cord injury from the level Th2 rostral to the medulla could exercise without support. This was the first coordinated arm and leg movement she could perform by herself. It improved her psychological state.
Anatomy: Structural damage caused by the edema following the accident
According to the MRIs of Figure 44A, B, Rafaela suffered an injury of the spinal cord and the caudal brain stem. The injury extended from the thoracic spinal cord segments Th1/Th2 rostral through all cervical segments to the caudal part of the medulla oblongata. A detailed analysis of Rafaela’s spinal cord and medulla oblongata injury (Figure 50) is important if one wants to understand why certain functions are lost or impaired, to adapt the therapy to the injury and to learn what structures can be repaired by movement-based learning.
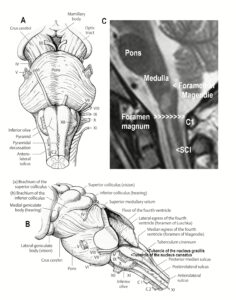
Figure 50: Ventral (A) and lateral (B) view of the medulla. The MRI (C) shows that the spinal cord injury (SCI) extends into the medulla, where many important functions are located. The vagus (X), the accessory (XI) and the cervical spinal nerves or their nuclei were damaged.
The brain stem is the phylogenetic oldest portion of the brain with its repair mechanisms and is subdivided into midbrain (mesencephalon), pons and medulla (oblongata). The caudal part of the medulla was damaged probably by the pressure caused by the edema around the foramen magnum. The foramen magnum limited the space around the medulla and spinal cord when the edema was building up and pressure was exerted in similarity to the occurrence of brain pressure during bleeding and edema in the brain. The probably blocked or reduced blood supply had secondary caused a hypoxic spinal cord and medulla injury. Since nerve cell bodies are more sensitive to hypoxia and pressure than nerve fibers, more injury has to be expected from nerve cell damage.
Since the brain stem is practically “packed densely” with neurons, which contains the essential autonomic regulatory centers as cardiac activity, circulation and respiration, even a small injury can produce neurological deficits of different types.
The cranial and spinal nerve deficits can be classified as supra-nuclear, nuclear and fascicular. The damage in Rafaela will be mainly nuclear and indeed she had no deficits in sensory functions, because the nerve cell somas lie in the ganglia outside the CNS and afferent fibers did not become damaged or only little on their way to the thalamus. The rostral intercostal nerves supply larger skin areas of the ventral body (Figure 51A) than is given schematically in Figure 51B. The spinal cord injury level is therefore sometimes misjudged. The caudal intercostal nerves, on the other hand, follow more the segmental trunk innervation.
Figure 51: A. Rostral intercostal nerves. Note that the anterior pectoral cutaneous branches and the medial mammary branches lead quite much caudally, especially of the intercostal nerves Th II and Th III, and can give rise to a misjudgment of the SCI level. B. Segmental innervation.
The breathing of Rafaela was impaired at the breathing center (mainly inspiration), at the intercostal nerve and at the phrenic nerve levels. Looking at the MRI’s of Figure 44 and Figure 45, it can be seen that the caudal part of the breathing center was damaged. When disconnected from the respirator, she breathed only once and stopped then breathing. The rhythmicity of breathing was lost. But also, the motoneuron somas of the first two intercostal nerves were damaged according to the MRI’s. Looking at her breathing movements five years after the accident, it could be seen that the first two intercostal nerves were not fully working, because the ribs were not moving properly. Further, the phrenic nerves, activating the diaphragm, were probably also not working fully, because they arise from the damaged cervical cord segments C3 to C6, which were damaged. Looking at her breathing, it was not normal and also not fully a paroxysmal breathing. Paroxysmal breathing is the breathing with only the diaphragm, typical for patients with a cervical spinal cord injury at the level C5/6. The thorax (rib cage) is getting smaller instead of increasing with inspiration and the stomach is coming up. Lucky that Rafaela could survive with this complicated injury and could breathe sufficiently by herself again after two months, when the caudal breathing center became repaired partly spontaneously. The rhythmic breathing had returned. The rhythmic firing of the network oscillator had repaired itself, probably stimulated by the rhythmic ventilation of the respirator.
After the injury, Rafaela could not speak. When CDT was started five years after the accident, she could speak but only quietly. The power for loudly speaking was absent.
Because the nucleus of the vagus nerve was damaged (Figure 45), she got repeatedly stones in the kidneys. The motoneurons of the accessory nerve, arising from the C2 to C4 segments (Figure 52), were also damaged because the trapezius muscle was activated too little (Figure 46). The functions of the glossopharyngeal nerve seemed not to be impaired.
Figure 52: Comparison between a spinal cord/medulla injury, quantified by an MRI (B), and a picture of the anatomy of the spinal cord and the medulla (A). The trapezius and the sternocleidomastoid muscles are innervated by the accessory nerve (A). The somas of the motoneurons of the accessory nerve are damaged (B). Also, the nucleus ambiguous (X) was probably damaged (A). The dorsal nucleus of the vagus nerve (X) (A) was probably not damaged because it is located more dorsally in the medulla.
The main functions to be improved were therefore walking, arm and hand movements, breathing and the autonomic functions as for example the kidney and full urinary bladder functions. Kidney functions and breathing are essential for life. With shallow breathing a lung infection is at risk.
Coordination Dynamics Therapy (CDT) repairs by coordinated arm, leg and trunk movement’s not only somatic functions, but also by learning transfers (see System Theory of Pattern Formation) the autonomic functions. If damaged CNS parts or nerves are included more directly in the movement-based learning, then most likely, the efficacy of repair is higher. In this patient the trapezius and sternocleidomastoid muscles were trained more directly when the patient performed strong rotational movements on the special CDT device (Figure 53).
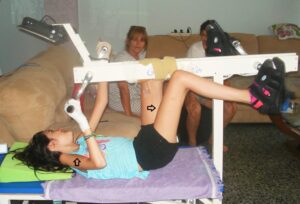
Figure 53: Patient with cervical spinal cord and medulla injury during the training of rotational movements. In the photo the left shoulder is lifted. Note the scar of the unnecessary orthopedic operations (marked with arrow at the left leg). The with little power functioning right hand is also fixed to make longer periods of training possible.
But how can the vagus nerve have trained more directly? The urinary bladder can be trained more directly when jumping on springboard. But in this way the sacral parasympathetic nervous system (Figure 20) is mainly stimulated and not the cranial portion. One is left, for the time being, with the exercising on the special CDT device, which activates the whole parasympathetic division.
The breathing center of the brain stem can be repaired more directly when pushing the patient to her limits by increasing the load (Newton), when more deep breathing becomes necessary. The hematocrit will increase then (good before an operation for not needing blood from other persons), but also the breathing center in the reticular formation is forced to improve its efficacy.
The rotational trunk movements, when exercising on the special CDT device, were used in the SCI patient Nefeli (above) to repair the caudal parts of the trunk via the rostral part. In Rafaela it was tried to repair the upper part of the trunk by inducing the trunk movement from the lower part. The rotational movements should best be induced volitionally. Such rotational movements are forced by having the handles in a high position (Figure 53).
Probable cause of the spinal cord and medulla oblongata damage
By comparing the spinal cord damage, shown in the MRI (Figure 54B) made two days after the accident (Figure 44A), with the arterial network of the spinal cord (Figure 54A), it is likely that the cord damage was due to ischemia caused by lack of blood supply through the anterior spinal artery and may be the vertebral arteries (Figure 55). The damage of the cross-sectional area in the anterior-center area (Figure 54B) fits the supply territory of the anterior spinal artery (Figure 54A). Since the cord damage extended caudally till to the Th2 level, it is likely that the great radicular artery of Adamkiewicz (Figure 41B) and the posterior intercostal artery of T4-T6 were supplying the spinal cord (Figure 41B, C), but their blood supply could not reach the cervical spinal cord.
The arterial network of the spinal cord and nerve root feeder arteries can partly be seen in Figure 41. There is quite a variation in the blood supply of the spinal cord. Why the posterolateral spinal arteries (Figure 41A) could feed the spinal cord and the anterior spinal artery could not is unclear. May by it has something to do with dentate ligaments, which keep the cord in the middle of the spinal canal to surround it by cerebrospinal fluid for safety. Only proper diagnostic at the time, shortly after the injury, would have told us what pathologic processes really took place, but the diagnostic was not done. The posterolateral spinal arteries are pictured in Figure 54A in a simplified way. As real preparations show (Figure 41), there is quite a variation also in the location of the posterolateral arteries.
In the picture of the blood supply of the spinal cord (Figure 54A) there are also spinal tracts indicated. All the tracts are given in Figure 54C. These tracts were not or only partly damaged by hypoxia. A communication of the brain with the caudal spinal cord was therefore possible what makes a repair by movement-based learning possible. But since the neural networks for arm functions are damaged in the cervical spinal cord, problems with arm and hand functions have to be expected in accordance with the clinical picture of the patient (Figure 46). The pyramidal tract was more damaged for the arm function than for the leg functions (Figure 54C). Since the neural networks of the intrinsic spinal cord apparatus was probably also damaged, especially the fasciculus proprium (dotted in Figure 54A), difficulties have to be expected to connect and coordinate arm and leg movements. In the rostral spinal cord, also the caudal part of the reticular formation lies in the territory of the anterior spinal artery (Figure 54A) and more caudal also partly the reticulospinal tract (Figure 54C). The patient had and still has substantial breathing problems.
When Rafaela tried to walk, she lost balance and fell to one side. The vestibulospinal tract could also be damaged (Figure 54C). But with such severe injury of the spinal cord and caudal brain stem there are many possibilities for loosing balance.
Figure 54: Arterial blood supply of the spinal cord (A) in relation to the spinal cord damage, measured by an MRI (B). Note that the feeder territory of the anterior spinal artery is similar to the cross-sectional spinal cord damage, indicating that the damage was caused by a lack of blood supply from the anterior spinal artery. Note further that the reticular formation and the fasciculus proprium are located in the damaged area which was probably caused by hypoxia.
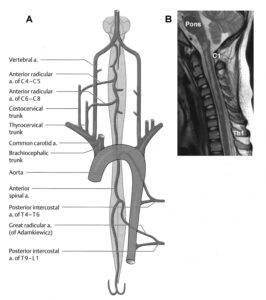
Figure 55: Contribution of segmental feeder arteries to the arterial blood supply of the spinal cord (A) in comparison to the incomplete spinal cord injury from Th1/2 to the medulla (B). The comparison indicates that the spinal cord injury was caused by a lack of blood supply through the rostral part of the anterior spinal artery.
A possible injury mechanism is that ischemia was caused around the foramen magnum. The edema caused an occlusion of the anterior spinal artery and reduced blood supply through the vertebral arteries (Figure 56). The ischemic attack probably lasted as long as the edema. When the MRI was made two days after the accident, the dead spinal cord and medulla tissue were mainly already liquefied and resorbed and the cystic cavity filled with cerebrospinal fluid. Collateral blood circulation through other pathways did not work, probable because the whole area around the foramen magnum was compressed. The MRI, made 6 months after the accident (Figure 44D), showed approximately the same injury of the spinal cord. Only the medulla seems to show repair already two months after the accident.
Figure 56: Anatomy of the arterial blood supply of the brain stem and rostral spinal cord. Already a transient inadequate perfusion of the medulla and rostral spinal cord, caused by pressure or transient infarction, will have tremendous consequences for brain stem functioning.
Medical malpractice in Rafaela
If following a head or neck accident, the patient loses consciousness, then there are dangerous pathologic processes in progress as bleeding, edema or embolic infarction.
Standard medicine would be to make an MRI, which would reveal ischemia within a few minutes. Diffusion-weighted MRI detects ischemia highly reliable anywhere in the brain. Digital subtraction angiography (DSA) (i.e., visualization of the blood vessels) provides the best morphological view of pathologic changes in the extra-cranial and intracranial vessels. Even ultrasound techniques would show that brain tissue damage is in progress. But just putting the patient in the hospital into bed and waiting that she recovers from coma is not standard medicine. “Lucky that it was recognized in the university clinic that the breathing of Rafaela arrested and that she was connected to a respirator”. Medical malpractice of such extent cannot be compensated for by the best brain repair methods, if available, and is not in accordance with the ideas of Hippocrates.
Stage of repair five years after the accident when CDT was started
When CDT was started, she could just exercise by herself on the special CDT device in the recumbent position (Figure 53), which is the first movement such a patient can do by herself. She could perform one or two steps alone before losing balance. She could not exercise in the sitting position and the coordination dynamics, the CNS functioning, could not be measured. She was still incontinent. She could speak but quietly. But she could write (Figure 48B) and use the telephone with the right hand.
Improvement of CNS functioning within 3 months of coordination dynamics therapy
After three months of CDT, Rafaela could stand up by herself from the sofa with a few trials (Figure 43B, C) and could exercise in the sitting position (Figure 57A), so that CNS functioning, by means of the coordination dynamics (CD) values, could be measured.

Figure 57: The patient Rafaela with a SCI and a brain stem injury became able to exercise on a special CDT device in the sitting position (A). When training with the Author, the hands were not fixed (B).
When comparing Rafaela’s face expression after three months of CDT (Figures 43D, 57) with those before the therapy (Figure 46), it is obvious that her mental state had improved. She laughed when she managed to exercise on the sky-walker in the standing position (Figure 43D) and also when the Author exercised with her (Figure 57B). Also, the positioning of the shoulder (trapezius muscle), the arms and hands improved a bit (Figure 43B, C) in comparison to before starting CDT (Figure 46). The continence improved substantially, but the repair was not fully achieved. She still had only a few minutes from the first desire to void to the spontaneous micturition.
As long as the Author supervised the therapy, no stones occurred in kidney and bladder. Therefore, CDT repairs not only bladder functioning, but it improved also kidney functions. Since movement-based learning improves cardio-vascular performance and the immune system functioning (building of NK cells [24]), CDT protects and helps the bladder-kidney system.
Rafaela became able to manage better at school. She has a good prognosis for further CNS repair if her therapy is continued aggressively, because her severe CNS injury was balanced, that means all regulatory functions worked a bit.
Disappointing for the Author, Rafaela and her mother were, after three months, not interested any more to be supervised by the Author. Not all patients and their families have sufficient mental discipline to fight with 20 hours therapy per week for a better future.
Continence repair in brain injury/malformation
Continence repair in cerebral palsy (brain malformation)
In traumatic brain injury, apart from extreme severe ones, the continence is not disturbed. In cerebral palsy, on the other hand, the continence is mostly impaired. But it needs mostly only a few months of CDT to be repaired. And even these patients appreciate it very much to become continent at day and night.
The girl Sophie had from birth an atrophy of the cerebellum and ponds. At an age of 5.5 Coordination Dynamics Therapy (CDT) was started. At the beginning, Sophie could not perform any movement accurately (Figure 1E). She was incontinent and her speech was very poor. She could not walk, run or jump. Her protection automatism patterns, when falling, were not working. The first fundamental progress in repair was achieved when the protection automatisms appeared in Sophie at an age of 6.5 years. The parents were not so much afraid anymore when trying to make her walking and Sophie tried now to walk by herself. The protection automatisms became operational at a time when she became able to exercise by herself on a special CDT device. When the protection automatism occurred, the learn to walk became much easier and less risky. Sophie was not afraid anymore to fall and she tried herself to walk without falling and she liked it. At an age of 8 she was able to walk without falling. Sophie’s trot gait crawling improved. The pace gait crawling became possible with poor performance. At an age of 9, Sophie could creep, crawl, upright, walk (Figure 1G), jump and play with the ball. She was fully continent. Her speech had improved, so that the Author started to understand her. Her writing got better and her higher mental functions improved. She had still problems with the balance, but she managed without falling. She was not able to run. The mother, teacher herself, taught her at home. Through 5 years of CDT, at an age of 11 years, Sophie could fully manage the balance and she became able run (Figure 1H). But the performance of walking and running was not fully normal, but she became able to move independently.
Figure 58 shows the now 13-year-old Sophie during training on treadmill to reduce the stride length during walking. Due to the cerebellum atrophy, she was still overswinging the legs.

Figure 58: Sophie during training on treadmill to reduce the stride length during walking. Due to the cerebellum atrophy, she was overswinging the legs. In A she tries to coordinate her walking pattern with that of her young cousin in front of her. In B an older cousin tries through backward walking to improve the interpersonal coordination to draw Sophie more strongly in a more physiologic walking pattern. The mother also helps to improve Sophies walking pattern through interpersonal coordination. The Sophie was able to walk and run freely.
Continence repair in severe brain injury
In brain injury, bladder functions are normally not impaired, but in very severe they are. In the patient Benjamin (Figure 88), who suffered a very severe brain injury, the continence was repaired within three months of CDT. The motor and cognitive functions were not fully repaired within 20 years of CDT. As said before, among continence, motor and cognitive functions, the continence is easiest function to repair.
Discussion
Limitations of basic science research
There are many inadequacies in the current research in spinal cord injury (SCI), brain injury and brain malformation. Animal research is mainly concerned with structural repair and the regeneration of the spinal cord in animals. The aspect of functional recovery is rarely understood and never analyzed. Even though structural repair is important in SCI, the researchers rarely discuss the difference in the potential of regeneration between animals and humans. In the goldfish, for example, the spinal cord regenerates spontaneously. But what inferences can be derived from this regeneration that can be relevant and applicable to humans? Presented gross human anatomy may make a casual reader believe that the animal data are indeed applicable to humans. However, the functional recovery is not measured thoroughly. Morphological data at the cellular level, like the degree and extent of growth and regeneration of axons across the injury site, is often not correlated with the functional recovery. The functioning of newly grown axon has to be proven in the context of neuronal network functions and not only by the improved movements of the treated animals, because in animals there exists also spontaneous recovery and functional reorganization, especially if only 50% of the cord was destroyed. Actually, fiber counts in different ascending and descending tracts would be needed to assess what percentage of tract fibers needs to be regenerated for meaningful recovery in humans.
Need for scientific and clinical human approach
The diagnostics at the beginning, during, and at the end of therapy is of particular importance to differentiate between spontaneous recovery and the improvement achieved by the treatment. Since spontaneous recovery may occur for up to 1 year after the injury, long-term studies are needed. In the case of a partial SCI, most of the recovery occurs between 2 to 6 months after injury and to a lesser degree up to 1 year. In the patient Nefeli, the treatment was started 4 years after the injury and lasted for a few years; thus, the recovery cannot be attributed to spontaneous recovery.
In the case of complete SCI (as assessed by MRI), only very limited spontaneous recovery occurs and the potential for functional reorganization is very low, since most of the distal cord is disconnected from the supraspinal control. In such an injury even the combination treatment of stem cell therapy and CDT did not bring about significant improvement. However, most of the spinal cord injuries are incomplete. Even in the case of a severe injury, a significant part can be salvaged by immediate removal of compression of the spinal cord by surgery, what was not done in Nefeli and Rafaela and the stabilization of the spine to minimize progressive mechanical injury.
There are many unscientific reports, claiming miraculous recovery in SCI patients, which could very well be attributed to a spontaneous recovery. Most of the rehabilitation centers focus mainly on care and general maintenance of patients and administer inefficient and arbitrary treatment that does not utilize the full potential of recovery in patients with partial SCI. Patients want more choices than the ‘no hope’ approach of conventional rehabilitation centers and the ‘false hope’, given by some physicians and researchers working in the field of animal research, and cell and molecular biology.
Over the last 30 years no significant conceptual or real methodical progress has occurred in the field of neuro-rehabilitation. The current science of neuro-rehabilitation is mostly outdated, as no real review of human neurophysiology has been done for many decades. The lack of any perceived need for development and in turn the absence of any efforts in that direction has perpetuated the inefficient, outdated and ineffective treatment modalities in the treatment delivery system. This is particularly relevant considering the fact that the young patients who suffered a SCI have 50 years of their remaining life span that they have to live with a very poor quality of life, while only getting care from the current rehabilitation centers. On the other hand, with optimal and intensive CDT for a few years after the injury, their quality of life can be enhanced substantially in order to achieve independence in daily activities, and most importantly physiological bladder control and probably improvement of the sexual function.
Animal experiments are removed from human reality
Recently it was reported on the spontaneous recovery of a SCI in rhesus monkeys and the important role for primate models in translational disease research [56]. A few comparisons will show that animal experiments are far away from human reality and normally cannot contribute to translational medicine or human disease research.
- In human patients, as can be seen from Figure 37D, the injury is often in the center of the spinal cord, and not just a half of the spinal cord is damaged as in the monkey experiment (Supplementary Figure 2 of [56]). Neurons in the spared grey matter of monkeys may function as relay neurons. The blood supply is probably less impaired in the monkey.
- Since in 50% SCI the spinal cord can mainly be repaired upon CDT [11] and the patient can be brought back to normal everyday life (Figure 37D), there is not much sense of after-developments in monkeys, unless the experiments are designed to be close to the clinical human setting to obtain parameters which cannot be measured in humans. Is it actually justified to kill monkeys for an after-development? Not to cite existing qualified clinical literature for the repair of a 50% SCI is also not convincing, especially when claiming that their research is a contribution to translational medicine.
- The biggest problems in human SCI are the urinary bladder functions [12] and the occurring pressure ulcers due to insufficient blood supply. Before World War II a majority of SCI patient mortality was due to continuing urinary bladder infections. By cutting only one half of the spinal cord in animals one is not getting urinary bladder and skin nutrition problems. The challenge of the future is to cure the 95% or complete SCI. – The neurosurgeon L.W. Freeman (USA) stated in the 1960’s after performing very many SCI repair experiments on dogs, “it is difficult to find persons who do research in paraplegia and to get money for such research; probably World War II is already too far away”. If one cuts the whole spinal cord in animals, then one would have to manually empty the bladder by pressing the urine out. Such work seems to be unpopular among researchers. In human patients the bladder is for example emptied approximately every 5 hours by intermitted catheterization (and other ways), introduced by Guttmann from Breslau [50] in the Second World War.
- The improvement of hand function is also very important in cervical SCI; I have not seen animal experiments tackling the hand and finger problem. A ‘tetra’ (tetraplegic patient) cannot empty his urinary bladder by intermittent catheterization without sufficient hand and finger functions. Hand functions can be improved in C5/6 SCI upon building of new motoneurons in the human spinal cord, but it needs several years of optimal CDT [46].
- The repair of the fundamental functions such as breathing, swallowing, and speech in high cervical SCI has also not been tackled by animal research. Obviously, animal research is far removed from the human patient’s real problems.
- Humans need years for development and repair and not only a few months. Animal experimentalists are therefore working on the wrong time scale with respect to development and repair to translate their data to human reality. Again, it seems not to be popular among researchers to do experiments which last over several years.
- Translational medicine makes only sense if the researchers have knowledge in animal and human research. A translator can only translate from German to English if he has knowledge in both languages. Also, for peer reviewed journals in translational medicine it would be important to have reviewers which are competent in animal and human research. The competence has to be documented by publications in international journals in both fields.
- When I was for postdoc education with Sir Bernard Katz at University College London, I learned that there should be no more than 4 authors on genuine research publications. 14 authors of a publication are typical for clinical research where clinicians do not have enough time for research and putting their names as much as possible on the publications of friends and assistances to increase the number of their own publications. By avoiding real research, professors in the clinical field may have insufficient understanding of qualified research and are less open to new developments in medicine.
