Publication Information
ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Biopreservation of Meat by Using Antimicrobial Proprieties of Essential Oil from Laggera Aurita in Burkina Faso
Henriette B. Mihin1*, Marius K. Somda1,2, Donatien Kabore3, Souleymane Sanon4, Agbémébia Y. Akakpo1,5, Alfred S.Traore2, Aboubakar S. Ouattara1
1Laboratory of Microbiology and Microbial Biotechnology, Research Center in Biological Food and Nutrition Sciences (CRSBAN), Department of Biochemistry and Microbiology, University Joseph KI-ZERBO. 03BP:7021, Burkina Faso.
2Laboratory of Food Technology, Department of Biochemistry and Microbiology, University Joseph KI-ZERBO. 03BP:7021, Burkina Faso.
3Department of Food Technology, National Center of Scientific and Technological Research. 03BP:7047, Ouagadougou, Burkina Faso.
4National Center of Research and Training on Malaria, National Center of Scientific and Technological Research. 01 BP 2208 Ouagadougou, Burkina Faso.
5Laboratoire of Quality Control and Normalisation (LCQN), Institute of Agronomical Research of Lome. BP: 1163, Togo.
Received Date: 21 May, 2019; Accepted Date: 31 May, 2019; Published Date: 10 June, 2019
*Corresponding author: Henriette B. Mihin, Laboratory of Microbiology and Microbial Biotechnology, Research Center in Biological Food and Nutrition Sciences (CRSBAN), Department of Biochemistry and Microbiology, University Joseph KI-ZERBO. 03BP:7021, Burkina Faso. Email: mhenrietteb@gmail.com
Citation: Mihin HB, Somda MK, Kabore D, Sanon S, Akakpo AY, Traore AS, Ouattara AS (2019) Biopreservation of Meat by Using Antimicrobial Proprieties of Essential Oil from Laggera Aurita in Burkina Faso. Adv Nutri and Food Sci: ANAFS-134.
Abstract
Essential oils are natural substances which can be used as natural conservators for foods. The present work aimed to evaluate biological proprieties of essential oil of Laggera aurita and its effect on shelf-life of beef. The extraction was carried out by hydro-distillation. Antioxidant activity was determined by method of DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging and FRAP (Ferric reduction antioxidant power). Antimicrobial activity was determined by microdilution and diffusion. Eleven pathogens strains were tested. The conservation test of ground meat using essential oil was carried out by monitoring the evolution of different microbial groups. Extraction showed 0.22 % yield of essential oil. The DPPH assay gave IC50 of 10.562 ± 0.3305 µL. The essential oil of Laggera aurita showed inhibitory activity on Bacillus cereus (50.000 ± 2.143 mm), Listeria monocytogenes (48.167 ± 2.472 mm), Staphylococcus aureus (47.667 ± 1.027 mm) and Clostridium perfringens (30.167 ± 1.472 mm). The MIC varied from 0.065 ± 0.009 % to 2.083 ± 0.139 % and the MBC from 0.312 ± % to 5.000 %. Biopreservation assay using essential oil revealed an important decrease of microbial charge in the ground. The results demonstrated that essential oil from Laggera aurita could be used as natural conservator for food industry.
