Publication Information
ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Comparative Study on Antioxidant Activity of Brassica rapa var. parachinensis Leaves and Seeds
Mona Ghanad*, Rosimah Nulit, Rusea Go, Christina Yong Seok
Department of Biology, Faculty of science, University Putra Malaysia, Malaysia
Received Date: November 15, 2019; Accepted Date: January 09, 2020; Published Date: February 06, 2020
*Corresponding author: Mona Ghanad, Department of Biology, Faculty of science, University Putra Malaysia, Malaysia. Email: m.ghanad.upm@gmail.com
Citation: Ghanad M, Nulit R, Go R, Seok CY (2020) Comparative Study on Antioxidant Activity of Brassica rapa var. parachinensis Leaves and Seeds.
Abstract
The aim of this study was to assess the antioxidant activities of B. rapa var. parachinensis leaves and seeds. The aqueous extraction of leaves and seeds were analyzed for total amount of phenolic, flavonoids, ascorbic acid, chlorophylls, carotenoids, 2, 2-diphenyl-1-picryl-hydrazil (DPPH) radical scavenging activity and reducing power capacity. The total amount of biochemical compounds showed different variety between leaves and seeds. The content of vitamin C was incredibly higher in seeds while the quantity of chlorophyll, carotenoids and phenolic contents was higher in leaves. DPPH scavenging activity of seed extraction displayed higher percent of inhabitation in comparison with leaves. The reduce power of seed and leaves at 10 mg extract were0.46±0.024 and 1.03±0.058, respectively. The data were analyzed by one-way ANOVA multiple comparison test followed by Duncan’s multiple range test.
Keywords: Antioxidants, Brassica, Disease, Vegetables assay
Introduction
Regular consumption of vegetables and fruits as sources of natural antioxidants which consist of many different antioxidant components such as vitamins C and E, carotenoids, and phenolic compounds have been recognized reduce the risk of chronic diseases [1]. Studies approved that an antioxidant rich diet has a very positive health impact in the long run [2 3]. Antioxidants play a very important role in the body defense system against ROS [4]. Halliwell (2007) [5] reported that an antioxidant is “any substance that delays, prevents or removes oxidative damage to a target molecule.
Antioxidants can scavenge free radicals and protect the human body from the oxidative stress that eventually, protect human body from cardiovascular disease, cancer, high blood pressure, diabetes and obesity. Cruciferous vegetables, in particular those included into the Brassica genus are good sources of a variety of nutrients and health-promoting phytochemicals [6].
Several epidemiological studies have been reported that there is an inverse connection between consumption of Brassicaceae and risk of cancer [7-9]. Frequent consumption of cruciferous vegetables such as broccoli, cauliflower, leaf mustard, cabbages, radishes, turnips, and kale reduce the risk of developing chronic disorders, including cardiovascular diseases and in protecting against cancer [10, 11], stroke [12], diabetes [13], and even cancer [14, 15].
The antioxidant activities of some Brassica vegetables such as cabbage, broccoli, cauliflower, kale, turnip have been studied extensively. However; no study had been conducted on antioxidant components and antioxidant activity of Brassicarapavar. Parachinensis which is the main and common food at some countries specially Malaysia. Therefore, this present study was undertaken to measure and compare the composition and antioxidant activity of B. rapavar. Parachinensis leaves and seeds [5].
Material and Methods
Plant Materials
The plant was grown in the mixture soil with the ratio 3 top soil and 1 river soil at Taman Pertanian University, University Putra Malaysia from January – August 2014. Mature seeds were collected according to method by Delmas et al., 2013.
Preparation of Plant Extract
The amount of 100 mg expanding leaves and mature seeds were taken for each replicate. Samples were heated in 1 ml 80% ethanol for 20 minutes. The supernatant was collected and the residue was re-extracted until the samples turned to white.
Measurement of Total Chlorophyll
The extraction and measurement of total chlorophyll was conducted according to method reported by Lichtenthaler and Wellburn (1985) [16]. The chlorophyll was measured spectrophotometrically at 470nm, 645nm and 663nm. Total chlorophyll and carotenoids were calculated according to the formula:

Measurement of Total phenolics
The content of total phenolic was measured spectrophotometrically using the Folin-Ciocalteau method [17]. Sample extraction(5 µl)) was added to tubes containing 120 µl ultrapure water, 12.0 µl of Folin-Ciocalteau reagent and 47 µl of saturated Na2CO3 (20g Na2C03 in 100 ml distilled water). The mixture was mixed, followed by incubation for 1 hour at room tempreture and measured spectrophotometrically at 760nm. The total phenol content of pant parts was expresses as miligrams of tannic acid per equivalent per 0.1 gram of fresh sample (mg TAE/0.1g FW) from a cilibration curve.