- In mouse experiments, half of the spinal cord is cut and treatment success is demonstrated by the staining of neurons and nerve fibers and demonstrating that the mouse can run for example on a horizontal ladder [57]. Whether the regenerated fibers were really functioning was not measured. Something like 50 years ago, there was a treatment for thoracic SCI with which patients learned to move the legs by moving the arms. The information from the upper (arms) to the lower part of the body (legs) took place most likely by skin traction. In a frog, I could not see from the morphology whether axons and synapses were functioning or not; when trying to understand regeneration, I used morphology and electrophysiology. Sir Bernard Katz, the specialist for the neuromuscular endplate, explained that sometimes denervated motor endplates survive very long and still look quite normal (as if they would really still function). In mouse treatment, success was measured by morphology and making the mouse move on a horizontal irregular ladder and arguing then that the progress is going from animal experiments to human treatment [57, 58]. In the human patient with the 50% cervical SCI, treatment progress was demonstrated by letting the patient run and jump, measuring the improvement of running and jumping, measuring the coordination pattern dynamics (CD) values for different loads and measuring motor programs and single motor unit firing (neuron level) with surface electromyography (sEMG) (Figures 17). And the scientific basis for the repair is the understanding of CNS functioning and repair at the neuron level in human [1-3]. For the time being the progress in SCI is coming from human research and not from animal research. Human data need to be translated to animal data [28]. Universities and research institutions world-wide seem to have an anxiety to touch human neurophysiology and qualified clinical research.
- In severe incomplete SCI there is the problem of muscle power generation. If not sufficient motoneurons can be activated following SCI, the body tries to compensate for by axonal sprouting to innervate the (denervated) muscle fibers of not functioning motoneurons. With such collaterals the motor units increase in size. In human the motor unit can be increased by approximately 50%. One motoneuron can, for example, supply 6000 muscle fibers when it normally innervates 4000 muscle fibers. In rat the motor unit can be increased up to 400%. Therefore, frankly speaking, a rat can walk already with a few functioning motoneurons, with poor coordination. Further, the power of regeneration is much higher in animals.
- The problem of smart-phone mania, which disturbs treatment, one is not having in rat experiments.
Consequences of false hope
In Chapter I of [2] it was shown, based on frog data that exogenous stem cell therapy in its present application is not working. The main problem seems to be that the newborn cells cannot be integrated in the existing neuronal networks for various reasons. Cell communication needs to be better understood. During competition a motocross athlete suffered a clinically complete SCI at the thoracic 11/12 levels. Six weeks after the accident the subject began intensive CDT at an up-to-date therapy center. After 6 months of therapy, when further improvements were only marginal, the patient opted for hematopoietic stem cell therapy. During two years of stem cell therapy, including 4 sessions of stem cell application, and on-going CDT, improvement remained marginal – no more than what would have been achieved with continuing only CDT. This hematopoietic stem cell therapy did not have any beneficial effect on the repair of the spinal cord in this patient. When it turned out that the costly stem cell therapy had no beneficial effect on the regeneration of his spinal cord, the patient lost the belief in SCI treatments and stopped also CDT. He only continued with some training, according to his opinion, to reduce spasticity. Another patient with a probably complete thoracic SCI was sent by a physiotherapist to the Author to get advice concerning a stem cell therapy in addition to the administered movement therapy. The patient did not consult the Author, because he wanted to have the stem cell therapy administered, whatever the arguments of the Author would have been. He even raised the argument that the Author may be against progress in SCI. Up until two years after the start of the stem cell therapy there was no real progress in repair. The patient stopped the therapy in the physiotherapy place and was doing then his own treatment at home. It seems that he had lost the belief in any treatment. During the treatment at the physiotherapy place no MRI was performed in this patient (messy treatment and diagnostic situation (Switzerland)). It was argued that this is not possible because of the metal fixation. But with a Titan fixation, which is nowadays mostly used, an MRI is often giving quite a good anatomical information about the severance of the injury of the spinal cord. This can be seen if one compares the MRI’s of a patient with Titan fixation (Figure 37C) and after its removal (Figure 37B).
Instead of raising false hope that the animal data can be easily used to cure diseases of the human CNS, the universities and research institutions should first do their duty, namely organizing qualified human neurophysiologic research, especially treatment research, and also support such research.
Ethics of SCI research, treatment, and clinical trials – false hope from animal treatment research
‘Transplantation of both embryonic stem cells and embryonic stem cell-derived neural (neural or glial) progenitors is able to efficiently promote CNS regeneration in preclinical models of stroke, myelin deficiency, acute SCI [59, 61] and Parkinson’s disease [60]’. This sentence of an article makes the reader believe that the research made already the step from the animal research to human research and treatment is already administered to humans. Looking up the references one finds the title: ‘Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury’. Patients with SCI or journalists think immediately that the SCI problem has been already solved! I have learned that it has to be written in the title on what species the research has been performed. In this case it was the rat. Animal researchers often deliberately choose the words in a way to make the reader believe that this research has already solved the SCI problem in humans. A well-known journal in medicine is even redefining medicine. It makes the reader believe that measurements in mouse are a part of medicine [62]. I have learned in my medical study that medicine has something to do with the cure of diseases in human. Specialist journals and general audience media need to set reasonable expectations of the safety and efficacy of potential therapies to avoid raising and then dashing the hopes of those living with SCI or those in government, those carrying out research, or the general public [62]. High ethical standards are required by researchers, clinicians and journalists to ensure that results are communicated to the general public in a manner that honestly reflects the safety and efficacy of a potential therapy [62].
Medicine is very successful if natural existing repair mechanisms are supported like in surgery or immunology. But if repair mechanisms do not exist, then medicine is not very successful. The repair of the nervous system is very limited and depends on the site of the injury. An exogenous stem cell therapy must be very sophisticated to be better than nature with respect to repair. The integration of new-born cells into the existing neuronal networks is by far not solved. Further, the scientific basis for doing neurobiology is out-of-date. The understanding of the functioning of the CNS in animals and humans has to be upgraded. In physics, for example, the basis is continuously upgraded. In Chapter I of [2] and [28] it was shown how complicated the innervation of two kinds of muscle cells by two kinds of motoneurons is (4-cell communication). The innervation and innervation changes during development and repair are probably much more complicated in the human CNS. It is too simple thinking to believe that an addition of cells can repair the CNS what the CNS cannot do by itself. CDT, on the other hand, rests on learning which is natural for humans. Academically accepted articles are generating false hope by allowing authors to state that stem cells can potentially be used in different CNS diseases including SCI. The authors are stating it to get funding for their animal research, by making believe that their research has direct consequences for the treatment of human patients. No wonder that practitioners also want to participate in getting money. Instead of warning that some clinics in Asia may look more for profit than for qualified stem cell therapy, the editorials should first think over their own research policies which are giving rise to such unqualified medical treatment. It is not enough to state “The medical promise of stem cells remains real, but largely unrealized for now. The excitement must not be left to dissolve into a muddle of disappointment, frustration and fear because of the practices of a few irresponsible profiteers” [4]. There are principal problems to be solved for stem cell therapy in humans. A few years ago, there was a big propaganda in the TV and other mass media in Switzerland about the suppression of grow-inhibiting properties for the regeneration [57, 58] of the spinal cord. It was stated that SCI could be cured in a year’s time. It seems to be popular among neurobiologists to raise false hope in society concerning treatment in human patients, as if they have not understood much about treatment of human patients.
If we want to repair function, we also have to measure function in a qualified way and one powerful tool is the electrophysiology, because the nervous system is mainly functioning by electric currents and potentials. It is difficult to understand why electrophysiology has mainly been destroyed world-wide, even though this tool was very successful in neurophysiology in the past. One reason could be that although the younger generations are capable of handling computers or electronic equipment very good, they do not have the manual skill any more to perform complicated electrophysiology. Statistics are needed concerning treatment success beyond the placebo effect, including the occurrences of cancer and CNS instabilities (seizure disorders). But more is needed in medical research than just statistics. Hopefully the globalization does not give rise to the coordination in thinking (in German: ‘Gleichschaltung der Denkweise’).
It is depressing for a qualified therapist that ‘there is an abundance of patients desperate for miracle cures, and one stem cell treatment can bring in tens of thousands of US dollars’ [63]. Western scientists and clinicians would argue that controls are necessary to identify unambiguously whether a therapy is safe and effective, some clinicians have claimed that withholding a potential therapy from a patient with SCI is in itself unethical [64]. Many SCI units know about CDT. But the patients are not informed that such a therapy exists! A real change from care to cure seems not to be in the interests of the neuro-rehabilitation centers. More money can be earned with care.
There is too much false hope generated by animal physiologists, neurobiologists, and researchers working in the field of genetics. Without detailed knowledge of the human physiology and pathophysiology, the animal knowledge is only of limited help to cure diseases in humans. False hope also stops the patient from fighting for improvements which are needed for everyday life. Why should a patient fight for three years or more with a movement-based learning therapy to get urinary bladder and some motor functions repaired, if in a few years’ time walking can be returned with miracle cells or pills? Such false hope is coming from qualified researchers, when they make believe that the animal data can be easily used to repair the human CNS. Qualified human research is needed and has to be organized. Only if there is an overlap between animal and human research, there is the possibility of rather safe transfer of knowledge from animals to humans. As long as human research is not organized properly, the patients have to suffer or even to die (for example Christopher Reeve). Even monkey experiments are far away from human reality. A monkey cannot speak, write or read, and will never be able to solve ‘differential equations’ (mathematics). The power of the human CNS is its learning capacity, which is outstanding among different species. A fly demonstrates with how little brain matter sophisticated fly tasks are possible. Qualified human CNS research, including at the single-neuron level, is needed for understanding the functioning and repair of its neural networks.
Authors of recent review articles presented approximately 1000 citations on the repair of the human spinal cord following injury [60, 64, 65, 66]. Less than 10% of the citations were from human research. Interestingly, the author was not cited, even though his work is widely available online. I learned a lot from these brilliant research articles with respect to animal research. But with respect to human research and applicability to human patients they were out of date by 25 years.
‘Miracle’ treatments are not the only dangerous for patients and the freedom in research. It is the worldwide research, treatment, and teaching system, that does not allow qualified human research, which is urgently needed to cure diseases and make humans live longer with a better quality of life [3, 67]. And if there are really operations with unbelievable success, then the treatment before those operations was wrong.
For example, a paraplegic patient came by wheelchair from a well-known German rehabilitation center to a neurosurgery department, was operated and walked 2 weeks later out of the neurosurgery department. A second patient was cured in the same way. The reason for this progress was caused by a mistake of the rehabilitation center. Bones from the spinal canal caused pressure on the spinal cord. The spinal cord stopped working but was damaged only little. A laminectomy freed the spinal cord and the spinal cord started to work again. Follow up MRI’s are needed which are normally not performed in rehabilitation centers. The argument is that it has no clinical consequence, because the patient is staying anyway in the wheelchair for the rest of his or her life. This argument is wrong since for example cysts can build up in the injured spinal cord which enlarges the spinal cord and pressure symptoms will occur and the patient loses further functions. The injury level may then rise for example from C6 to C5. And, of course, SCI can partly be repaired.
Out-of-date of the clinical treatment systems
On the clinical side the human research situation is not better. The diagnostic in clinics is good till sophisticated, because it is organized and money can be earned. Sometimes too much diagnostic is performed. But the cervical SCI patient Kadri (Figure 37E, F) said, why should I go to the neurologist. He is telling me what is wrong in my body, but he is not telling me how to repair the lost or impaired functions. Apart from exceptions, one cannot repair the human nervous system with drugs or operations. Movement-based learning, on the other hand, is a causal therapy which repairs the neural networks. But the physiotherapy to repair the nervous system is mainly out-of-date and inefficient. When in Switzerland the physiotherapy education was upgraded from school to academy, only the names were upgraded, not the education itself. Physiotherapists do not learn, for example, to perform electromyography (EMG) on patients. When the Author demonstrated surface EMG to physiotherapists in a course, they all liked it, because one could really see pathologic motor programs and spasticity on the screen of the scope. When seeing their own volitional muscle activation, physiotherapists were impressed.
Being at the international conference for pediatric acquired brain injury (IPBIS2018), really interest was only coming from one physiotherapy student who wanted to do research, a physician who was interested in neural network learning and two lawyers, who were supporting and supervising the families of brain injured children. But all the physiotherapists, rehabilitation physicians, neurologists or neuropediatric did not want to get informed about new developments in CNS repair. Robotics were of interest to them. When the Author is seeing a picture where a child with a nervous system injury is in the wheelchair (Figure 1A), he is getting angry and depressive. When the Author asked neuropediatric from New Zeeland whether they are not getting depressed when they diagnose all the deficits of a brain-injured child but cannot offer treatment, he did not get an answer and next day they did not come to the Authors poster (Figure 59) to get informed and discuss problems. The members of the conference seem to be afraid to see that the nervous system of children can partly be repaired. A physician, who treated Nefeli and Sophie (pictured on the lower part of the poster, Figure 59) in a rehabilitation center, did not want to see the outcome of his former patients on the long-term. Interesting is further that no member of the conference wanted to try out the special CDT device placed besides the poster (Figure 59). And no physician wanted to get a reprint or wanted to look in some publications or books (Figure 59). When the Author exercised at the entrance of the conference building on the special CDT device, after some time he was pushed away by the administration (the organizer of the conference) so that nobody can see his exercising on the device (out of view – out of mind). Students and stuff of the nearby School for Management, who were passing the Author during exercising on the special CDT device, were more interested what the Author is trying to demonstrate than the members of the conference. When the Author gave long ago a talk at a Nobel institute for Neuroscience in Stockholm (Prof. Grillner [67], pattern generator), he was not allowed to discuss with all the assistances, even though at that time they were thinking of a rat experiment to repair the spinal cord. They were planning at that time a rat experiment what the Author had mainly done already on human.

Figure 59: Poster of the Author Schalow G (Number 38) at the international conference IPBIS2018 in Belfast 2018: Pediatric acquired brain injury repair. The poster is not especially good because of lack of money. But the repair progress of the nervous system in children can clearly be seen It is also shown that human anatomy and physiology is needed for repair.
When really some junior physicians get motivated to try new repair strategies of the brain, they are punished by the establishment. Why to develop new treatment to repair the human brain, when it is impossible to bring it to the patient? Moderators in TV are often open to new developments. But first, explanations have to be that simple that a differentiation between qualified research and hocus-pocus is not possible and secondly, the neuro-rehabilitation is still going on with their out-of-date treatment whatever the progress is in their field.
New organizations and predatory organizations organize journals and conferences without sufficient knowledge. For both knowledge and money is needed. They screen the market and choose what sounds nice. But they are not blocking new developments. Researcher can now choose between not publishing or publishing in a new journal which may be predatory. But a real research worker has to publish because it is part of the profession and secondly, in human neurophysiology and clinical research, apart from statistics, many patients are suffering and dying and that puts load on the clinical researcher. It was estimated that by shifting the new Berlin airport from the South of Berlin to the periphery of Berlin, approximately 11 inhabitants will die per year caused by aircraft noise. By not using CDT, which improves health of patients in general, there may die a million people on earth every year.
Movement therapies are not popular because mental discipline is needed. Probably more than half of the population on earth has overweight. Especially young ones, who alternate between fast food and smart phone, have massive overweight. On Kriti (Greece), there are many children between 5 and 10 years which have already very much overweight and move therefore very little. In the class of Nefeli (Figure 1) there were 2 of the 18 children who had no overweight! But movements like walking, running and jumping are necessary for a healthy development. Many new diseases will therefore occur in the future due to the too little movements performed by children. Patients and healthy people only enjoy movements, if they have no overweight. Also, the elegance of walking is lost with overweight.
Universities and rehabilitation centers are with respect to human neurophysiology approximately 30 years out-of-date. Just now an internet organization (academia.edu) tells what papers of the Author are read most. These papers are the ones which the Author published 10 to 20 years ago (Classification scheme of human peripheral nerve fibers). That the neuro-rehabilitation is out-of-date is generally known. The Author was even told that for bringing progress to the rehabilitation, an agreement with the devil is justified. But how is it, most likely, with the so-called progressive institutions? Astronauts are brought into space and because of missing gravity it has to be looked for their health. Astronauts get for example osteoporosis from which they do not recover again on earth. Their physical exercise in space is for the time being the walking on treadmill. Exercising on a special CDT device would at least also be needed. One could measure then additionally the organization of their CNS first on earth, then in space and afterwards on earth again and see what had changed of CNS functioning and health in space. By measuring at the same time heart rate variability and split it by Fourier analysis into sympathetic and parasympathetic contributions, one could see whether the astronauts have stress and reduce it, if possible, by exercising. Further, because CDT improves also coordinated finger movements, astronauts could better perform complicated experiments, in which coordination is needed.
Plenty of money is given to enhance artificial intelligence and necessary infrastructure is built. The highest intelligence is by far still generated in the human brain. Why the researchers in the field of artificial intelligence are not trying to learn from the organization of the human neural networks for their artificial neural networks? The human brain is very fast and is most likely not using iteration processes for network changes. It seems that not only disabled children have a “tunnel-view”.
Aging men are worried to get prostate cancer. It is likely that prostate cancer growth can be inhibited by CDT in similarity to breast cancer [22] and anaplastic oligodendroglioma [23]. In TV it was recently analyzed what operational strategy is better, to operate only with the hands or using additionally a robot. But on prevention of prostate cancer, namely to reduce the probability of prostate cancer occurrence by physical activity (including CDT), it was not reported of.
Children with SCI are hoping for better treatment to become like the other children again
The patient Nefeli was saying to the Author: “Giselher, I want to be again like the other children”. The Author answered: “Nefeli, then we have to train very much at the limit”. And Nefeli answered: “Giselher, but I also want to play now. When I am grown up, I cannot play anymore”.
After the critic on the present research and treatment system, the functioning of the human urinary bladder will be presented in the second part. At this level of basic medical research, progress in medicine seems possible. A short abstract has been published [91].
Chapter 2
Human Neurophysiologic Basis for Bladder Functioning and Repair at the Single-Neuron Level
Human neurophysiology of bladder functioning
Clinical urinary bladder function test (Urodynamics)
To understand learning transfer, defined within the framework of System Theory of Pattern Formation for Repair, from movements to urinary bladder functions with human electrophysiology at the single-neuron level, it is started with the clinical diagnostic of the urinary bladder: urodynamics. The functioning of the urinary bladder can be evaluated by measuring the pressure in the bladder and in the abdomen (colon) and the electromyographic (EMG) activity of the external sphincters and/or functionally associated pelvic floor muscles. Such bladder diagnosis is called urodynamics (Figure 60). Especially the simultaneous activation of the detrusor (line detrusor pressure in Figure 60B) and the sphincteric and/or pelvic floor muscles (line pelvic floor EMG in Figure 60B), the so-called detrusor-sphincter-dyssynergy, can be measured, which may destroy the kidneys in the long term.
Upon retrograde filling of the bladder in a patient with a spinal cord injury, the pressure in the bladder and colon is measured, and the electromyographic activity (EMG) of the pelvic floor recorded with surface electrodes (Figure 60). The detrusor pressure is obtained by subtracting the abdominal pressure from the bladder pressure. The continence status of the patient is diagnosed by the reports of the first feeling of bladder fullness, the desire to void, and the leaving of fluid out of the bladder. The EMG activity of the pelvic floor informs when the external bladder and anal sphincters are activated. Upon knocking, pressing, coughing, and stimulating bladder reflexes, the bladder status is obtained. This patient of Figure 60 with a spinal cord injury subTh12 had a detrusor-sphincteric dyssynergy of the bladder, because the EMG activity of the external sphincters increased (the sphincters became activated) with the increase of the detrusor pressure (activation of the detrusor). Improvement of urinary bladder function, mainly due to therapy, could be quantified by repeated urodynamics measurements (Figure 60A, B).
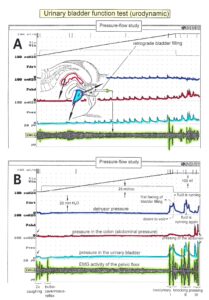
Figure 60: Improvement of urinary bladder functions mainly due to therapy, quantified by urodynamics in a 30-year-old female patient. A. Three months after the accident in the mountains with resulting paraplegia sub Th12 following spinal cord lesion. B. 12 months after the accident (lesion level lowered to sub L3). In A, the detrusor pressure (Pdet) is generated by the contracture of the bladder wall, as the pressure difference between abdominal pressure (Pabd, measured in the colon) and the bladder pressure (Pves, measured in the bladder). Electromyographic recording obtained with surface electrodes from the sphincters and the pelvic floor (EMG) is shown; the external sphincters and the functionally correlated pelvic floor muscles show similar EMG activity (the rhythmic pressure peaks in A do not originate in the bladder). In A, the detrusor shows nearly no activity with retrograde bladder filling at 25 ml/min; in B, the detrusor shows first activity at 360 ml bladder filling. A detrusor-sphincteric-dyssynergy occurs, because the detrusor pressure peaks occur at the same time as the sphincter EMG activity peaks (B) (bladder and sphincter contract at the same time, so that fluid can only emerge from the bladder at high bladder pressure; there is a danger of reflux through the ureter into the kidneys). The EMG peaks are a bit irregular, probably because the fluid, leaving the bladder, shunts transiently the EMG electrodes. Exact functional description of B: 2x coughing (B below) increases the EMG activity and passively the pressure in the abdomen and in the bladder (marked by the small arrows, physiologic). The bulbocavernosus reflex (induced by pressure applied to the clitoris) increased the EMG activity of the sphincters (physiologic). Conclusion: The reflex arch is in order; sacral nerve roots and nerves have not been damaged in the accident. I (bottom right): The patient feels an increase of involutional detrusor pressure (first feeling of bladder pressure at 360 ml). She tries to contract the sphincters to stop the bladder emptying. Shortly after the desire to empty the bladder, as the detrusor pressure decreases, fluid is leaving the bladder. II: Due to tapping onto the bladder, the bladder reflex is activated (detrusor activated, nearly no abdominal pressure); fluid is leaving the bladder. III: Due to the abdominal muscular pressure, the pressure in the abdomen increases as does passively the pressure in the bladder (the detrusor is not activated); fluid left the bladder. With a delay, the detrusor was activated by the bladder reflex. – The urinary bladder of the patient is partly functioning. It has to be further improved by therapy induced reorganization of the CNS: (1) An earlier feeling of bladder filling, (2) an increase of the time difference between the feeling of the first bladder filling and the involuntary emptying of the bladder (for the time being, approx. 10 min, in dependence on whether the patient is physically active (such as walking) or not, (3) further learning how to activate the detrusor on volition, and (4) the physiologic coordination between the bladder and the external sphincter functioning (to stop the detrusor-sphincter-dyssynergy). In cooperation with U. Bersch, Urology, Swiss Paraplegic Center Nottwil, Switzerland.
Limitation of urodynamics and need for human electrophysiology for causal repair
The evaluation of bladder functioning by means of urodynamics gave the information that the patient had a dyssynergy of the bladder. Repeated testing informed about changes in bladder functioning. But such bladder function tests are giving no information on the pathology of the CNS organization and how to repair urinary bladder functioning causally.
By performing similar bladder tests under operational conditions and recording single-nerve fiber action potentials from sacral nerve roots (Figures 2-4), the pathology of CNS organization can be explored in patients with spinal cord injury and ideas found how to repair the injured CNS causally through learning.