Keywords: Laggera aurita, Essential oil, Biopreservation, Biological activities, Meat
Introduction
The sanitary quality of a food is one of essential bases of its ability to satisfy the safety of the consumers. A food exposed to microbial pooling could lose its organoleptic, nutritional and sanitary qualities [1]. Despite the improved techniques for preserving food, the kind of preservatives remains one of the most important issues for public health [2]. One of main problems in food industry is to ensure safe food preservation for consumers [3]. To cope with the problems of oxidation and contamination of foodstuffs, new chemicals compounds have often been used to prevent the deterioration of food [4]. Preservatives such as butylate hydroxyanisole (BHA), butylate hydroxytoluene (BHT) and tert-butylhydroquinone (TBHQ) used in food preservation have been limited in several countries due to their undesirable toxicological and carcinogenic effects at short or long time [5]. Also, the current trend of consumers to seek a more natural diet has increased. The demand of customers for natural preservative has directed to researchers interest to develop protective methods for reliable foods having better nutritional and organoleptic proprieties with high microbial quality [6]. Aromatic plants have interesting properties due to metabolic substances during its secondary metabolism [5]. These essential oils are getting more interest for industries and scientific research due to antioxidant, antibacterial and antifungal activities [7]. They are also useful as natural preservatives in agro-food industries [8, 9]. Essential oils are a source of natural preservatives for perishable dairy products in Sub-Saharan Africa who’s the capacity of food preservation by cold chain is very limited. Among commodities, meat products are very susceptible to deterioration. Poorly preserved meat can lead to economic losses and health consequences in most of developing countries. In Burkina Faso, beef is a food of choice because of its important intake protein. However, rapid deterioration can be observed due to microbial contamination. Some microorganisms can be pathogens and harmful for consumers health. To overcome or stop meat spoiling, it is important to use natural preservatives such as essential oils. Although the antimicrobial activity of essential oils derived from plants has been proved by in vitro tests, many researches are required in order to investigate their activities on food. The present study aimed to investigate the biological properties of essential oils from Laggera aurita as well as it capacity to prolong shelf-life of meat.
Material and Methods
Plant Material
The leaves and the flowers of Laggera aurita were collected in different areas of Ouagadougou. The identification of the plant (Laggera aurita) was carried out by the Laboratory of Plant Ecology Biology in University Joseph KI-ZERBO. Fresh plant material was dried at room temperature for 72 hours. The dry matter was reduced to powder and packaged in bags for extraction.
The antimicrobial activity study focused on eleven (11) microbial strains:
- Nine (09) bacterial strains including 5 Gram-negative (Escherichia coli ATCC8739, Salmonella typhi, Salmonella paratyphi, Shigella dysenteria, and Pseudomonas aeruginosa ATCC9027) and 4 Gram-positive strains (Staphylococcus aureus ATCC25923, Listeria monocytogenes, Clostridium perfringens and Bacillus cereus)
- Two (02) fungal strains (Candida albicans and Aspergillus niger).
Food matrix: Ground beef
Ground beef was purchased at Zogona market (Ouagadougou). The meat samples were placed in icebox to maintain refrigerated conditions and transported to the laboratory.
Essential oil extraction
The essential oil was extracted from the plant material by hydro-distillation according to the method described by Baser and Buchbauer [10]. 500 g of dry matter was immersed in a Clevenger type apparatus containing about 5 L of distilled water. The mixture was boiled for three (03) hours. The obtained essential oil was collected in a sterile amber glass bottle for preservation. Yield, expressed as a percentage (%), was calculated using the following equation:
Yield = (Mass of essential oil (g)) / (Mass of dry matter (g)) × 100
Analysis of the organoleptic properties of essential oil
Organoleptic properties such as color, appearance and odor have been determined directly by the sense organs. The essential oil was first transferred to a transparent bottle.
Determination of biological activities of essential oil
Antioxidant activity
DPPH radical scavenging assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) test was performed according to the method described by Joshi and Collaborators [11]. Different volumes of essential oil (5, 10, 15, 20 and 25 μL) were mixed with 5 mL of ethanolic solution of DPPH (0.004% weight/volume). The mixture obtained was incubated in dark space for 30 minutes and absorbance was read at 517 nm using a spectrophotometer (Jasco, Japan). Butylhydroxytoluene (BHT) (0.05M), ascorbic acid (0.005M) and quercetin (0.05M) (5, 10, 15, 20 and 25 μL) were used as standard antioxidants and a negative control was also prepared in the same conditions like the samples. The tests were carried out in triplicate.