Total Flavonoid
The measurement of total flavonoid was conducted with using aluminum chloride colorimetric assay [18]. The amount of 2% AlCl3 in 2 ml ethanol was added to 2 ml extraction, mixed well and left in darkness for 1 hour. Then, the mixture was measured spectrophotometrically at 420 nm and prepared reagent was used as the blank. The total flavonoid content is expressed as milligram of quercetin (QE)/0.1 g fresh weight.
Ascorbic Acid
Vitamin C content was determined by the titrimetric method as described in the Association of Official Analytical Chemists (AOAC,1995).Fresh plant (0.1 g)was extracted with 10 ml of 1% phosphate citrate buffer (28.39g Na2HPO4 was solved in 19.21g citric acid in 1 liter) using through chilled pestle and mortar. The homogenous extraction was filtered through filter paper (Whatman filter paper no. 1). The extraction was added to 1 ml of 1.7 mM 2, 6-Dicholorophenol Indophenol sodium (DCPIP) and mixed well followed by incubation at room temperature for 15 minutes. Ascorbic acid was measured spectrophotometrically at 520 nm and 3 ml of DCIP-reagent as blank. Vitamin C content was expressed as mg ascorbic acid (AAE) equivalent/0.1 g of plant on a fresh weight basis.
Antioxidant Assay
Extraction Method for Antioxidant Activity
Leaves and seeds (0.2 g) was harvested and extracted in 10 ml of deionized water. The mixture was left at room temperature for 1 h in the dark place with occasional agitation. The aqueous extract was obtained by filtering the mixture through filter paper (Whatman No. 1) and the filtrate was assayed for antioxidant activity.
DPPH Radical Scavenging Activity
The antioxidant activity was measured according to the method described by Wong et al. (2006) [19]. The initial absorbance of 3 ml of 0.1 m methanolic DPPH reagent was measured at 515nm. Leaves and seeds extraction (40 µl) was added to DPPH reagent and kept at room temperature. The differences in absorbance were measured after 30 minutes at 515 nm. The antioxidant capacity based on the DPPH free radical scavenging ability of the extract was expressed as µmole Trolox equivalent per gram and aascorbic acid was used a positive control. The percentage of the DPPH radical scavenging is calculated using the equation as given below:
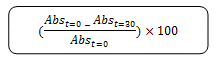
Abs t=0 is the absorbance of DPPP at time 0
Abs t=30 min is the absorbance of DPPP at time 30 min of incubation
Reducing Power Assay
The reducing power of crude extract was measured according to the method by Oyaizu (1986) [20]. Crude extracts dissolved in 1 m of distilled water and mixed with 2.5 ml of 0.2 mol/L phosphate buffer (pH 6.6) and 2.5 ml of 1% (w/v) potassium ferricyanide. The mixture was incubated at 50°C for 20 min. Following this, 2.5 mL of 10% (w/v) trichloroacetic acid were added and the mixture was centrifuged at 1750 x G for 10 min. A 2.5 ml aliquot of the upper layer was combined with 2.5 ml of distilled water and 500 µL of 0.1% (w/v) FeCl3. The absorbance of the reaction mixture was measured at 700nm. The capacity of samples extract to reduce the ferric-ferricyanide complex to the ferrus-ferricyanide complex of prussian blue was determined by measuring spectrophotometrically the absorbance at 700nm after incubation. Increased absorbance of the reaction mixture indicates greater reduction capability [21].
Analysis of Data
All data were analyzed using SPSS Windows version 21. T-test at significant level, p<0.05 was carried out to determine the significance difference between leaves and seeds. Tukey multiple comparison test was applied to determine which means are statistically difference if the ANOVA was significant.
Results and Discussion
Total Phenolic Content
Phenolic compounds, containing one or more acidic hydroxyl residues attached to an aromatic arene (phenyl) ring, are one of the most active constituents that contribute to the antioxidant activities of plant foods [22]. Therefore, it is great to measure phenolic content and determine its contribution to the antioxidant activity of plant materials. The total phenolic content of leaves and seeds was found to be 0.027±0.0005 and 0.016±.0012, respectively (Table 1). The total phenolic content was calculated using the following linear equation based on calibration curve of Tannic acid (y=13.283x, R2=0.997). Then, the phenolic contents are around two times higher on seeds in comparison with leaves. Studies on phenolic are increasing specially in the food industry because they delay oxidative degradation of lipids and thereby improve the quality and nutritional value of food will be improved [23].