Human neurophysiology for a deeper understanding of bladder repair by learning
Since learning and learning transfer during movement-based learning may need a few years, it is of great importance to use such learning treatment (behavioral information) which can really repair the urinary bladder. The proper behavioral information for learning transfer was developed by using the single-nerve fiber action potential recording method. By analyzing natural impulse patterns in sacral nerve roots from the somatic and parasympathetic nervous system upon bladder, bowel and skin stimulations, intravesical stimulation, and movements, it was found that jumping on a springboard and exercising on the special CDT device are especially efficient to induce learning transfer from movements to bladder functions. The free jumping or jumping on springboard are specifically designed to repair patterns, including transfer of learning from somatic to autonomic functions, discussed in detail below, and the exercising on the special CDT device is primarily used to reduce the variability of phase and frequency coordination and to increase thus the stability of network states by decreasing pattern fluctuation. Movements are exercised to generate a physiologic attractor layout of patterns again and the coordinated firing of neurons and neural sub-networks is trained to increase pattern stability by reducing the fluctuation of pattern states.
The innervation of the bladder is schematically shown in Figure 61. Nerve fiber counts of myelinated nerve fibers indicate the thickness of nerve roots and nerves. Even though single-nerve fiber action potentials can also be recorded from peripheral nerves, high-quality recordings can only be obtained from thin and long sacral caudal nerve roots. The very thin coccygeal nerve root contains mainly skin afferents to the coccygeal dermatome and is ideally suitable to analyze skin receptors properties. A recording is shown in Figure 5. To avoid adding up single-nerve fiber action potentials, the summed activity has to be low to be able to split up the multi-unit recording into several natural single-nerve fiber impulse patterns (Figure 4). The lower sacral nerve roots are suitable for that. An original recording from a S4 root upon anal catheter pulling to stimulate the somatic and parasympathetic nervous systems is also shown in Figure 61. Afferent (main amplitude upwards) and efferent single-nerve fiber action potentials (amplitude downwards) can be seen. The ventral S4 root is an ideal root to record single-nerve fiber action potentials from efferent (running out of the spinal cord) and afferent fibers (leading into the cord) (Figure 6). In caudal sacral nerve roots, there are also afferent fibers in ventral roots and also efferent fibers (motor fibers) in dorsal roots. The innervation of the bladder from the sacral micturition center is leading through the nerve roots S2 till S5.
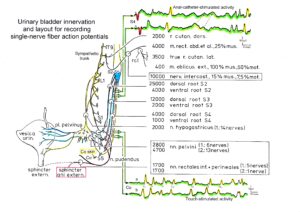
Figure 61: Urinary bladder innervation (anatomy; structure) and recordings of single-nerve fiber action potentials from a S4 and a coccygeal root (electrophysiology; function). Number of myelinated nerve fibers of the nervi rectales inferiores and perinales, the nervi pelvini and the nervus hypogastricus. T = thoracal; L = lumbal; S = Sacral; rcl = ramus cutaneus lateralis, no pure skin nerve; %mus = % of nerve fibers leading to muscles; %mot = % of nerve fibers which are motoric; 1 and 2 = Nerve fiber counts from cadavers 1 and 2. Number of nerves in the bracket gives the number sub-nerves of which the nerve consisted. The innervation pathway of the external bladder sphincter is unclear. The S4 root recording informs about single-nerve fiber activity running into the spinal cord (from the bladder receptors) and running out of the cord to the urinary bladder (bladder efferents) upon natural stimulation. Functions of the urinary bladder functions and the sacral micturition center in the spinal, as a part of the human CNS, can therefore be analyzed. The Co root (no efferents; something like a skin nerve) recording informs about skin receptor activity in the coccygeal dermatome.
Since the repair of bladder function in patients with SCI probably also requires the reorganization of pathways outside the spinal cord, the connection of the plexus pelvinus to rostral plexuses is shown in Figure 21. The picture allows understanding of just how complex and distributed the autonomic nervous system is. The bladder is mainly innervated by the somatic and parasympathetic nervous system and probably a little by the sympathetic nervous system (internal bladder sphincter). In Figure 20 the innervation of the human organs is shown via the sympathetic and parasympathetic divisions. By recording single-nerve fiber action potentials from caudal sacral nerve roots, only natural impulse patterns in somatic and parasympathetic nerve fibers can be expected to be obtained because it was recorded from the S3 to S5 dorsal and ventral nerve roots.
The complexity of the autonomic nervous system is tremendous (Figure 21). The thin ventral S4 root (motor root) in itself shows quite a complexity (Figure 7B). The thick myelinated nerve fibers are prominent. By enlarging the scale, also the non-myelinated nerve fibers become visible (Figure 62). Their number is in the range of 5-times higher than those of the myelinated fibers. The diameter of the thinnest myelinated fibers from which it is possible so far to record single-nerve action potentials is in the range of 3 to 4μm (Figure 8). To record from these thinly myelinated fibers is difficult but important. To repair of urinary bladder by learning, we must be able to record also from parasympathetic nerve fibers and distinguish their activity from the activity of the different groups of γ-motoneurons to understand bladder function.
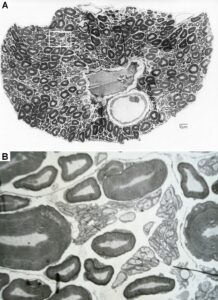
Figure 62: A. Montage of several electron microscope photographs of the S4 ventral root from HT1. Single arrow marks a nerve fiber with a comparably thin myelin sheath; double arrow marks a nerve fiber of same size with a comparable thick myelin sheath. Scale not corrected for shrinkage. B. Enlarged scale to make unmyelinated nerve fibers visible.
Since parasympathetic fibers are thin their single-nerve fiber action potentials are small and the identification of their action potentials in the summed impulse traffic is difficult. Also, the γ-motoneurons are thin and its different groups are difficult to separate, especially when the recording temperature is low. In the conduction velocity distribution histogram of Figure 63Da, the parasympathetic fiber groups cannot be distinguished from those of the γ-motoneurons, making it difficult to identify impulse patterns of single γ-motoneurons and parasympathetic efferent fibers.
Identification of peaks of γ-motoneurons and parasympathetic fibers in conduction velocity distributions on log scale
On a linear scale the conduction velocity peaks may fuse and can be separated by their different functions only partly (Figure 63Da, Ea). By constructing first histogram classes for conduction times however and plotting them on a log scale from the column values, the velocity peaks separated. Figure 63D, E shows that the fused γ-peaks of the linear plots (Da, Ea) split up into different peaks on the log scale (Db, Eabc). Since first histogram classes of conduction times were constructed using a linear scale, it was also possible to study the dynamics of peaks or peak values upon stimulation. The group identification of single-nerve fiber action potentials is necessary to explore human CNS self-organization.
Figures 63Db and 63Eb show several distribution peaks in which the γ1 and γ21-peaks could be identified from earlier functional considerations. By comparing the velocity distributions obtained upon stimulation with those without stimulation (Figure 63E), and reasonably assuming that the dynamic intrafusal motoneuron peaks are higher upon stimulation (Figure 63Ed) and the static ones are higher with no stimulation (Figure 63Ec), a second static γ2-peak (γ22) can be identified and at least one further rather dynamic motoneuron peak (para) can be seen. This peak distribution observed in the paraplegic patient could also be found in the measurements in HT6 (Figure 63Db). That the “Para” peak contains activity from parasympathetic fibres will be shown later. The identification of parasympathetic efferents contributes to the understanding of urinary bladder functions under physiologic and pathophysiologic conditions and its repair by CDT. The nerve fibre groups obtained from conduction velocity distributions for single fibres are in accordance with the sub-compound action potentials of compound action potentials [68]. Compound action potentials could also be recorded by electrostimulation from the bladder (Figure 65).
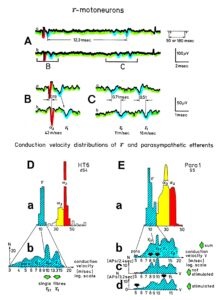
Figure 63: A. Activity from 2 g-motoneurons and an a2-motoneuron. The g1-motoneuron may have fired in the oscillatory mode for a few cycles with a period duration of 90ms or 180ms. Interspike intervals of the possible impulse train, consisting of 2 APs, are indicated (12.3ms). The insert shows the possible oscillation cycle period. HT6; dS4. B, C. Time and amplitude expansions of A. Conduction velocities are indicated. Note that with the increasing conduction velocity, the AP amplitude increases and the duration decreases. D, E. Conduction velocity distributions of motoneurons for the brain-dead human HT6 (D) and paraplegic 1 (E). To make intrafusal motoneuron and parasympathetic peaks visible, the main g-peak of Da and Ea was plotted on a log scale in Db and Eb. The distribution peaks are labeled with the groups, they most likely represent. In E, the distribution Eb is split into the distribution upon no additional stimulation (Ec) and upon additional stimulation (Ed). Note that in the non-stimulated distribution (Ec) the static g-motoneuron peaks (g22, g21) are highest, whereas under stimulation (Ed) the parasympathetic (Para) and the dynamic g-motoneuron peaks (g1) are highest. When plotting the velocities in Db and Eb logarithmically, the conduction times were first grouped by a conduction time histogram and the column values were then used (conduction distance = 8 mm) to construct conduction velocity distribution curves.
In the recording from a paraplegic patient in Figure 64 the different groups of parasympathetic fibres and γ-motoneurons do not fuse, probably because of the higher recording temperature during an operation to implant an electrical bladder stimulator. Such a procedure is now obsolete because the bladder can be repaired by CDT.
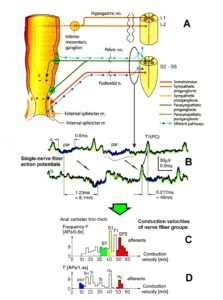
Figure 64: A. Innervation of the rectum [83]. B-D. Sweep piece of recording (B) and conduction velocity distributions (C, D) taken from time intervals following a change of a thin anal catheter (Æ = 12 mm) for a thick one (Æ = 20 mm). Note the manifestation of the parasympathetic peak.
Electrical Intravesical Stimulation
In Figure 66A, the recording and stimulation layout for electrical intravesical stimulation is shown. The negative electrode was connected to the tip of the bladder catheter but was in no direct contact with the bladder mucosa. The indifferent positive electrode was attached to the leg of the patient. Following stimulation with 30V compound action potentials (CAPs) of stretch (S1) and tension receptor afferents (ST) could be evoked. In the recording from the root dS2 (Figure 65A), mainly two CAPs follow the stimulation artefact, with latencies of 7.4 ms and 9.5 ms.
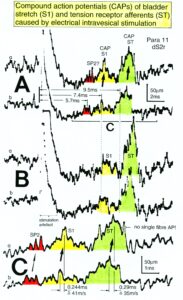
Figure 65: Compound action potentials (CAPs) of urinary bladder stretch (S1) and tension receptor afferents (ST) following electrical intravesical stimulation. Stimulation in B was slightly stronger than in A. The recordings in C represent a time-stretched portion of A. Mean conduction times and calculated mean conduction velocities of CAPs are indicated. In C, three single secondary muscle spindle afferent fibres are marked (SP2). The stimulation pulse (not rectangular) was 0.3ms long; no earthing between recording and stimulating electrodes. Earth electrodes positioned 5 to 10mm away from the recording electrodes reduced stimulation artefact and single-fibre AP amplitudes and were therefore not used. Horizontal line of stimulation artefact is due to the limited input range.
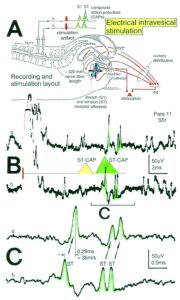
Figure 66: A. Recording and stimulation layout for electrical intravesical stimulation (electro negativity in bladder). B. Recording from an S5 root following electrical stimulation. Compound action potentials (CAPs) of S1 and ST afferents schematically redrawn from Figure 9A. C. Time-stretched sweep piece of the recording in B. Single tension receptor afferent APs (ST) are indicated.
The CAP conduction velocities in Figure 65C were 41 and 35 m/s. According to Figure 10C, the stretch receptor afferents contributed to the CAP with a latency of 7.4 ms and the tension receptor afferents to the CAP with a latency of 9.5 ms. The faster conducting S1 afferents give rise to the CAP with the shorter latency, as they would be expected to. From the latency and the group conduction velocity, it can be calculated that the afferent activity travelled approximately 320 mm to reach the recording electrodes, as indicated in Figure 10A. The surgeon estimated a distance of 300 mm.
With a slightly increased electrical stimulation, the S1 and ST CAPs increased slightly (Figure 65B), probably because more afferents were stimulated in the bladder wall. A recording from root S5 (same electrical stimulation) showed no pronounced CAPs. By schematically plotting S1 and ST CAPs from Figure 65A into Figure 66B and by calculating the conduction velocities of the single-fibre APs in the latency range of the ST CAP, it was found that the single-fibre APs were conducted by tension receptor afferents, as can be seen from the time-stretched Figure 66C. Thus, at least the ST CAP was composed of the single-fibre APs of the ST afferents. Normally, subpeaks in CAPs do not represent single-fibre APs, as is indicated in Figure 65C. The arithmetic addition of single-fibre APs of different triphasic AP waveforms often results in large amplitude potential changes, which do not represent single-fibre APs, as can be learned from recordings of high skin afferent activity. To obtain CAP recordings from stretch and tension receptor afferents a substantial number of bladder afferents have to be stimulated simultaneously, through the nerve root that is used to record from.
Location and stimulation of receptors for continence
The receptors of skin, urinary bladder and anal mucosa, the muscle spindles which were innervated by secondary spindle afferents, and the muscles which most likely were innervated by a-motoneurons in paraplegics 7 and 9 and in the brain-dead human HT6 are marked in Figure 33 in the lower pelvis. The (rhythmic) activation of these receptors by anal and bladder catheter pulling and skin stimulation of sacral dermatomes, to stimulate simultaneously the somatic and parasympathetic divisions to induce learning transfer, can be simulated and achieved during therapy, if a patient with severe cervical spinal cord injury jumps rhythmically on springboard with no weight support.
Following retrograde bladder filling urinary bladder stretch (S1) and tension receptor afferents (ST) can be stimulated and measured. In Figures 10, 11G the conduction velocity distribution histogram shows the activation of S1, ST and secondary muscle spindle afferents (SP2) for a bladder filling of 750ml. Jumping on springboard with a full bladder or colon and rectum activates the somatic and parasympathetic divisions much more because more receptors are activated. Before exercising on the special device or jumping on the spring-board, patients often first empty their bladder and rectum to stay continent. To understand urinary functions, it is important to study bladder filling at the neuron level.
Understanding bladder repair based on human neurophysiology
At a time when the spinal shock fades following injury, most patients with a severe spinal cord injury (SCI) present with a spastic bladder. Some patients with a less severe injury have a small storage volume in the bladder. Many also have to rely vegetative symptoms to detect bladder fullness. Some patients learn a reflex bladder to empty the bladder. But only patients with incomplete SCI can feel when the bladder is full and have the desire to void. Simultaneous bladder synergy and dyssynergy is a repair stage or a sign of incomplete SCI with tract fibers left to the urinary bladder. Kadri’s bladder functioning changed upon repair from the state of a complete SCI to an incomplete SCI (Figure 38). The repair change from dyssynergy to synergy can be understood by comparing the interplay between the somatic and the parasympathetic nervous systems. In brain death, the sacral micturition center is not disconnected from pontine micturition center and synergy should therefore be present, which was actually the case. In patients with severe SCI on the other hand, a dyssynergy is present. By measuring the different organizations of neuronal networks of the parasympathetic and somatic divisions in both cases, we can learn what has changed into pathologic functioning following the SCI. Then we will look for possible pathways for repair.
Activity of bladder afferents to retrograde bladder filling
First, we shall discuss the afferent input to the bladder upon bladder filling in the physiologic (brain-dead human) and pathologic case (SCI). Can some pathology in the bladder input be seen in the patient with SCI?
For measuring bladder afferent activity increase upon retrograde bladder filling, the bladder was emptied and then filled while recording. With the groups of nerve fibers identified from conduction velocity distribution histograms (Figure 67B), the increase in activity of the urinary bladder stretch (S1) and tension receptor afferents (ST) to retrograde bladder filling was measured. Figure 67 shows the activity increases following filling of the bladder of Para 7, via a catheter, with up to 400 ml fluid. The bladder was filled to the maximal acceptable value as determined pre-operatively by compliance (bladder filling volume / bladder pressure) measurements. Figure 67B shows the sum of the individual velocity distributions for different filling stages for afferents and efferents. The peaks are identified by two calibration relations; a2-motoneurons conduct with the same velocity as the secondary muscle spindle afferents (~50 m/s); and a3-motoneurons conduct approximately with the same group velocity as the stretch receptor afferents S1 (41 m/s) at about 35.5°C. Figure 67A gives velocity distributions for certain bladder filling stages. The most important obvious feature is that the bladder stretch and tension receptor afferents fire considerably even with no bladder filling.
By setting up conduction velocity borders of the S1 and ST groups according to the distribution curve, the activity of each group could be measured for different filling volumes and plotted (Figure 68A). As can be seen in Figure 68A, the stretch (S1) and tension receptor afferents (ST) in the left root S5 fired with 15 APs during 0.2s at no bladder filling. The bladder afferents increased their firing with the increasing bladder storage volume. At 160 ml filling, a transient very strong firing of ST afferents occurred. This was the filling volume at which before the surgery the detrusor was activated in an urodynamic measurement. It was not possible to activate the detrusor with retrograde bladder filling during surgery, even though the light anesthesia as paraplegics with a complete spinal cord lesion feel no pain.

Figure 67: Conduction velocity distribution histograms for stretch (S1) and tension receptor afferents (ST) and secondary spindle afferents (SP2) for different retrograde filling stages of the urinary bladder (A). Summed histograms for afferents (a) and efferents (b) are plotted in B. a1, a2, gb, g1 represent velocity distributions of a1, a2, gb and g1-motoneurons. Para 7, left nerve root S5.
From a ventral S3 root in paraplegic 11, a similar undulating activity increase of S1 and ST bladder afferents was obtained with retrograde bladder filling. When the bladder was empty, the afferents (S1 and ST) fired already with 10 APs/0.2s. At 50 ml filling, they fired with 23 APs/0.2s, at 200ml with 6 at 250ml with 25 and at 500ml with 12 Aps/0.2s. The detrusor was activated pre-operatively at a filling volume of 80ml (continuously undulating contractions (0.08Hz) started). As in the para 7, the first high afferent input falls approximately together with the onset of the pre-operative detrusor contraction, when filling the bladder. The occurrence of bladder afferent APs in the ventral S3 root was higher (166 APs/2.2s) during the bladder filling than those of a-motoneuron APs (70 APs/2.2s); the secondary muscle spindle afferents fired with 64 APs/2.2s. The speed of filling in Para 11 was 300ml/min. In this ventral S3 root there were at least a few percent of the fiber’s afferent.
Comparison of bladder afferent activity increase to retrograde bladder filling between a paraplegic and a brain-dead human
The pathology of the bladder afferent activity in the paraplegic 7 analyzed in Figure 68A, with dyssynergy of the bladder, is fully revealed by a direct comparison with the activity increase of the bladder afferents following retrograde bladder filling in brain-dead human HT6 (Figure 68B). The dependence of the bladder afferent activity on the bladder filling volume in paraplegic 7 shows four main differences in comparison to those in HT6. (1) The bladder afferents already fired with no bladder filling which was not the case in HT6. (2) The activity increased more smoothly in HT than in the paraplegic, even though bladder filling was stopped twice in HT6 but was not stopped in Para 7. (3) In the Para 7 the tension receptor afferent activity was higher than the stretch receptor afferent activity which was not the case in HT6. (4) The bladder afferent activity was higher in the paraplegic than in the HT6. By taking into account the different measuring times of the activity, the ST-activity in Para 7 was approx. 40 times higher than those in HT6 (210APs/1.2s in Para 7 against 5APs/1.2 in HT6). The recording conditions are not directly comparable. However, there are on average more bladder afferents in a root dS4 (HT6) than in a root S5. Still, the 40 times higher tension receptor afferent activity is probably overestimated. It is nevertheless safe to conclude that in some paraplegics the bladder afferent activity, especially from tension receptor afferents, was several times higher than that in the brain-dead human, the latter probably representing the physiologic case in this respect. The finding of increased afferent activity in two paraplegics is in accordance with the experience that in brain-dead humans’ activity from bladder afferents was recorded only occasionally, whereas in paraplegics the activity from S1 and ST-afferents was always obvious and prominent in the velocity distributions, rather independent of the extent the bladder was filled. Paraplegics with dyssynergy of the bladder suffer from high bladder pressure. This increased afferent activity will have consequences on the excitation of the CNS activating the striated sphincters of the bladder and the rectum.
Increased bladder afferent activity a reason for urinary bladder dysfunction
A dramatic increase in the activity of the stretch (S1) and tension receptor afferents (ST) was shown with certainty in two paraplegics (Figure 68). Also, in several other patients’ bladder afferent activity seemed increased, as the bladder afferent activity was prominent for even small bladder filling volumes. Nearly all patients who underwent surgery had a much smaller storage volume of the urinary bladder, but not necessarily a reduced compliance (filling volume / pressure ratio). Following urinary bladder de-afferentiation the bladder storage volume increased as did the compliance. However, there are also paraplegics with a storage volume of 50 ml and no increased compliance. They are said to have hyper-reflexia of the bladder. Thus, there are patients with a stiff and infected bladder in whom the afferent activity of the bladder is much higher. The afferent activity is even often present with an empty bladder. The increased bladder afferent activity causes the detrusor being activated too early. On the other hand, there are patients with no increased compliance and no infection, and a detrusor which already contracts at filling volumes of 50 ml. It will be shown that there are important changes in the somatic and parasympathetic neuronal network of the sacral micturition centre and in the coordination between them; this can account for an early activation of the detrusor and the external sphincter. As was shown above most of the complications of urinary bladder functions can be avoided and urinary bladder functions be cured if CDT is started early after the spinal cord injury.
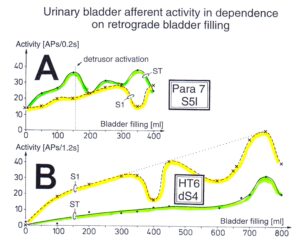
Figure 68: Afferent activity increase of stretch (S1) and tension receptor afferents (ST) to retrograde bladder filling in paraplegic 7 (left root S5) in relation to those of the brain-dead human HT6 (dorsal root S4). APs = action potentials. The speed of filling was 130 ml/min in para 7 and 100 ml/min in HT6.
Premature activation of sphincter motoneurons in paraplegics following too high input from tension and stretch receptor afferents
Since with the single-nerve fiber action potential recording method the activity is recorded simultaneously from afferent and efferent fibers in thin sacral nerve roots, it is possible to analyze bladder functions with the inclusion of the activity of sphincteric and pelvic floor motoneurons. It can be measured at what filling volumes the sphincteric motoneurons start to fire in response to retrograde bladder filling in patients with SCI and in brain-dead humans. It can be measured with what afferent input to the sacral micturition center, induced by bladder filling, the motoneurons, securing continence, are activated. In consequence, also dysfunction of the sacral micturition center can be analyzed.
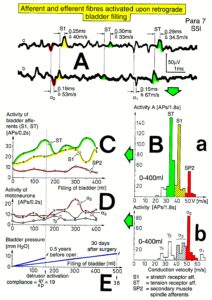
Figure 69: A. Action potentials (APs) recorded from a1 and a2-motoneurons and stretch (S1) and tension (ST) receptor afferent fibres. Conduction times and conduction velocities are indicated. B. Conduction velocity distribution histograms for afferent (a) and efferent fibres (b). C. Activity level changes of bladder afferent fibres (S1, ST) in dependence on bladder filling. D. Activity level changes of a2– (FR) and a3-motoneurons (S) (no single fibres) in dependence on bladder filling, recorded simultaneously with the activity of bladder afferents. E. Detrusor pressure (bladder pressure) in dependence on bladder filling, before and after the surgery; redrawn from urodynamic recordings. Paraplegic 7.
In Figure 69A, a recording from an S5 root of paraplegic 7 is shown. Conduction velocity distributions of afferent and efferent fibers were constructed (Figure 69B), and nerve fiber groups were identified by the velocity ranges of the peaks. The identification of the nerve fiber groups rested upon the calibration relation, namely that secondary muscle spindle afferent fibers (SP2) conducted with the same velocity as the a2-motoneurons (FR) and stretch receptor afferents (S1) conducted with the same velocity as a3-motoneurons (S). Figure 69 is the extension of Figure 68 to include the motoneuron firing.
The activities of bladder afferents and a2 and a3-motoneurons during retrograde bladder filling are shown in Figure 69C, D. The activity values of afferent and efferent fiber groups were obtained from histograms similar to those shown in Figure 69B. It can be seen that the motoneuron activity slightly increased during the bladder filling (Figure 69D) as did the stretch and tension receptor afferent activities (Figure 69C). The most important point, namely that bladder afferents fired already quite strongly when the bladder was empty, shows no characteristic consequences on the firing levels of the motoneurons. Most likely, the mixing of the activities from sphincteric motoneurons with those from other motoneurons, not associated with continence (and innervating, e.g., leg muscles), weaned off specific properties. A similar loss of specific properties of the activities of motoneurons with different functions was observed in dog sacral nerve roots (Figure 2 of [69], no specific properties in similarity to urodynamic recordings). In that case, motoneurons may have innervated leg, tail and continence muscles. The mixing of activities of the motoneuron firing in different modes, namely oscillatory and occasional, may have contributed to the loss of specific functions.
Specific properties of sphincter motoneurons could be revealed separating impulse patterns of single motoneurons from the summed impulse traffic by waveform comparisons [70] (Figure 4). Figure 70A,B illustrates the activity increase of motoneurons, innervating the external bladder sphincter, upon retrograde bladder filling and illustrates simultaneously the activity increase of motoneurons with tension receptor afferent activity changes in a brain-dead individual and in the paraplegic 7.
In the brain-dead human, the sphincteric motoneurons show only a little activity in the occasional firing mode for a bladder filling smaller than 500 ml (storage phase). The activity of the bladder tension receptor afferents is nearly zero for no bladder filling. In paraplegic 7, the sphincteric motoneurons a2(1) and a2(2) fired already oscillatory to generate high activity levels when the bladder was still empty. Since in the paraplegic the activity of the tension receptor afferents was already very high for the empty bladder, the high excitement of the sphincteric motoneurons was most likely caused by a high bladder afferent activity (Figure 69C), especially from the tension receptors of the bladder wall.
With increasing bladder filling, the activity of the sphincteric motoneurons increased in a manner similar to that observed in the brain-dead individual, if only at much smaller filling volumes. By comparing the activity increases of sphincteric motoneurons and tension receptor afferent fibers in paraplegic 7 with those in the brain-dead individual, it is concluded that the sphincteric motoneurons in the paraplegic behaved similar to the motoneuron in the brain-dead, the latter possibly representing the physiologic case in this respect. Only the sphincteric motoneurons in paraplegic 7 were activated too early because of a too high afferent input. The too high activity of the bladder afferents in the paraplegic mimics a rather full bladder, even though the bladder is still empty. The storage phase of the bladder is lost because of the too high afferent input. If the detrusor is also activated at certain bladder afferent inputs, similar to the sphincteric motoneurons, then the detrusor will also be activated at too small storage volumes (see below). This means that the detrusor in this paraplegic was activated too early because of the too high bladder afferent activity at small bladder filling. The so-called hyper-reflexia of the bladder, namely the automatic detrusor activation for too small bladder volumes in this case, can be explained by the too high bladder afferent activity, especially from the stretch receptors of the bladder wall.
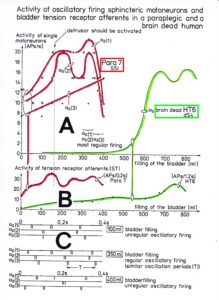
Figure 70: A. Activity of single a2-motoneuron (FR) in dependence on retrograde bladder filling, in paraplegic 7 and brain-dead human HT6. B. Activity of tension receptor afferent fibers (ST), recorded simultaneously with the motoneuron activity in “A” in paraplegic 7 and the brain-dead human HT6. Note that the a2(1) and a2(2)-motoneurons in paraplegic 7 show similar activity increases as the a2-motoneuron in the brain-dead individual, with only the storage phase of the bladder being lost. Note further that with respect to the activity levels of the tension receptor afferent fibers (B), the a2(1) and a2(2)-motoneurons in paraplegic 7 are not activated earlier than the motoneuron in the brain-dead human HT6 (A). C. Discharge patterns of the single a2(1), a2(2) and a2(3)-motoneurons at 100, 350 and 400ml bladder fillings. Paraplegic 7, nerve root S5.
In the brain-dead human the sphincter a2-motoneuron fired in the occasional firing mode at low bladder filling, in the transient oscillatory firing mode at bladder fillings between 500 and 600 ml, and in the continuous oscillatory firing mode at high bladder filling (Figure 11). In the paraplegic, the a2(1) and a2(2)-motoneurons, innervating the external bladder sphincter, fired continuously oscillatory for any bladder filling (Figure 69D).
The motoneurons innervating the external bladder sphincter are rather specifically driven by the bladder afferents (Figure 12). The secondary muscle spindle afferents (SP2), activating motoneurons that innervate the external anal sphincter (Figure 12), did not increase their activity with the increasing bladder filling (Figure 69C) and, for little bladder filling, did not contribute to the high activity of the a2(1) and a2(2)-motoneurons innervating the external bladder sphincter. The little understood gb-motoneurons (Figure 69D) showed no specific properties upon bladder filling.
During surgery, the detrusor was not activated upon bladder filling (see discussion in [71]). Before the surgery, the detrusor was activated at approximately 160 ml bladder filling (Figure 69E), at a volume when the sphincteric motoneurons a2(1) and a2(2) showed their first activity peak. If the parasympathetic division had not been suppressed by anesthesia, it is likely that the detrusor would have been activated at that filling volume during surgery. The motoneurons normally are activated strongly at high bladder filling to secure continence. At those bladder fillings, the urge to void is probably high. If in paraplegic 7 the bladder sensibility had been preserved, the urge to void would have been strong for small storage volumes. The relative extensive de-afferentation of the bladder – cutting of dorsal roots S2 to S5 for implanting an electrical bladder stimulator (S5 root was sometimes not cut, if many parasympathetic fibers were contained), ventral root afferent fibers and possible bladder afferents running through the plexus hypogastricus remain preserved – increased the storage volume from 160 to 500 ml and the compliance from 19 to 38 (Figure 69E).
In Figure 70A, there is another a2-motoneuron (a2(3)) that increased its activity upon bladder filling. Since this a2-motoneuron (FR) showed a different activity increase than the a2(1) and a2(2)-motoneurons, it is likely that this motoneuron did not contribute to continence. It was activated by reflex or response generalization. In Figure 70C, the impulse patterns of the a2(1), a2(2) and a3(3)-motoneurons are shown for different bladder fillings. The motoneurons fired mainly rhythmically with impulse trains consisting of two action potentials (APs), in accordance with the expected pattern of bladder sphincter motoneurons (Figure 1 and Table 2 of [33]). In accordance with the measurements in the brain-dead individual (Figure 11), the oscillatory firing is most regular for the highest motoneuron activation and less regular for smaller bladder fillings and probably overfilled bladders. But the oscillatory firing neuronal network, driving the motoneurons, was dysfunctional, as the oscillation period was strongly changing. In paraplegic 9 the disorder in the neuronal network of the functionally disconnected spinal cord was so grave that even the oscillation period (Figure 30) and the number of APs per impulse train changed.
In the next section, the interaction between the somatic and the parasympathetic neuronal networks will be considered at points where the detrusor activation is expected to inhibit the motoneurons innervating the striated (external) bladder sphincter. Again, recordings obtained from the brain-dead individual HT6 with bladder synergia will be compared with those from a paraplegic with detrusor-sphincter dyssynergia.
Coordination impairment between the somatic and parasympathetic nervous system divisions in the human sacral micturition center following spinal cord injury
After having shown that the identification of somatic and parasympathetic nerve fibers by the group conduction velocity and the group nerve fiber diameter is possible (Figure 63) and had not changed following spinal cord injury (SCI) [1], it will be analyzed here, on the basis of natural impulse patterns, travelling into and out of the sacral micturition center, what became pathologic in the organization of the CNS following spinal cord injury.
Normal voiding is a voluntary act which results in sustained contraction of the bladder (detrusor) and relaxation of the urethra until the bladder is empty. To enable fluid flow along the urethra it is necessary that the pressure in the urinary bladder exceeds that within the urethra lumen. Under normal circumstances, in order to initiate micturition, a fall in urethral pressure immediately precedes a rise in pressure within the lumen of the bladder. Usually, this pressure rise is produced by active contraction of detrusor smooth muscle at the onset of micturition. The extensive vesical part of the pelvic plexus (Figure 21) and the profuse distribution of autonomic motor nerves emphasize the importance of the autonomic nervous system in initiating and sustaining bladder contraction during micturition. Immediately prior to the onset of micturition, the tonus of the striated external bladder sphincter is reduced by central inhibition of its somatic motor neurons located in the third, fourth and fifth sacral spinal segments. This inhibition is mediated by descending spinal pathways originating in higher centers of the CNS probably mainly the pontine micturition center. The central integration of the nervous control of the bladder and urethra is essential for normal micturition.
Immediately following complete SCI there is a period of spinal shock that lasts about three weeks or longer. This period is characterized by muscular flaccidity and a loss of spinal reflexes. The recovery of reflex activities below the level of the injury occurs at different times. If bladder reflexes reappear, they differ in some important respects from those in a normal individual. The ‘autonomic bladder’ contracts in response to distension, but the power is rarely that of the normal bladder, and the residual volume is increased. Injuries above the level of the brain stem centers lead to involuntary voiding in which the detrusor contraction is coordinated with sphincter relaxation (detrusor-sphincter synergy). Injuries below the level of the brain stem centers, but above the lumbosacral spinal cord, lead, after a period of bladder paralysis associated with spinal shock, to involuntary reflex voiding in which the detrusor (bladder) contraction is not coordinated with sphincter relaxation (detrusor-sphincter dyssynergy). The coordination between the detrusor and the external bladder sphincter will now be compared between a paraplegic patient with an injury below the brain stem and detrusor-sphincter dyssynergy (pathologic) and a brain-dead human with partly destroyed brain stem and an at least partial detrusor-sphincter synergia (partial physiologic).
If the SCI is complete, it is believed that paraplegics develop vesico-sphincteric dyssynergy because of the disconnection from the pontine micturition center which is responsible for the coordination of the detrusor and the external bladder sphincter. In cases in which no detrusor-sphincter dyssynergy has developed, the spinal cord injury is believed to be incomplete, so that supraspinal centers can still coordinate bladder functions. The external bladder sphincter will not be ‘co-activated’ with the detrusor in response to distension. The view that the important function of coordination is performed by spino-bulbo-spinal pathways has incorporated anatomical ideas of a hierarchy of functions of increasing complexity, the further rostral one goes in the CNS. Still, the coordination for the mutual inhibition of detrusor and external bladder sphincter may be located in the sacral micturition center. This dyscoordination problem shall now be analyzed using the single-nerve fiber action potential (AP) recording method. As was shown in the patient Kadri with a SCI (Figure 38), such a dyscoordination can be repaired by movement-based learning. The human nervous system has sufficient complexity for re-learning. Rats and cats may not have a sufficient network complexity for such repair [72-74].
Learning transfer is successful in repairing urinary bladder function, since the somatic and the autonomic divisions dovetail with one another with respect to jumping on springboard and exercising on the special CDT device. The necessary connection to the pontine micturition center for the coordination control of the detrusor and the external bladder sphincter is achieved upon CDT by limited regeneration of the human spinal cord, recruiting ectopic pathways through root connections, sympathetic chain or plexus connections (misdirected haphazardly during development) and re-organization of plexus outside the spinal cord. It is believed that the autonomic division of the nervous system is an elaborately organized division of the CNS with representations at all levels. At each level the somatic and the autonomic systems dovetail with one another.
For animals, reviews by de Groat [75] provide a basis for understanding how the filling and voiding functions work. He states that the primary stimulus for micturition is bladder distension, which induces reflex activation of the parasympathetic excitatory outflow to the bladder, depression of the sympathetic inhibitory outflow, and depression of the somatic efferent output to the external sphincter; secondary reflexes elicited by the passage of urine through the urethra may reinforce these primary reflexes and facilitate the complex emptying of the bladder. When the micturition reflex is exceeded, the parasympathetic reflex pathway through the brain stem is active. For an introduction to the physiology of the lower urinary tract, see [76].
Time course comparison between the proposed parasympathetically induced muscle spindle afferent activity and the detrusor pressure
In detrusor-sphincter dyssynergy of the urinary bladder, the somatic external bladder sphincter is activated at the same time as the detrusor (smooth muscle) with the consequence that the bladder cannot be emptied. This dyscoordination between the somatic and parasympathetic nervous system divisions in the human sacral micturition center is reflected urodynamically (clinically) in the simultaneous increase of the detrusor pressure and the electromyographic activity of the pelvic floor (Figure 60B). Before analyzing the detrusor-sphincter dyssynergia at the neuron level an important correlation between muscle spindle activity and detrusor pressure, measured urodynamically, has to be done, namely that muscle spindles, also driven by the autonomic nervous system, show very similar activity changes than the detrusor pressure.
It was shown that the activity in parasympathetic efferents can be measured, identified and distinguished from the activity of γ-motoneurons in conduction velocity distribution histograms (Figure 63). Since the action potential amplitudes of parasympathetic efferents is small, it would still be very difficult to analyze the organization of the parasympathetic nervous system and it’s coordinated functioning with the somatic nervous system as in the control of the urinary bladder. But if some secondary muscle spindles in the parasympathetic range are also innervated by parasympathetic efferents besides somatic efferents (γ-motoneurons), then the activation of the parasympathetic nervous system can also be measured by the activity of secondary muscle spindle afferents. Since the action potentials of secondary spindle afferents are comparably large (thick fibers), the activation of the parasympathetic nervous system can be easily indirectly assessed. It will be shown now that at least some secondary muscle spindles, in the parasympathetic range, are innervated by parasympathetic efferents (parasympathetic fusimotors, with reassignment sympathetic fusimotors) [77-80]. The evidence is obtained by measuring the parasympathetic activation of the detrusor by detrusor-pressure changes and by measuring activity changes of secondary muscle spindle afferent fibers and compare the form of the changes of detrusor pressure with the activity changes of a secondary muscle spindle afferent fiber.
Figure 71 shows the regulation circuits for muscle length (A) and muscle tension (B). The Author does not know how morphologically the muscle spindles of external bladder and anal sphincters are innervated by the autonomic nervous system. Dr. Gladden [80], the specialist for muscle spindles, including human ones, tried to clarify it, but gave in for organizational reasons.
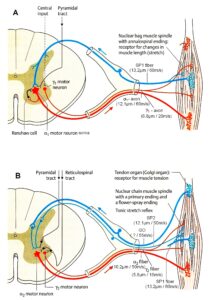
Figure 71: Regulation circuits for muscle length (A) and muscle tension (B). Nerve fibers are partly designated according to the classification scheme of human peripheral nerve fibers. This picture of muscle spindle innervation is not showing the real complexity of muscle spindle innervation. The innervation of the muscle spindles by the autonomic nervous system is missing. Figure partly taken from [83].
Upon retrograde bladder filling in patients with complete spinal cord injuries, the detrusor was reflex activated at rather small storage volumes, as could be measured through urodynamics by the detrusor pressure. In paraplegic 5, the detrusor was automatically activated at a bladder filling volume of approx. 350 ml, and in paraplegic 9 at approx. 10 ml. The reasons for the limited storage volumes are the increased afferent input from the bladder due to a bladder infection, for example, and the strong disorganization of the neuronal networks in the spinal cord. It was found for the paraplegics 7 and 11 that the first high bladder afferent activity occurred at bladder filling volumes in the operation, at which the detrusor was activated pre-operatively. In Para 7 the first high afferent input occurred at a filling volume of 150ml; the detrusor was activated at 160ml. In Para 11 the first comparatively high afferent input occurred at 50ml filling volume; the detrusor was activated at 80ml.
During surgery under light anaesthesia the detrusor never responded to the filling of the bladder in 120 patients. Also, quick filling with 4°C saline solution did not activate the parasympathetic division. Only in one instance, during surgery on a tetraplegic patient with a lesion sub C3 (artificial ventilation), with no tubes placed in the trachea and extremely light Propofol-anaesthesia, there seemed to be a slight detrusor pressure increase upon retrograde bladder filling. On the other hand, strong (painful) bladder catheter pulling activated the detrusor to contract in paraplegics with no anaesthesia, most likely activated the parasympathetic nervous system in the brain-dead human HT6 (no anaesthesia, possibly partial spinal shock) and seemed to activate the parasympathetic division during very light anaesthesia. It is concluded therefore that painful stimulation of the bladder under light anaesthesia activated the detrusor.
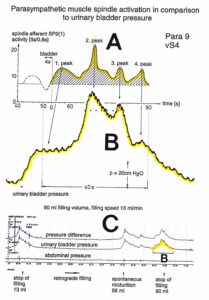
Figure 72: Comparison between changes in secondary muscle spindle afferent activity and detrusor pressure (measured before the surgery; for the implantation of an electrical sacral anterior root stimulator). Paraplegic 9. A. Activity changes of the afferent fiber SP2(1) from Figure 73B following bladder catheter pulling. Approx. mean activity level is represented by a dashed line at 7.5 IIs/0.8s ((APs -1)/0.8s). The activity above the mean is cross-hatched and is proposed to be due to parasympathetic activation. Root vS4. B. Detrusor pressure (pressure difference) changes taken from ‘C’. Note that changes in the detrusor pressure show almost exactly the same time course as do the activated changes of the secondary muscle spindle afferent fiber SP2(1) of ‘A’. Corresponding peaks are correlated by arrows. C. Abdominal pressure (measured as rectal pressure), intravesical pressure (urinary bladder pressure) and detrusor pressure (pressure difference) during retrograde filling before the surgery. One transient detrusor pressure increase, marked ‘B’, is used, after enlargement in ‘B’, to compare with the spindle afferent activity.
In Figure 72, the increase in detrusor pressure upon retrograde bladder filling before surgery is compared with the activity increase of the secondary muscle spindle afferent fiber SP2(1) following 4 times bladder catheter pulling during the operation. Figure 72A shows the undulating activity increase of a SP2(1) fiber. In Figure 72C the cystogram is shown. Upon bladder filling spontaneous micturition occurred several times. If parasympathetic fibers really activated muscle spindles, then the activity increase of the secondary muscle spindle afferent fibers following bladder catheter pulling may have a similar time course as does the bladder pressure increase due to detrusor activation following retrograde bladder filling. To check this similarity of time course, one undulating increase of detrusor pressure (Figure 72C) has been brought to the same time scale as the measured changes in muscle spindle afferent activity (Figure 72A) and transferred into Figure 72B for a direct comparison with Figure 72A. By comparing Figure 72A with Figure 72B, it can be seen that the occurrence of activity peaks of secondary spindle afferents is very similar in its time course to that of the peaks of the detrusor pressure. From the similarity of changes of spindle afferent activity and detrusor pressure (4 peaks) it can be concluded that some muscle spindles in the domain of the sacral parasympathetic nucleus are partly controlled by the parasympathetic division and that the muscle spindle and the detrusor activation have similar time courses. The other retrograde bladder filling induced undulating transient increases in pressure, shown in Figure 72C, had a similar time course.
Another correlation could be found between detrusor pressure and muscle spindle activation. The bladder pressure in paraplegic 5 increased steadily with no or only little undulations probably because the functions of the parasympathetic nervous system were still relatively preserved. The activity increase of a secondary muscle spindle afferent fibre in paraplegic 5 was also sustained and only little undulating.
Thus, there is some indication that some muscle spindles are partly driven by the parasympathetic division. The drive can be by parasympathetic fusimotor activity or more indirectly by somatic fusimotors (γ-motoneurons). Since in HT6, the somatic fusimotors did not change their activity levels strongly with the activation of preganglionic parasympathetic fibres and the activity increase of the secondary muscle spindle afferent fibre SP2(2) followed the transient increase of parasympathetic activity, it is likely that some muscle spindles are directly controlled by parasympathetic fusimotors. The direct control of some muscle spindles by parasympathetic fusimotors is supported by the slow activity decrease following very high activation [81] and the similarity with the time course of the detrusor pressure decrease following electrical stimulation of the preganglionic parasympathetic fibres during the surgery. The slow spindle afferent activity decrease would be difficult to explain by somatic fusimotor activation only. For further details see Chapter V of [1].
Pathologic organization of neuronal networks in the sacral micturition center following spinal cord injury
The detrusor-sphincter dyssynergia is analyzed now by comparing the natural impulse patterns of secondary muscle spindle afferents (SP2) contributing to continence and sphincter motoneurons in a brain-dead human with those in a patient with spinal cord injury (SCI). In Figure 72 it was shown that the changes of the SP2 fiber activity were similar to those of the detrusor pressure. We are therefore now in the position to measure indirectly the autonomic detrusor activation and compare it with the activity of the external bladder sphincter at the single neuron level.
In the brain-dead human the sphincter motoneurons, sub-serving continence, were inhibited at a time, when preganglionic autonomic efferents and a SP2 fiber increased their activity (physiologic). In the paraplegic the sphincter motoneurons were not inhibited (pathophysiologic). In the brain-dead human, an SP2 fiber showed doublet firing (interspike interval (II) 10 to 14ms) for low level parasympathetic activation and multi-ending regular firing for high parasympathetic activation. In one paraplegic with strong bladder dysfunction, the multi-ending regular firing was replaced by a repeated burst firing with a shortest II of 0.2ms (transmission frequency = 5000Hz) (see below). After SCI, the pathologic firing patterns of the SP2 fibers, the detrusor-sphincter dyscoordination, and the hyper-reflexia in paraplegics are most likely a result of neuronal network changes in the parasympathetic and somatic nervous system divisions of the sacral and pontine micturition centers. In every CNS injury, the phase and frequency coordination become impaired. In a paraplegic this is shown below by a comparison with the phase and frequency variability in a brain-dead human (Figures 87, 86). In the brain-dead the variability is small and in the paraplegic it is high. We shall now focus on the detrusor-sphincter dyssynergy.
Differences in the impulse patterns of secondary muscle spindle afferent fibres between paraplegics and the brain dead human HT6
The time course of the increase in the secondary muscle spindle afferent activity, induced by the sacral parasympathetic nervous system in muscle spindles contributing to continence, is very similar to that of detrusor pressure (Figure 72). The detrusor-sphincter dyssynergy is therefore analyzed by comparing the natural impulse patterns of single secondary muscle spindle afferents (SP2) and sphincter motoneurons in a brain-dead human (physiologic) with those in patients with spinal cord injury (pathologic). The parasympathetic nervous system was activated by painful bladder catheter pulling.