Vitamin C Content
Vitamin C or ascorbic acid is a water-soluble vitamin that can be found ubiquitous in fresh fruits and vegetables. Vitamin C has many nutritional and clinical benefits for human health. Vitamin C is a bioactive compound that is involved in healing wounds, iron metabolism, tyrosine metabolism, conversion of folic acid to folinic acid, synthesis of lipids and protein, resistance to infections, and cellular respiration. Moreover, vitamin C is an important antioxidant because ofsuppressive effects on oxidation of other compounds over donation of its electrons [24]. We observed that seeds have higher ascorbic acid contents (0.22b±0.019 mg/0.1g) as compared to leaves (0.022a±0.004 mg/0.1g). It is obvious from the present study that total amount of ascorbic acid is 10 times higher on seeds in comparison with leaves (Table 1). The values obtained from seeds were higher than total amount of ascorbic acid in Savoy cabbage (0.049.06 ± 7.52 mg/0.1g) that was reported by Fernandez-Leon et al., (2014) [25].
Total Chlorophyll and Carotenoid Content
The total chlorophyll and carotenoid contents of B.rapa var. parachinensis leaves and seeds are presented in (Table 1). Leaves contain significantly higher amount in total chlorophyll, carotenoid than seeds (t- test, p<0.05). Carotenoids have many physiological functions. Carotenoids are precursors of vitamin A and also contribute to the prevention of cancer and cardiovascular diseases. Epidemiological studies proved that people, who have been taken high level of β-carotene in their daily diets, significantly reduced the risk of lung cancer. In addition, carotenoids are included as one of the most important free radical scavengers that have efficient antioxidant activity [26].
Total Flavonoid
The antioxidant activity of flavonoids and also its benefits as human nutrition and health care are importantly considerable [27]. The total flavonoid content of leaves and seeds shown in (Table 1). There is no significance difference of total flavonoid between leaves and seeds (t-test, p>0.05).It is well-known that compounds such as flavonoid, which contain hydroxyl functional groups, are responsible for antioxidant effect in the plants. Part of plant (mg/0.1g) (mg/0.1g) (mg/0.1g) (mg/0.1g) (mg/0.1g)
Total Chlorophyll
Carotenoid
Phenolic
Flavonoid
Ascorbic Acid
Leaves
49.96a±2.26
31.120a±126.1
0.027a±0.0005
3.04a±0.16
0.022a±0.004
Seeds
1.15b±0.24
8.877b±6.52
0.016b±.0012
3.10a±0.05
0.22b±0.019
Values followed by the same letter in the same column are not significantly different according to Independent t-test at p<0.05.
Antioxidant Activities between Leaves and Seed of B. Rapa Var. Parachinensis
DPPH Radical Scavenging Activity
The DPPH assay is one of the most widely preferable methods to measure the radical scavenging activity of plant extracts. This assay is rapid, relatively simple, and easily standardized [28]. DPPH has a stable nitrogen-centred free radical that exhibit a violet colour in methanol solution. When DPPH free radicals do reaction with suitable reducing agents (for example, antioxidants), the solution loses its violet colour depends on the number of electrons taken up [29]. In present study, the radical scavenging activity of aqueous extraction of leaves and seeds, and ascorbic acid at five concentrations (0.5, 1, 2, 5 and10_mg/l) was tested by DPPH method (Figure 1, 2). All the extracts demonstrated a capacity to scavenge the DPPH radical in the test run. However, the extracts of seeds have relatively high DPPH scavenging activity comparing with extracts from leaves (ANOVA, p<0.05).Studies on the capacity of Brassica seed as antioxidants are very limited while this study discovered that the seed extraction of B. rapa var. parachinensis have dramatically higher capacity (> 80% at 10mg/assay) as a DPPH radical scavenger than leaves. Therefore, seeds have higher content of antioxidant compounds with greater potential than leaves.