Figure 73 shows the impulse patterns of parasympathetically driven secondary muscle spindle afferents in paraplegic 9 and the brain-dead human HT6. In the brain-dead individual (Figure 73B), the activity was low before strong bladder catheter pulling and increased strongly continuously and regularly following stimulation. In paraplegic 9 (Figure 73A), the activity level was higher before bladder catheter pulling and increased more slowly, in an undulating and irregular manner following catheter pulling. The burst-like firing seen in paraplegic 9 did not occur in HT6. The doublet firing was less regular in Para 9. Specific properties in the drive of the spindle seem to be lost in the paraplegic. Even though a burst-like firing also occurred once in HT6 (Figure 73Ba), the afferent fiber in HT6 could generate a sustained high regular activity level, whereas the fiber in paraplegic 9 could not.
The increase in the detrusor pressure in paraplegic 9 during retrograde bladder filling was very pathologic since there was nearly no storage phase and the pressure increased transiently and in an undulating way (Figure 72C). It is satisfying that the results obtained with the single-nerve fiber AP recording method from impulse patterns of afferent and efferent fibers support the results obtained urodynamically. The impulse patterns however will provide additional insight into the organization of the human nervous system under physiologic and pathophysiologic conditions.
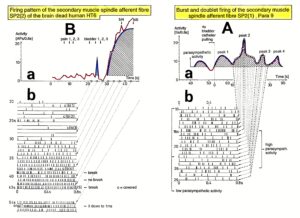
Figure 73: Comparison of the natural impulse patterns of single afferent fibers between paraplegic 9 with dyssynergia of the bladder (A) and the brain-dead human HT6 (B) with synergia of the bladder. Activity increase of the spindle afferent fibers following bladder catheter pulling (a) and the natural impulse patterns at different times following catheter pulling (b). A. Note that at activity peaks (a) there was burst firing in paraplegic 9, (marked by the arrows, 16s, 26s), and at low activity (a) there was no burst firing (10s, 23s). B. Note that even though burst firing appeared also in the brain-dead (25s), the activity increase sustained with similar interspike intervals (IIs). break = break of oscillation of sphincter motoneuron. c = covered = skin afferent action potentials covered SP2 fiber impulses due to bladder catheter pulling (bl 1 – bl 5).
Detrusor-sphincter synergy of the bladder in the brain-dead human HT6, and dyssynergy in paraplegic 9
The measurement of parasympathetic activation of the detrusor by activity changes of secondary muscle spindle afferent fibers (the spindle is innervated by autonomic fusimotors) allows an analysis of detrusor-sphincter dyssynergy using the natural simultaneous impulse patterns of secondary muscle spindle afferents and sphincter a2-motoneurons (and g-motoneurons).
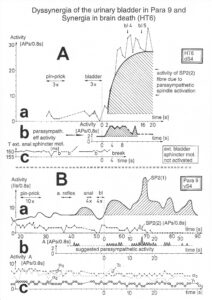
Figure 74: Direct comparison of secondary muscle spindle afferent activity and motoneuron activity between the brain-dead human HT6 with a synergia of the bladder (A) and the paraplegic 9 with a dyssynergia of the bladder (B). A. Simultaneous measurements of activities of secondary muscle spindle afferents (a), parasympathetic preganglionic motoneurons (b) and oscillatory firing (high activity mode) of a sphincter motoneuron innervating the striated anal sphincter (c). Note that with the transient activity increase of the parasympathetic fibers (b) the secondary muscle spindle afferent fiber increased strongly its activity (a) for minutes, and the oscillatory firing sphincter motoneuron discontinued its oscillation (c) to reduce strongly its activity. bladder 3x = 3 times bladder catheter pulling. T ext. anal sphincter mot. = oscillation period of the sphincter a2-motoneuron innervating the anal sphincter. For further details, see Chapter V of [1]. B. Simultaneous measurements of activity of secondary muscle spindle afferent fibers SP2(1) and SP2(2) following anal (anal 4x) and bladder catheter pulling (bl 4x) (a), and the activity changes of an a2-motoneuron (FR) and a3-motoneuron (S) and a dynamic fusimotor fiber (g1) (c). Note that following bladder catheter pulling (and probably parasympathetic activity increase), the spindle afferent fiber SP2(1) (most likely contributing to continence) increased its activity in an undulating manner (a), whereas the SP2(2) fiber did not (probably not connected to continence) (a), and the a-motoneurons did not reduce their activity (c). The dynamic fusimotor g1 transiently increased its activity similarly as in HT6 measurements. In similarity to ‘Ab’, the suggested parasympathetic activity increase is pictured (b). a. reflex = anal reflex stimulation. IIs = interspike intervals; IIs/0.8s = (APS – 1)/0.8s (the activity measures IIs/0.8s and APs/0.8s differ by ‘1’).
Figure 74A shows that in the brain-dead human HT6, whose parasympathetic preganglionic neurons increased activity (Figure 74Ab) upon bladder catheter pulling, the SP2(2) fiber activity increased strongly (Figure 74Aa), whereas the a2-motoneuron, innervating the external anal sphincter, discontinued its oscillatory firing (Figure 74Ac), which is a measure for a strong activity decrease. An a2-motoneuron, innervating the external (striated) bladder sphincter, was not activated. This means that with the activation of the detrusor, the sphincter motoneurons were relaxed by inhibition. Thus, the brain-dead human HT6 had a detrusor-sphincter synergy of the bladder.
In the paraplegic 9, the SP2(1) fiber showed no strong activity increase and there was no sphincter relaxation following bladder catheter pulling (Figure 74B). The secondary muscle spindle afferent fiber SP2(1) increased its activity in an undulating manner (Figure 74Ba). The parasympathetic fusimotors, driving the muscle spindle, innervated by the SP2(1) fiber, probably were not continuously active as suggested by Figure 74Bb, in contrast to the parasympathetic activity observed in the HT6 (Figure 74Ab). The other secondary muscle spindle afferent fiber in paraplegic 9 (SP2(2), Figure 74Ba) slowly reduced its activity upon bladder catheter pulling. This spindle afferent fiber was not connected to the continence of the bladder. It is likely that its spindle was not parasympathetically innervated and was sited in leg muscles or parts of the pelvic floor muscles not contributing to continence. The a2 and a3-motoneurons (Figure 74Bc) showed a high activation, which is expressed in their oscillatory firing, and probably contributed to the continence of the bladder and the rectum. These likely sphincter motoneurons did not reduce their activity following parasympathetic activation, as can be seen from the SP2(1) fiber activity, monitoring parasympathetic activity. These motoneurons were not inhibited and the external sphincters were probably not relaxed. The somatic fusimotor g1 (Figure 74Bc) increased its activity transiently and slightly upon painful bladder catheter pulling, in similarity to a g1 fiber in HT6 [34]. The measurements in paraplegic 9 indicate a loss of the inhibitory action of the detrusor onto the sphincter motoneurons.
The time constant for the activity decrease of a spindle afferent fiber following parasympathetic activation was 31s in a paraplegic and approx. 40s in a brain-dead human (Chapter V of [1]). It is concluded that the muscle spindles are unchanged following spinal cord injury. The pathologic firing patterns of the SP2 fibers are thus probably a result of neuronal network changes in the parasympathetic and somatic nervous system divisions of the sacral micturition center.
In conclusion, in the brain-dead human the sphincter motoneurons sub-serving continence were inhibited at a time, when preganglionic parasympathetic efferents increased their activity (physiologic) for 10s and an SP2 fiber increased its activity for several minutes. In the paraplegic with a strong bladder dysfunction, the SP2 fiber activity increased, due to parasympathetic activation, lasted for approx. one minute, showed undulations, and its amplitude was smaller than that measured in the brain-dead human. There was therefore no powerful continuous parasympathetic activation and the sphincter motoneurons were not inhibited (pathologic).
Neural network repair for improving bladder functioning
To reveal possible reasons for the loss of inhibition of sphincter motoneurons, simultaneous afferent and efferent impulse patterns were analyzed. In Figure 74, the impulse patterns of HT6 and paraplegic 9 were directly compared. In the HT6, the secondary muscle spindle afferent fiber SP2(2) showed an impulse pattern in which the interspike intervals (IIs) were rather similar (Figure 73Bb). Irregularities in the pattern occurred only when the number of recruited encoding sites changed. In paraplegic 9, the activity of the SP2(1) fiber was lower (Figure 73Ab) and less regular. Probably this reduced activity in the SP2(1) fiber was due to a reduced parasympathetic activation.
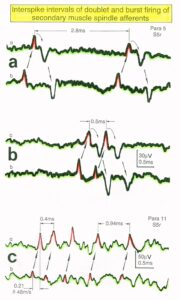
Figure 75: Doublet and burst firing of secondary muscle spindle afferent fibers. Recording a, b from paraplegic 5 and c from paraplegic 11. Interspike intervals (IIs) are indicated. In “c,” the single afferent fiber conducted with 48 m/s and the interspike interval increased from 0.4 to 0.94ms. The single-spindle afferent fiber in “a” and “b” is uniquely marked by performing a loop, which is demonstrated by an efferent-like AP time-locked to the afferent AP (see also Figure 11A).
As observed in the brain-dead individual, the low activity level of doublet firing (Figure 75a) and the high activity level multi-ending regular firing (Figure 73Bb) probably represent physiologic parasympathetic activation of secondary muscle spindle afferent fibers with the coordinated firing of several endings of the parent spindle afferent fiber. In paraplegics with detrusor-sphincter dyssynergia and spasticity, these firing patterns were changed and mixed. In paraplegic 5 with a slight bladder dysfunction, the multi-ending regular firing was somehow preserved. In paraplegics 9 and 11 with strong bladder dysfunctions, the multi-ending regular firing was replaced by a burst-like firing (Figure 75c). If one assumes that on average the spindles are activated in parallel with the activity of the preganglionic parasympathetic neurons, as suggested in Figures 74Bb, then the parasympathetic nucleus in the sacral micturition center seems to be unable to develop a sustained activation anymore. For further details see [1].
In conclusion, following SCI the neural networks of the sacral and pontine micturition centers and their connections are functionally damaged and have to be repaired in similarity to the repair of motor functions where the intrinsic apparatus of the spinal cord is damaged. Spasticity has to be reduced and movement performance improved by improving the functions of the intumescentia lumbo-sacralis and supra-spinal centers and their connections. Neural repair means, the networks have to be repaired.
The research performed above, to clarify human urinary bladder functions under physiologic and pathologic conditions, is the level to be performed the Author expects from human brain research, where plenty of money is going to in USA and EU. Human research at the neuron level is also needed. With the clinical diagnostics as for example electroencephalography (EEG), magnetic resonance imaging (MRI) and positron emissions tomography (PET) one will never find out how the human brain is really functioning. For sure, human electrophysiology is needed for finding out how the human brain is functioning. It is impossible to understand why the discipline electrophysiology is world-wide not organized and used any more. The neural networks of the human brain are mainly functioning on the basis of voltages and currents and for measuring them, electrical recordings are needed.
Functional repair stage of the urinary bladder in the SCI patient Nefeli
Voiding is a voluntary act which results in sustained contraction of the bladder (detrusor) and relaxation of the urethra with its internal and external sphincters until the bladder is empty. To enable fluid flow along the urethra it is necessary that the pressure in the urinary bladder exceeds that within the urethra lumen. Under normal circumstances, in order to initiate micturition, a fall in urethral pressure immediately precedes a rise in pressure within the lumen of the bladder. Usually, this pressure rise is produced by active contraction of detrusor smooth muscle at the onset of micturition.
With standard urodynamic recordings, the functional state of the bladder can be evaluated. The bladder is filled retrograde and the bladder pressure and the electromyographic (EMG) activity of the pelvic floor (for function of the external sphincter) are recorded. Such a recording of bladder pressure and sphincter activity is given here for a bladder dyssynergia.
In Figure 76 a typical detrusor-sphincteric dyssynergia of the urinary bladder is demonstrated in a patient with a spinal cord injury sub TH12 present for 2 years. Upon retrograde bladder filling, the intravesical pressure increased. At a filling volume of 350 ml, the detrusor contracted automatically in response to bladder distension. The (surface) EMG activity of the pelvic floor increased at a time when the detrusor pressure increased. If the external anal and the external (striated) bladder sphincters were activated at the same time, and this probably was the case, then the external bladder was activated at approximately the same time as the detrusor. This co-activation is called detrusor-sphincter dyssynergia of the bladder, since in normal humans the external bladder sphincter is relaxed (inhibited) upon the activation of the detrusor, so that urine can pass through the urethra under low pressure.
The loss of the sphincter relaxation during detrusor contraction results in a high bladder pressure during voiding. A high bladder pressure will result in a detrusor hypertrophy with an increased bladder stiffness, as estimated from the reduced compliance (and, possibly, an increased bladder infection rate). In addition, there probably will be an increase of stretch (S1) and tension (ST) and pain receptor afferent activity of the bladder wall resulting in a too early activation of the detrusor in response to it. The storage capacity of the bladder may thus become reduced to 50 ml or even less. Further, the increased intravesical pressure may cause ureteric reflux, and, due to infections, progressive renal impairment.
Upon further filling of the bladder (Figure 76) and a stoppage of the filling, movement artifacts indicate an occurrence of spasticity of the lower body. The avoidance of the co-activation of the detrusor, the pelvic floor and the striated bladder sphincter is an important clinical problem (continence) as well as an interesting scientific problem as analyzed above.
Figure 76: Standard urodynamic recording including intravesical pressure (urinary bladder pressure), abdominal pressure, detrusor pressure (pressure difference) and pelvic floor electromyography (EMG). Detrusor pressure is that proportion of the intravesical pressure which is produced by active and passive bladder wall properties. Detrusor pressure is calculated by electronically subtracting the value of abdominal pressure from that of intravesical pressure. This derivation is extremely important in order to be able to distinguish intrinsic and extrinsic contributions to intravesical pressure. Upon retrograde bladder filling the detrusor started to contract at approx. 350 ml. Abdominal pressure is the pressure around the bladder and this is measured as rectal pressure.
Since in the SCI patient Nefeli (Figure 1) only the pressure of the bladder was recorded with retrograde bladder filling (uro-water-cystometry), the diagnostic information obtained was limited. The normal desire to void occurred at 67ml water filling, the strong desire to void at 68ml and the urgency to void at 69ml. The residual urine was 10ml. These urodynamic measurements were in accordance with the fluid measurements performed at home. The time period from the desire to void till to the automatic micturition was only 20s in accordance with the small bladder volume increase from the normal desire to void to the urgency to void (69ml-67ml = 2ml). The measured fluid at home was between 50 and 60ml. Taking into account the 10ml residual urine, Nefeli had to go to toilet at bladder filling volumes between 60 and 70ml.
The bladder repair problem to be solved was to increase the bladder filling volume till to the desire to void (Figure 42) and to increase the bladder filling volume difference from the first desire to void till to the urgency to void and of course to reduce the number of bladder infections.
Through a few years of CDT, Nefeli had no bladder infections anymore and the filling volume reached 100ml. Because Nefeli had an incomplete spinal cord injury, she had a synergy of the bladder; that means with the detrusor contraction the sphincters relax and fluid could leave the bladder.
It should be emphasized, the biggest problem in SCI is the urinary bladder functioning, followed by the sexual function and the walking.
The therapy task to be solved is how to achieve this bladder functioning improvement by movement-based learning. Especially the desire to void has to occur at a larger bladder storage volume and the external bladder sphincter has to become more powerful to keep the continence. The healthy sister of similar size has a bladder storage volume of between 140 and 240ml till to the first desire to void. This means, more CDT treatment would be needed for Nefeli to increase the bladder storage volume.
Nefeli’s pathologic bladder problem is similar to that of elderly. Their bladders have a too small storage volume so that they have to go too often to the toilet and the time from the first desire to void till the micturition starts automatically is too short. The power to keep the external sphincter closed when the detrusor starts to contract and the internal sphincter opens, is too small. Such elderly persons get easily incontinent. In elderly, this slight pathologic dysfunction is partly caused by arteriosclerosis of the thick spinal cord feeding artery (artery of Adamkiewicz) (Figure 41B), which becomes extremely arteriosclerotic in elderly [41]. Also, the loss of nerve cells in aging will contribute to an increase of transient incontinence, because the neural control of the bladder is very delicate and vulnerable. At an age of 11 Nefeli will have no problems with arteriosclerosis and nerve cell death. Her dysfunction was caused by the injured spinal cord tracts and the impaired communication between the sacral and the pontine micturition center. To improve continence in elderly it may help to exercise on the special CDT device to improve the coordinated neural network organization of the sacral and pontine micturition centers.
The strategy to repair bladder functions by learning transfer is to change the efficiencies of the bladder receptors and sphincter motoneurons, to enhance the regeneration of afferent and efferent spinal cord tract fibers and to enhance the usage of other pathways especially those ones outside the cord, especially those ones connecting the different plexuses (Figure 21, yellow). But not only the neural control system of the bladder has to be repaired. Also, the bladder structure has to be improved as measured for example by the compliance. Too small filling volumes make the bladder shrinking, which has to be avoided as much as possible. The bladder has to be enlarged again. Nefeli had at this stage of repair only seldom bladder infections. Otherwise, there would be an increasing risk that bacteria would ruin the bladder epithelioma and the antibiotics may not have sufficient access any more to the bacteria.
The movements to be performed to induce learning transfer for bladder repair are mainly the exercising on the special CDT device and the jumping. The improvement of phase and frequency coordination when exercising on the special CDT device will improve the neural network organization of the sacral and pontine micturition centers and their communication. The jumping will activate the stretch (S1) and tension receptors (ST), the receptors responding to fluid movement (S2) and the mechanoreceptors (M, P) of the urinary bladder (Figure 33). This repeated physiologic input when jumping will help to repair the neural networks of the sacral and pontine micturition centers.
The emptying of the rectum (Figure 64) was mainly daily and rather normal in Nefeli. She felt gas in the rectum but could not always control it. Bowel pain before or after eating could be stopped by exercising on the special CDT device by less than one hour.
Yates [82] has constructively criticized the quality of research in the area of human urinary bladder physiology. In particular, he has recommended that investigators should look at the methods being used in parallel disciplines.
Continence repairs through neural network learning – a step into the neural network organization of the sacral micturition center
Natural firing patterns of proprioceptive afferents and a and g-motoneurons measured simultaneously, and the phase (and frequency) relations between them
Original recordings of the firing of a-motoneurons (oscillatory), proprioceptive and bladder afferents are shown in Figures 3 and 4. Due to catheters positioned in the anal canal and urethra, continence pattern states are activated. These continence states of the CNS are secured by the ongoing communication with the bladder and anal canal via the fibres in the S2-S5 nerve roots. Touch, pinprick, anal and bladder catheter pulling, and filling of the continence organs will change the natural impulse traffic in those fibers.
Figure 15 shows such schematic natural simultaneous impulse patterns of a static and a dynamic g-motoneuron, two secondary muscle spindle afferents and an oscillatory firing a2-motoneuron O2 in a dorsal S4 nerve root (there are afferents and efferents in dorsal or ventral lower sacral roots) during continence pattern changes. The small arrows and the dotted lines indicate existing relative phase coordination’s between the static and the dynamic g-motoneurons and motoneuron O2, and between g-motoneurons and secondary muscle spindle afferents. The dashed-circle line indicates a phase relation between the APs of the static g-motoneuron (g1) and the cross-correlation between SP2(2)-fiber (single ending one of the mother fiber) and SP2(5)-muscle spindle afferent fiber.
Including the phase relations between the firings of secondary muscle spindle afferents and the oscillatory firing motoneuron O2, we obtain interlaced loops of coordination’s between the firings of g-motoneurons and secondary muscle spindle afferents, and between secondary spindle afferents and a-motoneurons and between a-motoneurons and g-motoneurons (co-activity of a and g-motoneurons) (Figure 83). It becomes obvious from the correlations between the natural impulse patterns (including those of single encoding sites of spindle afferents) that the g-loop is not a single loop, but a network of loops, because of the divergent projections of g-motoneurons onto muscle spindles and the probably divergent and convergent projections of secondary muscle spindle afferents into the neuronal network of the spinal cord, consisting of a and g-motoneurons and interneurons.
More general, phase and frequency coordination can be seen among the natural firing patterns in the afferent and efferent fibres; this means that upstream in the CNS, there should also be phase and frequency coordination among neuron firing. More precise measurements will be presented below. Two phase relations have been observed to occur mostly between the APs of the secondary muscle spindle afferents and the oscillatory firing motoneuron per one oscillation period (Figures 81, 82, 84) (for somatic activation) in accordance with the “in phase” and “anti-phase” jumping on springboard and crawling. With this coordinated natural impulse traffic to and from the periphery, the change of integrative pattern states can also partly be understood from bladder to movement states, as will be shown.
Response of an oscillatory firing α-motoneuron to touch and painful pinprick stimulation (Neuronal network pattern change)
I will show now that a small change in the natural impulse patterns travelling into the sacral micturition centre will change there the neuronal network pattern organization. Ten schematically drawn sweep pieces of simultaneous impulse patterns of a2, a3 and g1-motoneurons and the secondary muscle spindle afferent fibres SP2(1) and SP2(2) are shown in Figure 77. Following a sequence of ten touch stimulations (Figure 77, upper right insert), the rhythmically firing motoneurons responded by a change in the duration of the oscillation period. Of interest is here how fast the a3-motoneuron reduced its oscillation period following the start of the touch stimulation (measured electrically) or following the volley of skin-afferent APs running through the ventral root skin afferent fibres. The delay of the first reduction of the oscillation period is defined in Figure 77 following touch 7. Beginning and end of the stimulations are marked. There is a possibility that the a3-motoneuron (S) is actually an a2-motoneuron (FR), and the a2 is an a3-motoneuron. Because of the pathologic oscillatory firing, the type of the motoneuron can no longer be safely identified from the firing pattern, and identification on the basis of conduction velocity is not safe as the distributions of conduction velocities of a2 and a3-motoneurons (and of other nerve fibre groups) overlap.
It can be seen in Figure 77 that the a3-motoneuron mostly reduced its oscillation period after a delay (measured from the beginning of the afferent volley) shorter than the duration of the oscillation period. The first reduction of the oscillation period is marked by a small arrow. The response times are analyzed quantitatively in Figure 78. Following touches 6 and 7, a partial transient synchronization of the firings of afferents and efferents occurred, as can be seen by the similar occurrence times of the impulses. Also, a slight partial synchronization followed touches 2 and 3. Since on the average, the delay in the shortening of the oscillation period occurred after a time interval shorter than the oscillation period, it is of interest to see whether the response times were similar to those for pinpricking. It has been established in humans that the difference between touch and pinprick stimulation is that for pinpricking, additional pain fibers are activated (Figure 5), and pain fiber activity may facilitate different pathways to the motoneural networks.
The touch sites 1, 2, 6 and 7 were inside the anal reflex area and the sites 4, 5, 9 and 10 were outside of it. The anal reflex area extended approx. 6 cm laterally from the anus.
After touching experiments with the touch stimulation at pen-marked sites 1 to 10 close to the anus (Figure 77), the touching sites were now pinpricked in a similar fashion approx. every 0.8s to 1s. After a delay, there again was a reduction in the duration of the oscillation periods.
The responses to pinpricking differed from those following touch stimulation in three aspects. Firstly, the response delay times were longer than the oscillation periods.