Seeds of tronchuda cabbage showed pro-oxidant activity for concentrations higher than 1.9 μg/ml, due to the high amount of ascorbic acid which exhibit higher antioxidant potential than its leaves [30]. The antioxidant capacity of tronchuda cabbage seeds higher than internal and external leaves. By measuring the DPPH activity, 2000 µg/ml of external leaves need to inhibit 95% and more than 4000 µg/ml of internal leaves need to inhibit 95%, compared with seed only need 400 µg/ml to inhibit more than 95%. This is not surprising, since seeds often contain the highest amount of lipids than any plant tissue, with high content of polyunsaturated fatty acids. The existence of high levels of phenolic compounds, and organic acids, namely ascorbic acid, in tronchuda cabbage seeds, indicate that these compounds protect storage lipids from oxidation, as observed with tocopherols [31], contributing to the viability of seeds and their rapid germination once oxygen demand during germination is high [32, 33]. The crude extract of mustard seeds showed more than 50% inhibition at 50µg/100µL assay [34]. Comparison studies also found that the antioxidant capacity of mustard seed higher (57% inhibition) than fenugreek seed (50.4 %) and poppy seed (44.0%) at 50µg/100µL assay. In the plant studied seeds displayed more than 66.0 % inhibition at the 2mg/ml assay tested that confirms others.
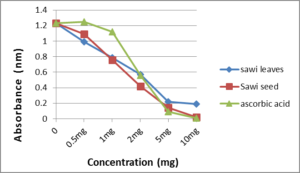
Figure 1: Scavenging effect of crude extracts of leaves and seed on 2,2-diphenyl-1-picrylhydrazyl radical as measured by changes in absorbance at 517nm.
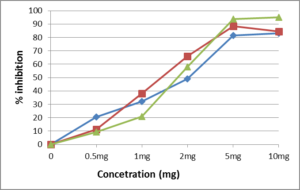
Figure 2: DPPH radical scavenging activity of leaves and seed of B.rapa var. parachinensis. Ascorbic acid used as positive controls. Each point averaged from triplicate measurements with standard errors falling within the data points.
Reducing Power
The reducing power between leaves and seed of B. rapavar. parachinensis and the reference compound (ascorbic acid) were determined. All extracts showed some degree of electron donation capacity in a concentration-dependent manner (Figures 3). The capacity of leaves was significantly lower (t-test, p<0.05) than seed. In addition, from (Table 2) shows that the reducing power of seed (1.03) significantly higher (t-test, p<0.05) than leaves (0.46) for 10 mg of extract in test. According to Ishtiaque et al. (2013) [34], the reducing power of mustard seed are higher (0.53) than fenugreek seed (0.47) and poppy seed (0.425) at 100µg/100uL. Methanol extractions of rapeseed (B. napus L) also have high reducing power (1.6) at 2.0mg/ml assay tested. Thus, B. rapavar.parachinensis seed has the high reducing power ability among the plants which studied with 0.78 reducing power at 1mg/ml assay tested.
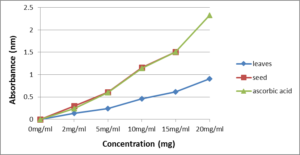
Figure 3: Reducing power of crude extracts of B. rapa var. parachinensis leaves and seeds. (10 mg/ml extract in test)
B. rapa var. parachinensis
Reducing Power
Leaves
0.46a ± 0.024
Seeds
1.03b ± 0.058
All the values are presented in mean ± SE (n=5). There was 10 mg of indicated extract in each sample. Standard ascorbicacid used and absorbance in 700 nm was 1.142 ± 0.000.
Conclusion
We evaluated the antioxidant activities and nutritional propertiesof leaves and seed of B. rapavarparachinensis. The results obtained in this study suggest that leaves and seeds of this plant constitute a good source of health-promoting compounds, namely, carotenoids, flavonoid, phenolic compounds, and ascorbic acid. It should be emphasized that leaves and seeds of this plant supply distinct phenolic. This could be of great relevance when biological activities are considered and deserves further studies. Nevertheless, the antioxidant capacity of leaves and seeds follows the order: seeds > leaves. The distinct chemical composition of these plant materials has a great influence in their antioxidant properties. Thus, the commercialization of standardized aqueous extracts of leaves and seeds to be used as antioxidants may be regarded as a possibility for both food and pharmaceutical industries. Seeds have a potential as a source of natural antioxidant in the food industry and pharmaceutical.
References
- Dembinska-Kiec A, Mykkanen O, Kiec-Wilk B, Mykkanene H (2008) Antioxidant Phyto-chemicals against Type 2 Diabetes. British J. Nutri 99: 109-117.
- Sin HP, Liu DT, Lam DS (2013) Life style Modification, Nutritional and Vitamins Supplements for Age-Related Macular Degeneration. Acta Ophthalmologica 91: 6-11.
- Willis LM, Shukitt-Hale B, Joseph JA (2009) Recent Advances in Berry Supplementation and Age-Related Cognitive Decline. Current opinion in clinical nutrition and metabolic care 12: 91-94.