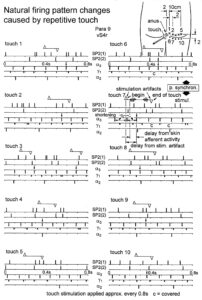
Figure 77: Changes of natural impulse patterns of a2, a3 and g-motoneurons and secondary muscle spindle afferents (SP2(1) and SP2(2)) in paraplegic 9 upon touching sites 1 to 5 (x) and 6 to 10 (·) (insert). The small arrows indicate the first shortening of the oscillation period of the a3-motoneuron following the beginning of the touch stimulation (marked by an artificially generated artifact). Note that partial synchronization (p. synchron.) of the firing of the a2 and a3-motoneurons and secondary muscle spindle afferents occurred upon touching sites 6,7. The a2 and a3-motoneurons were only identified by their conduction velocities; it is therefore possible that the a3-motoneuron was actually an a2-motoneuron and the a2-motoneuron an a3-motoneuron (overlap of velocity distributions); the firing patterns were very pathologic and could not be used for the motoneuron identification.
Secondly, the response to pain application had a longer duration and was stronger; mostly, the duration of two oscillation periods was reduced following pinprick stimulation and the reduction of the duration was larger. Following pinpricks 3, 7 and 8, a very short oscillation period occurred, which resembled a resetting of the oscillation cycle. Thirdly, the transient synchronizations of afferents and efferents following pinpricks 6 were more pronounced.
Latency difference in the reduction of the duration of the oscillation period following touch and pinprick stimulation
It can be seen from Figure 78A that in nine out of ten cases, the latency of the oscillation period shortening following touch stimulation of the perianal skin (see insert in Figure 77) was shorter in duration than the oscillation period (»100ms), represented by the dotted line. The shortest latency was approx. 10ms, when measuring from the afferent volley running in direction of the spinal cord. The latency of the first reduction of the oscillation period, measured from the skin touch stimulation artefact, was longer or shorter than the duration of the oscillation period (Figure 78B). The shortest latency was approx. 30ms. The difference of the latency, i.e., the time from the stimulation artefact (touching of the skin) to the moment when the skin afferent volley passed the recording electrodes, was approx. 20ms. According to the group conduction velocities of skin afferents (T1 skin afferents (PC) » 44 m/s at 36 °C), most of the time was lost in the touch receptors and the thin nerve fibres connected to them. With a conduction distance of 0.3 m, a conduction time of tcond = s/v = 300 mm/ 44 mm(ms)-1 = 7ms results. The latency until the reduction of the first oscillation period duration following pinpricking the sites 1 to 10 was mostly longer than the oscillation period itself, independent of whether measured from the skin afferent volley (Figure 78E) or from the stimulation artefact (Figure 78F). Since not every pinprick was strong enough to generate pain (stimulation of pain receptors), it is concluded that the latency from the pinpricking to the first reduction of the oscillation period was longer than the oscillation period. By comparing the latencies to the first reduction of the oscillation period following repetitive touch and pinprick stimulation of sites 1 to 10, it turns out that following touch stimulation the delay is shorter than the oscillation period, whereas it is longer following pinprick stimulation. It seems therefore that touching reinforced the sustained stretch reflex of the anal sphincter while pinprick did not.
The first shortening of the oscillation period in comparison to two preceding periods is plotted in Figure 78D, C (touch) and in Figure 78H, G (pinprick). The reduction of the oscillation period for touch stimulation was between 8 and 28ms and between 5 and 40ms for pinpricking. The pinprick stimulation generated a larger transient reduction of the oscillation period. When correlating the latency of shortening to the extent of shortening for pinpricking (Figure 78H), it seems as if the shorter latency correlated with a smaller reduction of the oscillation period (more touch-like), and the long latencies correlated with the greater reduction of the oscillation period (more pain-like). A similar relation was not found following touch stimulation (Figure 78D). It is most likely that the painful pinprick stimulation transiently replaced the sustained stretch reflex of the anal sphincter by a protective reaction of the anal sphincter against pain application; the time needed for the reorganization of the oscillatory firing neuronal network was at least one oscillation period long.
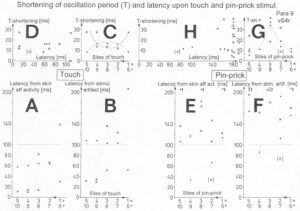
Figure 78: Shortening of the oscillation period T of the a3-motoneuron (perhaps an a2-motoneuron upon touch and pinprick stimulation). A, B. Latency of the shortening of the oscillation period (for definition, see Figure 77) measured from the skin afferent activity (A) and from the stimulation artifact (B) with successive touching of sites 1 to 5 (x) and 6 to 10 (·) as indicated in Figure 3. Note that the delay is often shorter than the oscillation period. D, C. Shortening of the oscillation period (T-shortening), with respect to two preceding oscillation periods, in relation to the latency of shortening (D) and in relation to successive touching of sites 1 to 10 (C). E.-H. Same description as for A-D, only with respect to pinpricking. Note that touch stimulation induced a shorter latency for the reduction of the oscillation period than did pinpricking.
The relation between the sites of pinprick (Figure 78G) or touch stimulation (Figure 78C) and the oscillation period shortening shows no clear correlation. The dotted and the dashed lines may show similar latencies. Perhaps the protective reaction against pinpricking of the anal canal and the pelvic floor is highest at sites 3 and 8 at the border of the anal reflex region.
Time needed for the change from the attractor state “continence” to the attractor state “protection”
By comparing the latencies from the moment when the skin afferent volley passed the recording electrodes to the first reduction of the oscillation period following repetitive touch and pinprick stimulation of the sites 1 to 10, it turns out that the delay following touch stimulation (≈ 10ms) is much shorter than for pinprick stimulation (≈ 110ms). It seems therefore that touching reinforced the sustained stretch reflex of the anal sphincter while pinprick stimulated the protection reaction against pinprick (pain). It can be evaluated how much time is needed for the reinforcement of the sustained stretch reflex and for the pattern change from the continence state (sustained stretch reflex) to the protection reaction. With a conduction distance of 0.1 m (distance between the recording electrodes and spinal cord), a conduction time for the fastest skin afferents (group conduction velocity of T1 skin afferents (Parcinian Corpuscle) » 44 m/s at 36 °C) of tcond = s/v = 100 mm/ 44 mm(ms)-1 = 2.3ms is resulting. The conduction time of the action potentials of the motoneurons to cover the distance from the spinal cord to the recording electrodes is resulting also to approximately 2.3ms (the group conduction velocities of the α2 and α3-motoneurons are 37 and 50m/s, respectively).
The processing time to reinforce the continence pattern of the anal sphincter was therefore approximately 5.4ms (10ms-4.6ms = 5.4ms). If we assume a synaptic transmission time of 2.5ms, the processing time in the spinal cord is sufficient for the transmission time of two synapses. The reinforcement of the sustained stretch reflex was may be just a di-synaptic pathway.
The conduction times to and from the spinal cord of the protection reaction are approximately 10ms (7.7 + 2.3), because the fastest pain afferent action potentials needed a bit more time than the touch afferent action potentials (group conduction velocity of the fastest pain afferents = 13m/s; tcond = s/v = 100 mm/ 13 mm(ms)-1 = 7.7ms). The processing time in the CNS to change from the sustained continence pattern to the protection reaction pattern is therefore approximately 100ms. This time would be enough to involve 40 synapse transmissions. For a reorganization of the neuronal networks for pattern change, conduction times of intraspinal axons and dendrites would also have to be taken into account. But it is obvious that the pain application induced a completely different CNS response.
That pinpricking resulted in a longer response time of motoneurons than touching of the perianal skin is not obvious and cannot be explained based on the reflex theory. Being a stronger stimulus, pinpricking (touch plus pain afferent activity, Figure 5) would be expected to have the same or slightly shorter rather than a much longer response time than touching of the perianal skin (touch afferent activity only, Figure 5).
It is concluded that the change from one network state to another one, restricted to the spinal cord (measurement in a paraplegic), needed approximately 100ms.
With respect to movement learning and learning transfer, strong pain has to be avoided during exercising, because a certain pattern (like jumping on springboard) has to be trained (and neuronal networks entrained) and not a mixture of a movement pattern and a protection reaction.
Attractor layouts for jumping on springboard and switching between continence and protection
For jumping on springboard (Figure 79Aa), the equation of motion of the collective variable φ (angle between the legs) could be solved in the HKB model (Figure 22) and an attractor layout constructed for the jumping in in-phase and in anti-phase (Figure 79Ab). In analogy, an attractor layout for bladder continence and protection reaction (Figure 79Bb) can be drawn, even though the anal continence was measured. The enhancement of the continence safety (deepening of the potential well) needed approximately 6ms, and the change from the pattern continence to protection reaction needed approximately 100ms.
It is therefore possible to analyze integrative patterns of the human CNS with natural impulse pattern of single-nerve fibers recorded from sacral nerve roots. Natural impulse pattern can explain integrative functions of the human CNS.
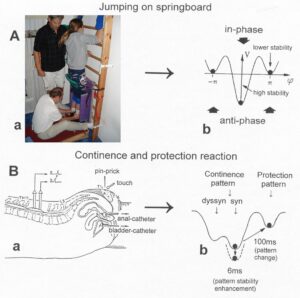
Figure 79: Attractor layout for jumping on springboard in comparison to that of continence and protection.
Relative phase and frequency coordination between the APs of α and γ-motoneurons and secondary muscle spindle afferents with no additional stimulation and upon touch, pinprick, and bladder catheter pulling
Above, it was shown that natural impulse pattern can explain pattern change, which means the generation of integrative patterns can partly be understood at the single neuron level. Natural impulse patterns were related to integrative CNS organizations. How can this aid in understanding why jumping on springboard contributes substantially to the repair of bladder functioning? A better understanding can be achieved to go deeper into the complexity of the cooperative and competitive interplay among neurons that means going deeper into the complexity of phase and frequency coordination of CNS self-organization. Changes of natural firing patterns have roughly been shown in Figure 15. The phase and frequency coordination of coordinated firing will now be analyzed more in detail.
Time correlations of afferent and efferent impulse patterns are easy to detect in the brain-dead individual as the oscillatory firing of a2-motoneuron (O2) was regularly like an inner clock (Figures 3, 4).
Figure 80: Measurement ranges and definitions of phases for the analysis of phase and frequency coordination’s between motoneurons and spindle afferents. Coordination (synchronization) between firing patterns can directly be seen in B. (A) Activity level of secondary muscle spindle afferent fibre SP2(1) in dependence on time. 10x touch = touching sites 1 to 10 shown in B; t. 5-6 = touching alongside the skin from site 5 to site 6; 10x pinprick = pinpricking sites 1 to 10; anal reflex = anal reflex stimulation; 4x anal = fourfold anal catheter pulling; 4x bladder = fourfold bladder catheter pulling; peak 1- peak 2 – 3 = first, second and third peak of spindle afferent activity due to parasympathetic activation; p. synchro = partial synchronization; syn = synchronization of a and g-motoneurons and secondary muscle spindle afferents. Note the synchronization of the firing patterns following pinprick 6 inside the anal reflex area. (B) A set of single impulse patterns of secondary muscle spindle afferents (SP2(1,2)) and a and g (intrafusal)-motoneurons and sites of stimulation. The small arrows in the impulse pattern of a3-motoneuron (S) point to a shortening of the oscillation period following pinprick 6 (pp6). The triangles indicate the beginning and the end of pinpricking. (C)(g,h,i) Definitions of the phases between the different motoneurons and spindle afferents in two sets of impulse patterns (g), and the corresponding sets of phase relation distributions (h,i). Para 9; vS4.
The phases of fusimotors and spindle afferent APs could be defined with respect to the impulses of that inner clock. In the paraplegic, the rhythmic firing was rather irregular. The motoneuron firing therefore cannot be used anymore as a time reference basis. More phases between the extracellular action potentials of the different fibers are necessary to fully describe the correlation between the simultaneous impulse patterns. In Figure 80Cg, the mutual phases between the APs of the different fibers are defined. Figure 80Ch,i shows the corresponding phase distribution histograms. Since too few phases occurred in a sweep piece of 0.8 s duration, phases occurring in certain time intervals were pooled and plotted in Figures 81 and 82.
In this special pathologic case, the a2 and a3-motoneurons fired rhythmically with impulse trains consisting of one action potential (AP), in contrast to the physiologic firing patterns [69], in which a2 and a3-motoneurons fire with impulse trains consisting of more than one AP (Figure 12). The identification of motoneurons by conduction velocity is not absolutely safe since group conduction velocity ranges overlap. It is very unlikely, nevertheless, that one of the motoneurons was an a1-motoneuron (FF) (missing high conduction velocity and high AP amplitude and no correlation to primary spindle afferent fibres), even though they fire physiologically with impulse trains consisting of one AP. It has been shown that oscillatory firing a1-motoneurons are mainly driven by time locked primary spindle afferent fibres [4, 87]. The firing patterns of the a2 and a3-motoneurons are strongly pathologic with respect to the length of the oscillation period [69, 86] and the impulse train length so that it is impossible in this paraplegic to identify the kind of motoneurons by their discharge patterns of oscillatory firing; this would be possible were the neuronal network driving the motoneurons to fire in a physiologic manner.
In Figure 81, the interspike intervals (IIs) and the phases are shown for similar time intervals. Before stimulation, within the time interval 1-6 s, the a3-motoneuron fired every 100ms, the g1-motoneuron every 100 to 130ms, and the SP2(1) fibre every 80 to 150ms (Figure 81Aa).
The a2-motoneuron mostly fired every 300ms and the SP2(2) fibre every 250ms. At that particular time interval, similar phases (phase relation of broad peak type) occurred twice per a3-oscillation period between the APs of the a3 and g1 axon between the g1 and the SP2(1) fibres, and between the a3 and the SP2(1) fibres (Figure 11Ba). One phase relation occurred between the impulses of the a3 and a2-motoneurons, and two between the a3 and the SP2(2) fibres. The broad phase relations between discharge patterns are interpreted as interactions between populations of neurons. Following different stimulations, interspike interval (II) distributions and phase relations changed with time.
Upon touching sites 1 – 5, the IIs of the almost oscillatory firing g1-motoneuron reduced in size to be more similar to those of the oscillatory firing a3-motoneuron (Figure 81Ab). The changing of the different phase relations indicates changes in the interactions between neuronal subnetworks (Figure 81Bb). Upon touching sites 6 to10 (especially sites 6, and 7 (inside anal reflex area)), the IIs of the almost oscillatory firing g1-motoneuron increased again (Figure 81Ac). A transient partial synchronization occurred between the different nerve fibres (Figures 81Bc, 84c). Upon pinpricking sites 1 – 5, the IIs of the almost oscillatory firing g1-motoneuron reduced again to have a similar II distribution as the a3-motoneuron.
Figure 81: Relative phase and frequency coordination between a and g-motoneurons and secondary muscle spindle afferents due to touching and pinpricking sacral dermatomes as in Figure 5G. A. Interspike interval distribution of spindle afferents SP2(1) and SP2(2), a2 (FR) and a3-motoneurons (S) and the dynamic fusimotor g1 for different time intervals upon touch, pinprick and anal catheter pulling. Interspike intervals (IIs) were collected from several sweeps of 0.8 s duration per second. External loop generation and frequency coordination of a and g-motoneurons and secondary muscle spindle afferents are marked by the semi-circle and the full circle. The large arrows point to the increase and decrease of the mean II of the distribution. Unsafe identification of a2 and a3-motoneurons (or vice versa) because of loss of specific oscillator properties. B. Histograms of the phases between afferent and efferent fibres for the time intervals indicated, upon different stimulation. Phases were collected from several sweeps of 0.8 s duration per second. The small arrows indicate phase relations. Phase coordination is indicated in a,e. Para 9; vS4.
The a3-motoneuron even slightly increased its IIs (decrease of activity), so that the II distribution of the oscillatory firing a3-motoneuron and the now oscillatory firing g1-motoneuron became very similar (Figure 81Ad).
Upon pinpricking sites 6 and 7 (inside the anal reflex area), a and g-motoneurons and secondary muscle spindle afferents showed similar II distributions (Figure 81Ae). Only one phase relation was organized per oscillation cycle between the different nerve fibres (Figures 81Be, Figure 84e). A synchronization between the APs of the different nerve fibres occurred as can be seen from the direct impulse patterns (Figure 80B). The occurrence of similar II distributions of, and transient constant phases between, the APs of the a3, g1 and SP2(1) fibres are interpreted in the way that, in its oscillatory firing the oscillatory firing, a3-motoneuron built up an external loop to the muscle spindle innervated by the g1 and SP2(1) fibres. The g-loop became integrated into the oscillatory firing of the a3-motoneuronal network. Before pinpricking, the g-loop, consisting partly of the g1 and SP2(1) fibres, also contributed to the oscillatory firing, since on the average there existed phase relations. With the pinpricking, however, also the II distributions assimilated, so that this g-loop was directly included into the oscillatory firing of the a3-network rather than only contributing to the drive of it. The building up of an external loop to the periphery by spinal oscillators is substantially used when a patient with a spinal cord injury is jumping on springboard (see Figure 85, below). Upon pinpricking sites 8, 9 and 10 (outside of the anal reflex area) and following pinpricking of site 10, the II distribution of the SP2(1) fibre shifted away from those of the a3 and g1 axon. The oscillatory firing a3-motoneuronal network had abolished its external loop, even though still getting drive from it. Upon anal reflex stimulation and catheter pulling, the external loop was not built up again (Figure 82).
Figure 82: Interspike intervals (IIs) and phase relations for time intervals indicated in Figure 80A. For legend, see Figure 81.
Following touch, pinprick and anal reflex stimulation, but not painful catheter pulling, mostly two-phase relations existed in paraplegic 9 between the activity of the a3, g1 and SP2(1) fibres per oscillation period (100-140 ms) of the a3-motoneuron (Figures 81, 82), but the phase relations changed with ongoing time. With the activation of the parasympathetic division, upon bladder catheter pulling (Figure 80A), three phase relations occurred per a3-motoneuron oscillation period (Figure 82Bc,e). At the peaks of parasympathetic activation, three phase relations occurred (Figure 82Bc,e), and only two phase relations were present with little parasympathetic activation (times between the peaks 1 and 2) (Figures 80A, 82Bd). Even though the functional units, consisting of fusimotor and a-motoneuron neuronal networks and spindle afferents fibres, were rather unstable in paraplegic 9, in comparison to the brain-dead human (see below), an important difference between skin (somatic) and bladder (parasympathetic) stimulation occurred. Another (third) phase relation per a3-motoneuron oscillation period occurred with the activation of the parasympathetic division. The activated parasympathetic neuronal network of the sacral micturition and defecation centre seems to have channelled input to the oscillatory firing somatic neuronal network.
Phase relation changes between the action potentials of the a and g-motoneurons and secondary muscle spindle afferents in paraplegic 9 through somatic and parasympathetic activation of the sacral micturition center
As shown in Figures 81 and 82, the number (and the values) of phase relations changed between the firings of the different nerve fibers upon different stimulations. In the brain-dead human HT6, two phase relations were found between the a2-motoneuron and the secondary muscle spindle afferent fibre SP2(2) and the a2 and the g1-motoneuron (Figure 5 of [31]). Also in the paraplegic, two phase relations often existed between the firings of the different nerve fibres. It was suggested above that probably a third phase relation occurred when the activated parasympathetic division channelled an additional input to the oscillatory firing somatic neuronal network.
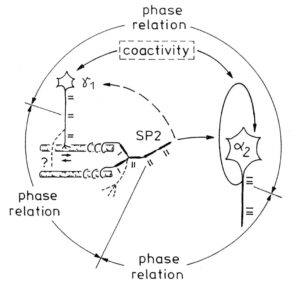
Figure 83: Schematized existing phase relation between a2 and g1-motoneurons and a secondary muscle spindle afferent fibre (SP2). Parallel existing phase relations between other parent afferents and the a2-motoneuron and between parent secondary spindle afferents are not shown. Phase relation means the increased occurrence of phases in ms in a certain phase range between the action potentials (APs) of the two compared nerve fibres. The complex afferent and efferent muscle spindle innervation was not attempted to be shown. Small arrows at intrafusal muscle fibre indicate local contraction, which is in nuclear chain fibres readily transmitted to the place of afferent innervation. A possible reason of the doublet firing of the SP2 fibre is pictured to occur from single APs (schematized by bars) of two myelinated endings, not necessarily from pacemaker switching. More endings of the parent SP2 fibre and g1-motoneurons are indicated by dashed line branches. “Coactivity” indicates a correlation between g and a-motoneuron spinal cord circuitries for higher activations.
It may therefore be worthwhile to further analyse the number of occurring phase relations per oscillation cycle upon different somatic and parasympathetic stimulations.
Since two phase relation occurred per oscillation cycle between the a3 and γ1-motoneurons and the SP2(1) fiber (Figure 81Ba) in paraplegic 9, and also their IIs were rather similar, it is concluded that the neuronal networks of the a3 and g1-motoneurons formed together with the spindle afferent fibre SP2(1) a part of a functional unit. The neural ensemble is built by efficiencies of synapses and projections between the convergence of several fusimotors on one muscle spindle and by the divergence of muscle spindle projections onto several rhythmically firing populations of neurons driving a and g-motoneurons. Such a functional unit is partly pictured in Figure 83 and schematized drawn by three circles in Figure 84. The a2-motoneuron and the SP2(2) fiber belonged to another functional unit (another ensemble) (longer IIs and the existence of only one phase relation). The two functional units (ensembles) are characterized in Figure 84 by two sets of three circles each. The two functional units interacted with each other, as there existed a phase relation between the a2 and a3-motoneurons (Figure 84).
Before stimulation (but with the anal and bladder catheters positioned), there were two phase relations in unit 1 (Figure 84a).
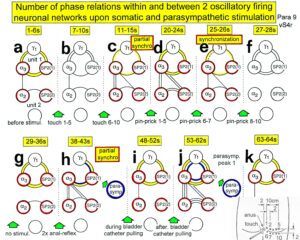
Figure 84: Number of phase relations within and between the two functional units a3/g1/SP2(1) and a2/-/SP2(2). Time intervals are those of Figure 80A. Note that in “a,” the functional unit 1 is with two phase relations per oscillation period in a stage similar to those seen in the brain-dead individual; with synchronization, only one phase relation occurred (e) and the parasympathetic division channelled an extra phase relation to interact with the somatic division (j).
When touching sites 1 to 5 (Figure 80), the skin outside the anal reflex area, only slight changes occurred in the two units with respect to the number of phase relations (Figure 84b). But when touching sites 6 to 10, the skin inside the anal reflex area, a partial synchronization occurred (Figure 84c), and functional unit 1 reduced the number of phase relations to one. When pinpricking sites 1 to 5, two phase relations occurred again in unit 1 (Figure 84d). Upon pinpricking sites 6 and 7, the number of phase relations between all the components of the two units dropped to one (Figure 84e), and synchronization occurred between the firing patterns (Figure 80B). Since in the brain-dead human two phase relations per oscillation cycle were observed in the functional units, it is possible that synchronization and the existence of only one phase relation for two to three seconds reflected a slight pathologic organization of the networks. Even though upon touching sites 6 to 10 (Figure 84c) or upon pinpricking sites 6 to 7 (Figure 84e) only one phase relation existed in unit 1, and synchronization occurred with both stimulations, it was shown above (Figures 78, 79) that the touch afferent input organized a different functional state of unit 1 than pinpricking. The response time until the shortening of the oscillation period was longer than the oscillation period (» 100 ms) for pinprick and shorter for touch. It was shown above (Figure 79) that repetitive touch stimulation (most effective inside the anal reflex area) reinforced the sustained stretch reflex of the anal sphincter (continence pattern), and repetitive pinprick stimulation replaced the continence pattern by the protection reaction of the anal sphincter. The number of phase relations alone therefore only provides limited information on the functional state of the organization of the neuronal networks of the human spinal cord. Measurements of a number of parameters are necessary to yield a rather complete description of the functional state of neuronal networks.
Following pinprick 8 and 10 and with no stimulation, two phase relations existed again in functional unit 1 (Figure 84f,g), in some similarity to pre-stimulation status (Figure 84a). Following two anal reflex stimulations, partial synchronization occurred in the components of the two units (Figure 5 of [84]) and mainly two-phase relations existed (Figure 84h). But the organizational state was still not very similar to the pre- (Figure 84a) or post-stimulation state in unit 1 (Figure 84g), since the parasympathetic division was slightly activated following anal reflex stimulation, as was measured by the impulse pattern (increase of doublet activity) of the secondary muscle spindle afferent fibre SP2(1) (Figures 5,7 of [8] (part 2)). Therefore, probably one phase relation was due to the somatic activation in similarity to Figure 84c,e, and the other phase relation was due to the activation by the parasympathetic division. During bladder catheter pulling (Figure 84i) and with no stimulation (Figure 84k), the number of phase relations and possibly the functional organization was again similar to the pre-stimulation state (Figure 84a). Following strong (painful) bladder catheter pulling with a strong activation of the parasympathetic division (time interval 53-62s (Figure 84j)), measured by the increased doublet firing (see Figure 5 of [84] (part 2)) of the SP2(1) fiber, the functional organization of the sacral micturition centre of the disconnected spinal cord changed completely. Functional unit 1 was now correlated by three phase relations per a3-oscillation cycle. The functional unit 2 also showed three phase relations per an a2-oscillation cycle, and interacted with functional unit 1 by three phase relations as well (between the a3 and a2-motoneurons; Figure 84j; 53-62s).
Between the first and second parasympathetic peak (Figure 80A) at the time interval 63-64s (Figure 84k), the organization form of the two functional units was similar to that before the first parasympathetic activation (49-52s) (Figures 84), only the values of the phase relations changed (Figure 82Bd). With the second strong activation of the parasympathetic division (parasymp. peak 2, time interval 65-72s), the functional unit 1 was bound together again by three phase relations (Figure 80A), in similarity to the first strong activation of the parasympathetic division [31] (part 3), measured by the burst firing of the secondary muscle spindle afferent fibre SP2(1) (Figure 8 of Ref.41 (part 2)) and the increased doublet firing of the SP2(1) fibre (Figure 5 of Ref.41 (part 2)). The functional unit 2 was disorganized, but phase relations still occurred between the a3 and the a2-motoneurons and the SP2(2) fibre [31] (Part 3). The a2-neuronal network and the fusimotor networks, driving the SP2(2) spindle afferent fiber, were integrated differently. After the second strong parasympathetic activation, in the time interval 73-76s (Figure 10A), the functional organization of the two functional units in the spinal cord was similar to that before the activation of the parasympathetic division. Functional unit 2 was slightly disorganized as the SP2(2) fibre strongly reduced its firing [31] (part 3). For further details see chapter V of [1].
This intricate analysis shows how complex neural network organization changes are. But such analyses are a first real step to understand human neural network organization and its consequences for disorders.
Building up of external loops to the periphery by premotor spinal oscillators
With the building up of simultaneous phase relations between a, g and SP2 fibres, an external loop of premotor spinal oscillators is built up to the periphery, which makes it possible to directly influence the firing of spinal oscillators by a rhythm training.
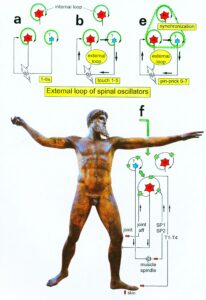
Figure 85: Spreading of oscillatory firing from a-motoneuron neuronal network to include muscle spindles (periphery) and synchronization of different a and g-motoneuron neuronal networks caused by touch and pinprick stimulation. a. a-motoneuron neuronal networks fired oscillatory (solid line loop), g-motoneuron neuronal network did not or did only partly (dashed line loop), upon no additional stimulation. b. Oscillatory firing a and g-motoneuron neuronal networks built up a phase relation with muscle spindle afferents and efferents (external loop to the periphery, indicated by thick arrows) upon touch. e. Oscillatory firing a (internal circuitry loop) and g-motoneuron neuronal networks (external loop) synchronized (broad peak phase relation) upon pinpricks. The dashed line loop represents synchronization. f. Oscillatory firing a (internal circuitry loop) and g-motoneuron neuronal networks (external loop). The open arrows indicate that it may be possible to synchronize spinal oscillators by rhythmic afferent input, generated by rhythmic movements (such as jumping on a springboard or running), and to re-preformat the neuronal circuitry by synapse remodeling to fire more physiologically oscillatory to reduce spasticity and improve locomotion. Extensive pathologic movement like tremor may entrain neuronal circuitry to increase tremor movement. The Greek good is a bronze statue of Zeus found close to the cape of Artemision 460 BC.
Upon jumping on springboard (Figure 79) (and other rhythmic movements like sky-walking (Figure 43D) or running), premotor spinal oscillators organize themselves to fire transiently oscillatory according to the motor pattern and build up an external loop to the periphery (Figure 85). If the frequency of the rhythmic movement has an integer relationship to the Eigenfrequencies of the premotor networks and more rostral networks, these premotor networks get entrained for more specific self-organization.
Entrainment of premotor spinal oscillator networks by rhythmic movement-induced afferent input and inputs from supraspinal centers
If one approximates, for high activation, spinal neuronal networks into premotor spinal oscillators [86] (driving the motoneuron) and propriospinal oscillators, then premotor spinal oscillators can be handled in a first approximation as single linear oscillators. The premotor spinal oscillators and the spinal pattern generating networks are self-organized and driven by peripheral afferent and supraspinal input. When training rhythmic, dynamic, stereotyped movements, the premotor spinal oscillators, approximated as linear oscillators, are driven by movement-induced afferent input from the periphery (mainly the legs) and surrounding pattern generating networks and possibly supraspinal inputs. These spinal oscillators and most likely their neuronal network can be entrained at least by use of the external loop for a better self-organization.
If one assumes that loop circuits do not only exist between the premotor spinal oscillators and the periphery, but are a general structure in the CNS, then motor learning involves the formation of loop circuits (or better loop network circuits) between the cortex and the periphery involving the sensory cortex and the thalamus. When a linear oscillatory system is driven by an external periodic input its response contains both frequency components (the own and the external one). This is also, in general, true with nonlinear oscillators. However, in this case, if the external frequency is close to the Eigenfrequency of the oscillator itself, then it is possible to have a response at the external frequency only. This phenomenon is known as entrainment or synchronization. It is of paramount importance with respect to biological oscillators because it allows them to “latch on” to the environment. Thus, a rhythm with a free-running period of 24.7 hours may be synchronized to 24 hours when exposed to the natural sequence of day and night.
Impaired organization of premotor spinal oscillator following spinal cord injury as an indicator for pathologic network organization
Following spinal cord injury, the oscillatory firing networks lose specific properties. The Eigenfrequencies of the premotor spinal oscillators change from a narrow to a broad frequency band (Figure 86).
Self-organized α2-oscillators fire physiologically at an Eigenfrequency (varying probably within a small frequency band as indicated with the hatched distributions in Figure 86) with impulse trains consisting of two to three action potentials. Following brain death, this Eigenfrequency band enlarges (black area in Figure 86). Following a spinal cord injury, the Eigenfrequency band enlarges strongly and includes in this case the frequencies between 4 and 14Hz for firing with two or three action potentials per impulse train (Figure 86). The premotor spinal oscillators have lost their specific properties and could now be excited at frequencies at which they physiologically would not be excited. This is one reason for spasticity.
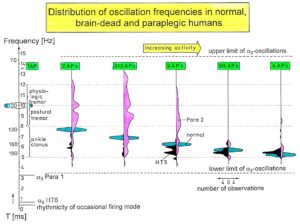
Figure 86: Distributions of oscillation frequencies of continuously oscillatory firing a2-motoneurons with increasing number of APs per impulse train (increased activity) in paraplegic 2 (open), in the brain-dead HT5 (filled), and in probably normal humans (cross-hatched). Frequencies and rhythmic activity changes in the occasional and oscillatory firing mode are indicated. Ranges of physiologic tremor, postural tremor and ankle clonus are also drawn. Note that frequencies for the brain-dead HT5 are too low, and the oscillation frequencies of the spinal cord isolated for a long time (Para 2) are too high and too spread as compared to the theoretically predicted frequency ranges of healthy humans (cross-hatched). T = oscillation frequency.
Difference of phase stability between a brain-dead human and a paraplegic
To make phase relation changes better recognizable with time from stable to unstable, a representation of phase relations is used, which comes from the measuring of the speed (frequency) of rotation.
The speed of rotation of a turning cylinder with a spot on its surface can be measured with a stroboscope.
If the stroboscope flashes light with the same frequency as the cylinder is turning, the spot on the circumference seems to stand still. There is a constant phase between the two frequencies (frequencies are same or multiples of each other). If the phase relation changes, the spot will move.
If no phase relation exists between the turning of the cylinder and the flashing of the light, no spot will be seen. In similarity to stroboscopic measurement of frequencies of turning cylinders, the phase relation between two oscillatory firing spinal oscillators is pictured in Figure 87A. A time axis is introduced on the horizontal line to make phase relation changes visible in dependence on time.
In Figure 87Aa, the loop excitation is pictured for this oscillator model. In Figure 87Ab, the phase relation between the SP2 fiber activity incidence and the oscillatory firing is pictured on the circumference of the oscillation period cylinder of the oscillator. Figure 87Ac,d,e shows different phase relations, namely a constant phase relation (c), a changing phase relation (d), and no phase relation (e). In Figure 87B, phase relation changes are plotted between an a2-motoneuron and the activity of a secondary muscle spindle afferent fiber and between an a2 and a g1-motoneuron. The data were taken from a brain-dead individual, probably normal with respect to the number of phases per oscillation cycle and with respect to phase changes. It can be seen that there were two phase relations per a2-oscillation cycle and that the phase relation changed only little with time. The phase coordination between the firings of the a2 and a g1-motoneuron and the secondary muscle spindle afferent fiber was stable.
In Figure 87C, D, in a patient with a spinal cord injury, different phase relation changes are shown with respect to the a3-oscillation cycle (C) and the a2-oscillation cycle (D). It can be seen that the different phase relations changed strongly in value over time (upon different stimulation) and that also the number of phase relations per oscillation cycle changed. The phase stability of the cooperative and competitive interplay among neurons became impaired. Whether the change of the number of phase relations from two to three following the activation of the parasympathetic nervous system in the sacral micturition centre is physiologic or not is not clear. See also Figure 84.
The most obvious difference of the phase relation changes between the brain-dead human and the paraplegic was that in the paraplegic the phase relations varied very much, whereas they changed only little in the brain-dead human. The strong phase relation changes in the paraplegic can be interpreted as instability in the organization of neural networks. The correlation of neural subnetworks was instable in relation to those of the brain-dead human. Assuming that the neural network organization and functioning was rather physiologic in the brain-dead with respect to the firing patterns of the premotor spinal oscillators, the functioning of the networks became instable following spinal cord injury.
The more frequent occurrences of changes of phase relations between the different nerve fibres in combination with the changing number of phase relations per oscillation in the patient with a spinal cord injury may mean that subnetworks reacted and interacted more quickly and easily with others according to the afferent input. Especially because the oscillatory firing networks lost specific properties, their resonance frequencies changed from a narrow to a broad oscillator frequency band (Figure 86), which means that the oscillators were not excited at a certain frequency anymore but by a broad frequency band. They could now be excited at frequencies at which they physiologically would not be excited. Over-activation and mass effects could be the result. On the other hand, certain networks could escape from driving afferent influence by their phase change: phase escape to avoid interaction. Functionally far away networks are not reached anymore, which also would result in a loss of specific properties. Therefore, because of the loss of specific properties, some interactions could have occurred more easily and other ones not at all.
W.R. Hess tried, in 1944, to compare biological order and human society [85]. In a society, the upper behaviour of spinal oscillators could be called “putting its flag to the wind.” There could be similarities between the organizations of the human nervous system and the organizations between very many individual nervous systems.
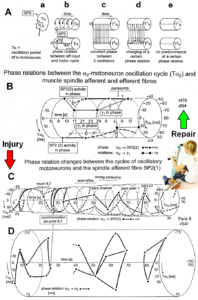
Figure 87: Phase relation changes from stable (brain-dead human HT6, B) to unstable by CNS injury (spinal cord injury, Para 9, C, D) with the consequence of incontinence. Through neural network learning (exercising on the special CDT device, jumping), continence and other patterns can be repaired by improving the phase stabilities and using plasticity. In A the measuring principle of phase relation changes with a stroboscope is shown.
Need for improving the stability of phase and frequency coordination to allow specific patterns formation and learning transfer
A young mother, with stress incontinence after giving birth to the first child, could well improve her continence status upon jumping on springboard in addition to other training, because her CNS is not injured; just the periphery has to be repaired by means of changing the CNS.
In severe cervical spinal cord injury, the jumping on springboard (Figures 22, 24) is not sufficient for bladder repair (the biggest problem in spinal cord injury). First, of course, the patient has to regain movement functions back (especially the trunk stability) to be able to perform the jumping on springboard. Further, the self-organization of CNS networks by phase and frequency coordination has to be improved to make learning transfer from movements to bladder functions possible, since in every CNS injury, the phase and frequency coordination is impaired. Large instabilities in phase and frequency coordination will not allow specific pattern formation as a basis for learning transfer. However, the stability of phase and frequency coordination can be improved when the patient is exercising on special coordination dynamics therapy devices, especially the one shown in Figure 27.
The importance of stable phase and frequency coordination to allow specific pattern formation and in consequence learning transfer to other patterns can be understood at the collective variable level (System Theory of Pattern formation) and at the neuron level. The behavioural information Finf of the coordination pattern dynamics, characterized by equations of motion of collective variables, dX/dt = Fintr(X) + ∑cinfFinf(X,t), affect the whole coordination pattern dynamics, including stability, rather than only certain coordination patterns. If the behavioural information includes the exercising of extremely coordinated, integrative movements, like exercising on the special CDT device for turning, then the quality of the CNS self-organization can be enhanced by improving the exactness of self-organization, namely the precision of phase and frequency coordination between neuron and neural assembly firing. By improving the precision of organization of the intrinsic dynamics Fintr(X), that is, the specific variability of the injured networks, certain patterns do then already reappear.
At the neuron level, neurons often serve more than one network pattern at the same time by time sharing of neuron firing and, in this way, give rise to learning transfer among the activated patterns. If subnetworks are improved in the organization of one pattern, the organization of the other pattern will also improve. Neurons involved in the organization of breathing and activating intercostal muscles, for example, are also involved in the organization of trunk stability. By reducing spasticity of the trunk (in patients with Parkinson’s disease), the breathing will also improve. Similarly, sphincteric motoneurons are involved in continence and pelvic floor weight bearing. If during pregnancy the (non-trained) pelvic floor is not trained, sometimes incontinence occurs and after birth stress incontinence.
Entrainment of oscillators through jumping on springboard
Upon jumping rhythmically on springboard (Figure 24), in addition to the stimulation of mechanoreceptors for movement control, also mechanoreceptors for bladder and rectum control are synchronously activated with the movement. Continence functions are synchronously activated with the jumping (coherent activation of bladder and movement patterns). Since, additionally, for high activation premotor spinal oscillators build up an external loop to the periphery, neural assemblies are directly entrained to improve their “Eigenfrequencies” and to coordinate their firing with other oscillators (Figure 85). The springboard has an Eigenfrequency (f ≈ 1Hz; ω=2πf), which makes a training in the entrainment region possible. A jumping frequency of 1 Hz is especially efficient for the entrainment of α3-oscillators because they have an “Eigenfrequency” also around 1 Hz (Figure 12).
Repair of phase and frequency coordination through training of very coordinated arm and leg movements on the special CDT device
A completely different contribution to learning and learning transfer is induced if the patient is exercising on the special coordination dynamics therapy device for turning (Figure 27). This training of very coordinated arm, leg, and trunk movements, imposed by the device, is especially improving the impaired phase and frequency coordination of neuron firing. Following spinal cord injury, the phase and frequency coordination becomes impaired, as turns out when comparing the coordinated firing of neurons in a brain-dead human (rather physiologic) with that of a patient with a spinal cord injury (Figure 87). The network organization becomes deteriorated and escapes entrainment, learning, and learning transfer. Following injury, the variability of the coordinated firing of neurons becomes too large to enable efficient and specific learning and learning transfer. But when patients exercise very coordinated arm, leg, and trunk movements on the special coordination dynamics therapy device for turning (on which a precise coordination between arm and leg movements is imposed by the device, to which the training individual has to adapt to), the CNS of patients can relearn specific self-organization, which means can relearn more exact phase and frequency coordination among neuron firings from the device. Since the CNS network is an open system, many different very coordinated integrative movements should be trained, not to allow the pathologic organization to escape from the repair/entrainment.
An impaired phase and frequency coordination between the firings of single neurons and neuron assemblies (Figures 86, 87) can be expected to have consequences in the coordination between arm and leg movements because motoneurons innervate muscle fibers. Rhythmic coordinated firing of single motor units has been measured electromyographically with surface electrodes (Figure 17). Indeed, the coordination between arm and leg movements is partly or fully lost following CNS (brain and spinal cord) injury and is often not taking place in the malfunctioning CNS.
This partly impaired phase and frequency coordination at the single neuron level, the assembly level and the macroscopic level can be measured macroscopically when the patient is exercising on a special coordination dynamic therapy device (Figures 88) on which arms and legs turn with a slightly different frequency (transmission 19 (arms) : 18 (legs)). The phase coordination between arms and legs is imposed by the device. The loss of phase and frequency coordination between arm and leg movements becomes visible and measurable by the arrhythmicity of turning. During a turning cycle the coordination between arms and legs changes between pace and trot gait and according to the difficulty of the coordination the turning frequency increases and decreases. This frequency variation (df/dt; f = frequency) can be recorded, quantified and displayed on a computer screen (Figure 88).
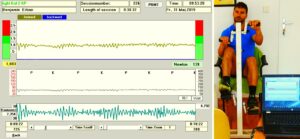
Figure 88: Coordination dynamics display. The brain-injured patient and disabled athlete Benjamin Erban is exercising on a special CDT device and the arrhythmicity of exercising (df/dt, lower trace) is displayed. At this moment, he is exercising at 139Newton. The mean value of arrhythmicity per minute (coordination dynamics value) is 6.792. Note that between K (trot gate) and P (pace gait) the amplitude of arrhythmicity is higher, but not between P and K. Upper trace = frequency of exercising, middle trace = load in Watt. After 20 years of CDT, the severely brain-injured patient runs 100m in 14s and jumps 4m long. In Grosseto 2016 he won a bronze medal in running for disabled.
During the functional reorganization of the injured CNS of patients, the relative phase and frequency coordination of neuron firing has to be entrained as exactly as possible by the movement induced afferent impulse patterns from the receptors (learning through feedback information) to restore coordination in the range between 3 and 5 milliseconds (Figure 13). The device has therefore to impose the exercising patient a coordination in the millisecond range for the different coordination’s of arm and leg movements between pace gait and trot gait. The easy pace and trot gait coordination’s, but not the difficult intermediate coordination’s, can often be performed by the patient easily. Therefore, the continuous change from the easy to the difficult coordination’s and backwards diagnoses the capability of the CNS to organize easy and difficult organizational states. If the movement state can be easily generated by the neuronal networks of the CNS, then the frequency variation of turning is small during the turning cycle (Figure 88, between P (pace gait) and K (trot gait)), and if the movement state is difficult to organize by the CNS, then the frequency variation is large (Figure 88, between K and P).
A healthy person can walk or crawl (automatisms) in trot or pace gait coordination, but not in a coordination in between. When exercising on the special CDT device, on the other hand, he is able to adapt to the intermediate difficult coordination’s. A rat or dog is probably not able to adapt to the difficult intermediate coordination’s between pace and trot gait, because of missing complexity of their neural networks. If one changes the nerve supply of flexor and extensor muscles, the rat cannot relearn, the monkey can after some time and the human can re-learn nearly immediately [72-74].
The cerebral palsy girl Sophie learned walking (Figure 1), balance and other motor functions through CDT. But when training even more complicated coordination’s between arm and leg movements, her higher mental functions improved strongly, so that she had not to be pushed so much anymore to train. She enjoyed it to train by herself with the family (Figure 90).
The improvement of CNS functioning can be measured by the coordination dynamics value (Figure 88) and plotted over time with ongoing therapy (Figure 89). In Sophie, the continence was achieved first, the motor functions are still progressing, but the big problem is to what extent the cognitive functions can still be improved [15].
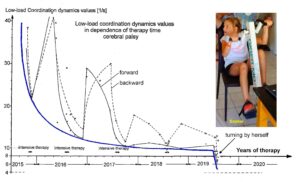
Figure 89: Improvement (lowering) of the low load coordination dynamics values with ongoing coordination dynamics therapy (CDT). During extensive therapy she improved strongly; with little or no therapy she got worse (higher values). The solid thick line connects the best values, which reached nearly a plateau of no further progress. When using a special CDT device with more complicated coordination’s between arms and legs, the CDT values substantially improved (lowered) further. With this improvement of brain functioning, the cerebral palsy girl Sophie started to turn by herself.
It seems therefore that if it possible to train phase and frequency coordination in the deep complexity of CNS organization. Therefore, more repair is possible, especially with respect to the higher mental functions. To improve sufficiently phase and frequency coordination and CNS organization in the deep complexity of neural network organization, the patient has to train also at higher loads (1), at higher coordinated complexity (2) for longer times (3). Mental discipline is needed. Further, also easy coordination’s have to be trained as walking or running for CNS stability. A balance between variability and stability is needed, because it can happen that CNS organization is drifting.

Figure 90: The cerebral palsy girl Sophie during training with cousins. Sophie trains to improve her brain functioning and the others to improve their general health and to motivate Sophie to train.
I in the injured pathologically functioning CNS of patients, often coordination’s other than pace and trot gait are the easy coordination’s. A coordination coordinate is needed to judge the coordination dynamics. The pace and trot gait coordination’s are used for the calibration, since both coordination’s between arm and leg movements occur naturally during rhythmic coordinated (automatic) movements like creeping, crawling, walking and running.
The imposed coordination of arm, leg and trunk movements, upon exercising on the special coordination dynamics therapy and measuring device (Figure 88), is in accordance with the coupling possibilities of premotor a1 (8-20Hz), a2 (5-9Hz) and a3-oscillators (0.1-4Hz), even though the frequencies are only a relative coordination parameter, whereas the phase is an absolute coordination parameter.