- Vivek KG, Surendra KS (2006) Plants as natural antioxidants. Natural. Production. Radia 5: 326-334.
- Halliwell B (2007) Biochemistry of Oxidative Stress Bioche- chemical Society Transactions 35: 1147-1150.
- Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. The Journal of Nutrition 134: 3479S-3485S.
- Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y (2004) Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. The Journal of Nutrition 134: 1134-1138.
- Heber D, Bowerman S (2001) Applying science to changing dietary patterns. The Journal of nutrition 131: 3078S-3081S.
- Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA (1996) Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiology Biomarkers & Prevention 5: 733-748.
- Prochaska HJ, Santamaria AB, Talalay P (1992) Rapid detection of inducers of enzymes that protect against carcinogens. Proceedings of the National Academy of Sciences 89: 2394-2398.
- Zhang YH, Kramer TR, Taylor PR, Li JY, Blot WJ (1995) Possible immunologic involvement of antioxidants in cancer prevention. The American Journal of Clinical Nutrition 62: 1477S-1482S.
- Gillman MW, Cupples LA, Gagnon D, Posner BM, Ellison RC (1995) Protective effect of fruits and vegetables on development of stroke in men. Journal of the American Medical Society 273: 1113-1117.
- Craig WJ (1997) Phytochemicals: guardians of our health. Journal of the American Dietetic Association 97: 199-204.
- Ray A (2005 Cancer preventive role of selected dietary factors. Indian Journal of Cancer 42: 15-24.
- Stan SD, Kar S, Stoner GD, Singh SV (2008) Bioactive food components and cancer risk reduction. Journal of Cellular Biochemistry 104: 339-356.
- Lichtenthaler H, wellburn A (1985) Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transaction 11: 591-592.
- Harborne J (1989) General procedures and measurement of total phenolics. Methods in Plant Biochemistry 1: 1-28.
- Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food chemistry 64: 555-559.
- Wong SP, Leong LP, William Koh JH (2006) Antioxidant activities of aqueous extracts of selected plants. Food Chemistry 99: 775-783.
- Oyaizu M (1986) Studies on products of browning reaction: Antioxidative activity of products of browning reaction. Japanese Journal of Nutrition 44: 307-315.
- Gülçin I, Elmastaş M, Aboul-Enein HY (2007) Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum Family Lamiaceae) assayed by different methodologies. Phytotherapy Research 21: 354-361.
- Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of Agricultural and Food Chemistry 46: 4113-4117.
- Aneta W, Jan O, Renata C (2007) Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry 105: 940-949.
- Suntornsuk L, Gritsanapun W, Nilkamhank S, Paochom A (2002) Quantitation of vitamin C content in herbal juice using direct titration. Journal of Pharmaceutical and Biomedical Analysis 28: 849-855.
- Fernandez-Leon AM, Lozano M, Gonzalez D, Ayuso MC, Fernandez-Leon MF (2014) Bioactive Compounds Content and Total Antioxidant Activity of Two Savoy Cabbages.Czech Journal of Food Science 32: 549-554.
- Machlin LJ (1995) Critical assessment of the epidemiological data concerning the impact of antioxidant nutrients on cancer and cardiovascular disease. Critical Reviews in Food Science and Nutrition 35: 41-49.
- Pourmorad F, Hosseinimehr SJ, Shahabimajd N (2006) Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. African Journal of Biotechnology 5: 1142-1145.
- Nanjo F, Goto K, Seto R, Suzuki M, Sakai M (1996) Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radical Biology & Medicine 21: 895-902.
- Umamaheswari M, Chatterjee TK (2008) In vitro antioxidant activities of the fractions of Cocciniagrandis L. leaf extract. African Journal of Traditional 5: 61-73.
- Sousa C, Taveira M, Valentão P, Fernandes F, Pereira JA (2008) Inflorescences of Brassicacea species as source of bioactive compounds: A comparative study. Food Chemistry 110: 953-961.
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. The Plant Cell Online 16: 1419-1432.
- Andarwulan N, Fardiaz D, Wattimena GA, Shetty K (1999) Antioxidant activity associated with lipid and phenolic mobilization during seed germination of Pangium edule Journal of Agricultural and Food Chemistry 47: 3158-3163.
- Randhir R, Shetty K (2005) Developmental stimulation of total phenolics and related antioxidant activity in light-and dark-germinated corn by natural elicitors. Process Biochemistry 40: 1721-1732.
- Ishtiaque S, Khan N, Siddiqui MA, Siddiqi R, Naz S (2013) Antioxidant potential of the extracts, fractions and oils derived from oilseeds. Antioxidants 2: 246-256.