When the hand levers are turned at between » 0.4-1.5 Hz, the resulting frequency difference in turning between arms and legs is approx. 8.5 Hz (low a1-oscillator frequency or high a2-frequency) for low hand-turning frequency of 0.5Hz (low a3-frequency). A slower turning of the hand levers would train directly more the premotor a2-oscillators (f < 8.5Hz). Faster turning of the hand levers (higher a3-frequency) would train directly the a1-oscillators in the higher frequency range (f > 8.5Hz). Therefore, similar frequencies appear with respect to the frequency of turning on the device for measuring CNS organization and reorganization as have been measured for premotor spinal oscillators.
Conclusion
Human repair-neurophysiology is a new discipline with which the human nervous system can be repaired. Since the nervous system is involved in nearly all body functions, the general health can be improved. Especially urinary bladder functions of the sacral micturition center can be analyzed in detail by using the newly developed single-nerve fiber action potential recording method and recording from the thin and long lower sacral nerve roots (Figure 3, 41) [1, 70, 88]. By combining human neurophysiology with the System Theory of Pattern Formation, there is a theoretical basis that through movement-based learning vegetative and cognitive functions can also be repaired through learning transfer [2]. Bladder continence is achieved via the repair of the vegetative (inner bladder sphincter) and the somatic nervous system divisions (external bladder sphincter) and their coordination. Bladder dyssynergia can be repaired [70, 89, 91]. An ePoster (Figure 91) from the conference “continence 2022” summarizes bladder repair.
Figure 91: ePoster 121 from the continence conference ICS 2022 of the international continence society.
Figure 91: Poster for continence repair through movement-based learning and learning transfer (Coordination dynamics therapy (CDT)).
Poster explanation. The continence can mainly be repaired through exercising on the special coordination dynamics therapy device (repair of phase and frequency coordination) and jumping and walking (plasticity). The scientific basis starts with the introduction of the new recording method, the single-nerve fiber action potential recording method. It becomes possible to measure bladder and rectum functions at the single-neuron level in human under physiologic and pathologic conditions. Following injury, malformation or degeneration, the bladder is functioning pathologically and also neural networks in the sacral and pontine micturition centers are functioning pathologically. First, because the receptors of the bladder wall are firing already strongly when the bladder is empty; there is no storage phase. Second, the neural networks of the sacral micturition center, in connection with the pontine micturition center, are functioning pathologically. The vegetative and somatic neural networks, activating the detrusor and the external bladder sphincter and its coordination, are impaired in its functioning and have to be repaired. This can be achieved through CDT. The impaired phase and frequency coordination of neuron firing is improved when exercising on the special CDT device. The neural networks are re-organized when training jumping, walking and other movements. Especially when activating bladder and rectum functions through pin-pricking sacral dermatomes, anal and bladder catheter pulling and bladder filling, the functions of the sacral micturition center can be explored specifically at the single-neuron level when using the single-nerve fiber action potential recording method.
In many CNS injuries the bladder can be repaired. The problems are that the patients have to work hard and the human research to repair the human nervous system is not organized. Animal research is no substitute for human research. Some details of such human research are given in this publication. The poster to summarize the progress (Figure 91) has to be enlarged to see details.
Epilogue
Ethics
After an initial financial support of this research project to repair the human CNS by the “Deutsche Forschungsgemeinschaft” (DFG), further support was refused because of professional and ethical doubts. In spite of the support by the former German president Richard von Weizsäcker, the DFG did not change its mind and stated that the Author cannot apply any more for funding of this research project. Also, the “Swiss National fond” and the “Max Planck Institution” refused support. For the impossibility to get support for a research project to repair the human nervous system see Epilogue of [1]. Because of the impossibility to get official funding for this research project, the Author went on with the research, mainly on personally saved money for 35 years to reach the present level.
When the Author wanted to work on brain-dead humans at the University of Greifswald (at that time German Democratic Republic, or Russian occupational zone (Figure 96)), mainly at the neurosurgery department [29], an ethical committee was founded and this committee decided that it is justified to work on brain-dead humans for repairing the human CNS. For developing the “single-nerve fiber action potential recording method”, the Author was supposed to get a professorship in Dresden (Figure 93, after being re-built). But after 10 years, with the fall of the Berlin wall, everything changed, the bad and the good things.
The ethics in research are important. But it is not only important what research on human is justified, but also what research is allowed to leave out. Here an important example. Through coordination dynamics therapy it is possible to live longer with a better quality of life for 10 to 20 years [25, 26]. If every hundreds person (10-2) on earth (world population = 7×1012) would have the mental discipline to train hard and live longer for 10 years (10-1), then every year a few million lives could be saved when assuming a life time of 100 years (7×1012×10-2×10-1= 7 million). That means qualified research on human has tremendous consequences.
War children (Kriegskinder)
Nightmares can partly be cured during CDT treatment of scoliosis and in cerebral palsy [9]. (The following is not easy to write for the Author).
However, the CNS of children cannot only suffer damage from a traumatic brain injury, infection or malformation, but also from the fallout of conflict zones, especially in the absence of parents. They can suffer nightmares which may last for the rest of their lives. Figure 92 shows Butscha in Ukraine and Figure 93 shows the ruins of Dresden following the bombing in 1945. Such situation may induce nightmares in those ones who survived.

Figure 92: Road in Butscha, Ukraine, 2022.

Figure 93: The Bombing of Dresden took place in the final months of the Second World War, when Hitler-Germany had lost already the war. In four raids between 13 and 15 February 1945 the bombing and the resulting firestorm destroyed over 6.5 km2 of the city. An estimated 25,000 people were killed. There was no army in Dresden. A pilot of the United States Air Force refused to bomb Heidelberg, because he had studied in Heidelberg. Dresden was a cultural landmark and is sometimes referred to as “Florence on the Elbe”. Culture is only protected by those ones who have culture.
The Author walked through Berlin like the child in Figure 94. The streets were cleaned by women (Trümmerfrauen). The bricks in the ruins seem to have been turned several times by the bombing.

Figure 94: Ruins in Berlin after World War II. (Scharoun was a famous German architect).
To imagine the criminality done in the war in Ukraine, we need a comparison to other wars, as the first and second world war. This is not especially the duty of medical research, but first medicine is always involved in wars, second the public in many countries is avoiding such comparisons, third as Kant argues “if justice perishes, it is no longer worth living on earth” (Figure 95), and fourth the Author’s families from the father’s side (Stettin) and mothers’ side (Regentin) were refugees (Figure 96), of which nobody wants to speak about.

Figure 95: Nobody wants to speak about the German refugees. “Those who know the truth and fail to call it by its name become the truth’s main enemy.” (Julius Rupp, Wer die Wahrheit kennt und nicht benennt, ist der größte Feind der Wahrheit).
Figure 96: Parcellation of Germany according to the Potsdam conference. Germany was divided into 10 parts. This division is in contradiction to the principle of the integrity of borders in the 20th and 21st centuries.
In Figure 97 some data of World War II are summarized with respect to Germany. There were 15 million German refugees, 3.5 million were killed on their way. 300 000 women were raped, abased or killed. Seven million Germans starved on hunger or disease in between 1946-1947. Eisenhower forced German soldiers to dig diches in wet ground (Rheinwiesen) to stay in and stopped the red cross to bring them food. One million German soldiers died in the hands of the USA after the war according to the statement of three persons of Figure 97.

Figure 97: From “Junge Freiheit”, 6.5.2022, Page 9.
Since the Author has been a theoretical nuclear physicist [90], he has to state the following. The physicists Heisenberg (see also collected works of Heisenberg, especially section C, volume 5, page 164), Einstein and Bohr had the agreement not to tell politicians about the possibilities of nuclear forces with respect to build an atomic bomb. But Einstein convinced Roosevelt in a letter to build the (first) atomic bomb. Following Hiroshima and Nagasaki, he felt sorry for that. The German Heisenberg did not build an atomic bomb for Hitler. After the war, Adenauer did not used the knowledge of Heisenberg to build nuclear power plants and Heisenberg (the father of Quantum Mechanics) was disappointed that his expert knowledge was not used. Who knows how the development of nuclear power plants would have gone with his knowledge? Not only the Author made the experience that expert knowledge (for example this article) is not of interest anymore.
References
- Schalow G (2013) Human Neurophysiology: Development and Repair of the Human Central Nervous System. Nova Science Publishers, Inc, Hauppauge NY, USA, 734 pp.
- Schalow G (2015) Repair of the Human Brain and Spinal Cord. Nova Science Publishers, Inc, Hauppauge NY, USA, 525 pp.
- Schalow G (2015) Neural network learning in human. Nova Science Publishers, Inc, Hauppauge NY, USA, 324 pp.
- Schalow G (2002) Stroke recovery induced by coordination dynamic therapy and quantified by the coordination dynamic recording method. Electromyogr. Clin. Neurophysiol. 42: 85-104.
- Schalow G (2002) Improvement after traumatic brain injury achieved by coordination dynamic therapy. Electromyogr. Clin. Neurophysiol. 42: 195-203.
- Schalow G and Jaigma P (2006) Improvement in severe traumatic brain injury induced by coordination dynamics therapy in comparison to physiologic CNS development. Electromyogr. Clin. Neurophysiol. 46: 195-209.
- Schalow G (2019) Regeneration of the human spinal cord via coordination dynamics therapy. Peertechz Publications, 97 pp. eBook.
- Schalow G (2009) Partial cure achieved in a patient with near-complete cervical spinal cord injury (95% injury) after 3 years of coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 49: 199-221.
- Schalow G (2002) Recovery from spinal cord injury achieved by 3 months of coordination dynamic therapy. Electromyogr. Clin. Neurophysiol. 42: 367-376.
- Schalow G (2003) Partial cure of spinal cord injury achieved by 6 to 13 months of coordination dynamic therapy. Electromyogr. Clin. Neurophysiol. 43: 281-292.
- Schalow G, Jaigma P and Belle VK (2009) Near-total functional recovery achieved in partial spinal cord injury (50% injury) after 3 years of coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 49: 67-91.
- Schalow G (2010) Cure of urinary bladder functions in severe (95%) motoric complete cervical spinal cord injury in human. Electromyogr. Clin. Neurophysiol. 50: 155-179.
- Schalow G (2021) CNS Repair in a Girl with a Spinal Cord Injury. Adv. Pub. Health Com. Trop. Med. 121: 201-226.
- Schalow G (2006) Cerebellar injury improvement achieved by coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 46: 433-439.
- Schalow G (2021) Cure-like brain-repair in a girl with atrophied cerebellum and pons through Coordination Dynamics Therapy. Adv Pub Health Com Trop Med 123: 1-47.
- Schalow G, Jaigma P (2005) Cerebral palsy improvement achieved by coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 45: 433-445.
- Schalow G (2006) Hypoxic brain injury improvement induced by coordination dynamics therapy in comparison to CNS development. Electromyogr. Clin. Neurophysiol. 46: 171-183.
- Schalow G, Pääsuke M, Ereline J and Gapeyeva H (2004) Improvement in Parkinson’s disease patients achieved by coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 44: 67-73.
- Schalow G and Nyffeler T (2001) Koordinationsdynamik-Therapie: Myelomeningozele (Spina bifida). Physiotherapie.
- Schalow G and Nyffeler T (2000) Koordinatiosdynamik-Therapie: Skoliose. Physiotherapy.
- Schalow G (2019) Permanent coma patient re-learned to speak via Coordination Dynamics Therapy. Arch. Clin. Med. Case Rep. 3(2): 33-50.
- Schalow G (2017) Breast cancer growth inhibition via Coordination Dynamics Therapy. In: “Horizons in Cancer Research. Volume 68”. Editor: Hiroto S. Watanabe. Nova Science Publishers, Inc, Hauppauge NY, USA, pp. 125-151.
- Schalow G (2020) Anaplastic oligodendroglioma WHO III brain cancer-patient recovered following operation, radiation and chemotherapy through Coordination Dynamics Therapy, which is also a Covid-19 treatment without ventilator. Int. J. Med. Clin. Imaging 5(2): 165-210.
- Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M and Hojman P (2014) Muscle dysfunction in cancer patients. Ann. Oncol. 25: 947-958.
- Schalow G (2020) To live longer with a better quality of life through coordination dynamics therapy especially in patients with severe brain injury and brain-cancer. Int. J. Med. Clin. Imaging 5(2): 118-155.
- Schalow G (2021) Euthanasia in organ donation can be avoided through Coordination dynamics therapy. Adv Pub Health, Com Trop Med: APCTM-132. Volume 3: 1-34.
- Schalow G (2022) Basal ganglia and cortex repair through human-repair neurophysiology 12 years after hypoxia during birth. In Schalow G (Ed) Human Repair-Neurophysiology, page 181-227. B P International.
- Schalow G (2022) Spinal muscular atrophy repair through Coordination dynamics therapy and Translation of frog neuromuscular innervation pattern changes caused by neurotrophins to human. Med.: AOASM-160 2022(2): 1-81.
- Schalow G and Lang G (1987) Recording of Single Unit Potentials in Human Spinal Nerve Roots: a New Diagnostic Tool. Acta Neurochir. 86, 25-29.
- Schalow G and Lang G (1989) Electrodiagnosis of human dorsal sacral nerve roots by recording afferent and efferent extracellular action potentials. Neurosurg. Rev. 12, 223-232.
- Schalow G (2009) The classification and identification of human somatic and parasympathetic nerve fibres including urinary bladder afferents is preserved following spinal cord injury. Electromyogr. Neurophysiol. 49, 263-286.
- Schalow G (2020) Classification and Identification of Human Peripheral Nerve Fibers by Conduction Velocity, Nerve Fiber Diameter and Natural Firing Patterns with Consequences for CNS Repair and Covid-19 Infection Treatment. Int. J. Med. Clin. Imaging 5(3): 231-314.
- Schalow G (1991) Oscillatory firing of single human sphincteric a2 and a3-motoneurons reflexly activated for the continence of urinary bladder and rectum. Restoration of bladder function in paraplegia. Clin. Neurophysiol., 31, 323-355.
- Schalow G (1993) Action potential patterns of intrafusal g and parasympathetic motoneurons, secondary muscle spindle afferents and an oscillatory firing a2-motoneuron, and the phase relations among them in humans. clin. Neurophysiol., 33, 477-503.
- Schalow G (2005) Phase and frequency coordination between neuron firing as an integrative mechanism of human CNS self-organization. Electromyogr. Clin. Neurophysiol., 45: 369-383.
- Schalow G (2021) Phase and frequency coordination improvement among neuron firing for improved CNS self-organization and neural repair in Parkinson and spinal cord injury. Int J Med Clin Imaging 6(1): 350-425.
- Kelso JAS (1995) Dynamic Patterns. The Self-Organization of Brain and Behavior. MIT Press, Cambridge.
- Haken H, Kelso, JA & Bunz H (1985) A theoretical model of phase transitions in human hand movements. Biological Cybernetics 39, 139-156
- Schalow G (2006) Functional development of the CNS in pupils aged 7 to 19 years. Electromyogr. Clin. Neurophysiol., 46, 159-169.
- Zanone PG and Kelso JAS (1992) Evolution of behavioural attractors with learning: Nonequilibrium phase transition. Journal of Experimental Psychology: Human perception and Performance, 18 (2): 403-421.
- Schalow G (1990) Feeder arteries, longitudinal arterial trunks and arterial anastomoses of the lower human spinal cord. Zentralbl. Neurochir. 51: 181-184.
- Schalow G (2009) Impaired coordination between oscillatory firing FF and FR-type motor units in Parkinson’s disease and patients with spinal cord injury. In: Berkovsky, T.C. (Ed.), Handbook of Spinal Cord Injuries, Chapter 15. pp 501-517. Nova Science Publishers.
- Deisseroth K, Singla S, Toda H et al (2004) Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42: 535-552.
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA and Gage FH (1998) Neurogenesis in the adult human hippocampus. Nature Medicine 4: 1313-1317.
- Praag van H, Kempermann G and Gage H (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 2(3): 266-270.
- Schalow G (2009) Building of New Motoneurons in the Human Spinal Cord upon Coordination Dynamics Therapy to Improve Finger Functions in Motoric Complete Cervical Spinal Cord Injury. In: Berkovsky, T.C. (Ed.), Handbook of Spinal Cord Injuries, Chapter 4. pp. 231-264, Nova Science Publishers.
- Drapeau E, Montaron MF, Aguerre S et al (2007) Learning-induced survival of new neurons depends on the cognitive status of aged rats. J. Neurophysiol. 27, 6037-6044.
- Zhao C, Deng W and Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132: 645-660.
- Schalow G (2010) Scientific basis for learning transfer from movements to urinary bladder functions for bladder repair in patients with spinal cord injury. Electromyogr. Clin. Neurophysiol. 50: 339-395.
- Guttmann L (1973) Spinal cord injuries. Comprehensive management and research. Blackwell, Oxford.
- Sramkova T and Fajtova R (2010) Sexual life after spinal cord injury. In: Berkovsky, T.C. (ed.), Handbook of Spinal Cord Injuries, Nova Science Publishers, New York, pp. 133-166.
- Courtois F et al (2010) Assessment of sexual potential and treatment of sexual dysfunctions in men and women with spinal cord injury. In: Berkovsky, T.C. (ed.), Handbook of Spinal Cord Injuries, Nova Science Publishers, New York, pp. 167-229.
- Koepchen HP (1990) Physiology of rhythms and control systems: An integrative approach. In: Haken H, Koepchen HP editors. Rhythms in Physiological Systems. Berlin, pp 3-20.
- Lambertz M & Langhorst P (1998) Simultaneous changes of rhythmic organization in brainstem neurons, respiration, cardiovascular system and EEG between 0.05 Hz and 0.5 Hz. J. Auton. Nerv. Syst. 68: 58-77.
- Schalow G and Zäch GA (2000) Reorganization of the human CNS. Theoretical basis for modern neurorehabilitation (31 case reports). Gen. Physiol. Biophys. 19 (Suppl. 1): 1-244.
- Rosenzweig ES et al (2010) Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nature Neuroscience, Advance online Publication.
- Maier IC and Schwab ME (2006) Sprouting and circuit formation in the injured spinal cord: factors and activity. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361: 1611-1634.
- Zorner B and Schwab ME (2010) Anti – Nogo on the go: from animal models to a clinical trial. Ann. N.Y. Acad. Sci., 1198, Suppl.1: E22-34.
- Keirstead HS et al (2005) Human embryonic stem cell derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 25: 4694-4705. (rat)
- Martino G and Pluchino S (2006) The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 7: 395-406.
- McDonald JW et al (1999) Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nature Med. 5: 1410-1412.
- Kerschensteiner M et al (2005) In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Med. 11: 572-577. (rat)
- Editorials: Order from chaos (2010) Nature 466: 7-8.
- Thuret S, Moon LDF and Gage FH (2006) Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7: 628-643.
- Bradbury EJ and McMahon SB (2006) Spinal cord repair strategies: why do they work? Nat. Rev. Neurosci. 7: 644-653.
- Harel NY and Strittmatter SM (2006) Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury. Nat. Rev. Neurosci. 7: 603-616.
- Grillner S (2003) The motor infrastructure: From ion channels to neuronal networks. Nature Reviews Neuroscience 4: 573-586.
- Schalow G and Zäch GA (1994) Nerve compound action potentials analysed with the simultaneously measured single fibre action potentials in humans. Electromyogr. clin. Neurophysiol. 34: 451-465.
- Schalow G (1992) Recruitment within the groups of g1,a2 and a3-motoneurons in dogs and humans following bladder and anal catheter pulling. Gen. Physiol. Biophys. 11: 101-121.
- Schalow, G., Bersch, U., Göcking, K. and Zäch, G.A. (1995). Detrusor-sphincteric dyssynergia in paraplegia compared with the synergia in a brain-dead human by using the single-fibre action potential recording method. J. Auton. Nerv. Syst. 52, 151-180.
- Schalow G (2009) Coordination impairment between the somatic and parasympathetic nervous system divisions in the human sacral micturition centre following spinal cord injury. Electromyogr. Clin. Neurophysiol. 49: 337-367.
- Sperry RW (1945) The problem of central nervous system reorganization and muscle transposition. Quart. Rev. Biol. 20: 311-369.
- Sperry RW (1947) Effect of crossing nerves to antagonistic limb muscles in the monkey. Arch. Neurol. Psychiat. (Chicago) 58: 452-473.
- Weiss P and Brown PF (1941) Electromyographic study on coordination of leg movements in poliomyelitis patients with transposed tendons. Soc. Exper. Biol. Med. 48: 384-387.
- de Groat WC (1975) Nervous control of the urinary bladder of the cat. Brain. Res. 87: 201-211.
- Torrens M and Morrison JFB (1987) The Physiology of the Lower Urinary Tract, Springer Verlag.
- Arbuthnott ER, Gladden MH and Sutherland F (1982) Autonomic innervation of muscle spindles from the hindlimb of the cat. Proc. Roy. Soc. 17: 1.
- Barker D and Banks RW (1986) The muscle spindle. In: Engel AE and Banker, B.Q. (Eds.), Myology, Vol. 1, Part 1, McGraw-Hill, New York, pp. 309-341.
- Passatore GM, Fillipi GM, Grassi G (1985) Cervical sympathetic nerve stimulation can induce an intrafusal muscle fibre contraction in the rabbit. In: Boyd IA and Gladden MH (Eds.), The Muscle Spindle, Stockton Press, New York, 221-226.
- Swash M and Fox KP (1985) Adrenergic innervation of baboon and human muscle spindles. In: Boyd IA and Gladden MH (Eds.), The Muscle Spindle, Stockton Press, New York, pp. 121-126.
- Schalow G (2009) Coordination impairment between the somatic and parasympathetic nervous system divisions in the human sacral micturition centre following spinal cord injury. Electromyogr. Clin. Neurophysiol. 49: 337-367.
- Yates FE (1980) Urodynamics and the study of female incontinence: the state of art as perceived by an outsider, with a modest proposal. In: Zinner NR, Sterling AM (Eds.). Female Incontinence, Prog. Clin. Biol. Res. 78, pp. 1-14.
- Baehr M and Frotscher M (2005) Duus‘Topical Diagnosis in Neurology. Thieme Verlag, Stuttgart. (a very good book for medical neuroscience since it correlates structure and function in human nervous system injuries).
- Schalow G and Zäch GA (1996) Reflex stimulation of continuously oscillatory firing a and g-motoneurons in patients with spinal cord lesion. Physiol. Biophys. 15, Suppl.1: 75-93.
- Hess WR (1981) Biological order and human society. In: Akert K (Ed.), Biological Order and Brain Organization – Selected Works of WR Hess, 3-15, Springer-Verlag, Berlin.
- Schalow G (1993) Spinal oscillators in man under normal and pathologic conditions. Clin. Neurophysiol. 33: 409-426.
- Schalow G (2001) Time axis calibration in human CNS organization for judging dysfunction. Electromyogr. Clin. Neurophysiol. 41: 485-505.
- Schalow G, Zäch GA and Warzock R (1995) Classification of human peripheral nerve fibre groups by conduction velocity and nerve fibre diameter is preserved following spinal cord lesion. J. Auton. Nerv. Syst. 52: 125-150.
- Schalow G, Bersch U, Michel D and Koch HG (1995) Detrusor-sphincteric dyssynergia in humans with spinal cord lesions may be caused by a loss of stable phase relations between and within oscillatory firing neuronal networks of the sacral micturition centre. J. Auton. Nerv. Syst. 52:181-202.
- Schalow G and Yamamura M (1971) Investigation of ground state correlation in an extended Lipkin-Meshkov-Glick-Modell. Nuclear Physics A161: 93-104.
- Schalow G (2022) Urinary bladder repair through Coordination dynamics therapy. https://www.ics.org/2022/abstract/121.