The pourcentage of inhibition (% I) was calculated according to the following equation:
% Inhibition = [(Ablank⎯Asample) / Ablank] × 100; where Ablank is the absorbance of the negative control and Asample is the absorbance of the essential oil.
The antioxidant activity of the essential oil was expressed by the 50% inhibitory concentration (IC50) which is the amount of essential oil needed to reduce the initial concentration of DPPH by 50%. The 50% inhibitory concentration was calculated using a regression line (% inhibition = f (concentrations)). The tests were carried out in triplicate.
Ø Ferric reduction antioxidant power (FRAP)
The reducing power of essential oil of Laggera aurita was determined according to the method of Singh and Collaborators [12]. Different volumes of essential oil (5, 10, 15, 20 and 25 μL) were mixed with 2.5 mL of phosphate buffer (200 mM, pH 6.6) and 2.5 mL of potassium ferricyanide (K3Fe(CN)6) at 1%. The mixture was incubated at 50 °C for 30 min then 2.5 mL of trichloroacetic acid (10%) were added to the mixture followed by centrifugation at 650 G for 10 min. The supernatant was collected (5 mL) and mixed with 5 mL of distilled water and 1 mL of 0.1% iron (FeCl3) chloride. Absorbance was then measured at 700 nm using a spectrophotometer. Ascorbic acid was used as a standard at 5 mg/mL, A negative control (blank) was also included in each test. The standard and blank were prepared according to the process of essential oil. An increase in absorbance indicates an increase in reducing power. A standard curve of absorbance of essential oil was designed. The tests were carried out in triplicate.
Determination of antimicrobial activity
Preparation of the inoculum
A suspension of each bacterial strain was prepared in 10 mL of Mueller-Hinton Broth for 18-24 hours at 37 °C. Using the sterile diluent (physiological saline), the concentration was adjusted in each tube to about 1.0 108CFU/mL comparable to that of the McFarland 0.5 standard according to Lennette [13]. For the fungal suspension, each strain was prepared in Sabouraud broth for 18 to 48 hours at 30°C. With a sterile diluent, the concentration was adjusted to about 1.0 108 CFU/mL comparable to that of the McFarland 0.5 standard [13].
Microbial strains
Ø Agar diffusion method
Petri dishes containing Sabouraud Chloramphenicol medium (for fungal strains) and Mueller-Hinton agar (for bacterial strains) were inoculated aseptically with inoculum. Seeding was done by flooding the Petri dish and the excess was aspirated. After drying the dishes, the agar was perforated diameter. A volume of 5 μL of Laggera aurita essential oil was injected into different wells. The dishes were exposed at room temperature for one (1) hour before incubation to promote diffusion of essential oil on agar plate. The dishes were incubated at 37°C for 24 hours for bacteria and at 30°C for 48 hours for fungal strains. The results were read by measuring the diameters of inhibition space [14, 15].
The activity of essential oil was ranged according to the diameter of inhibition values according Negreiros and Collaborators [23]. The microbial strains were ranged as non-sensitive when a diameter is less than 8 mm, sensitive from 9 mm to 14 mm, high sensitive from 15 mm to 19 mm and extremely sensitive for more than 20 mm.
Ø Determination of minimum inhibitory concentration (MIC): MIC values of essential oil against microbial cells were determined by method of broh dilution.
Determination of the minimum inhibitory concentration (MIC) was performed in a sterile 96-well microplate. For bacterial strains, Muller-Hinton broth supplemented with tween 80 was used to increase the solubility of the essential oil (HE). Thus, 190 μL of Muller-Hinton broth supplemented with tween 80 (0.5%) were introduced into the wells of line 1 and 100 μL into the other wells of the microplate from line 2 to line 12, then 10 μL of ssential oil were added to the wells of line 1. The contents of the wells of line 1 were well mixed. Cent (100) μL were collected from these wells (line 1) for cascaded dilutions in the other wells up to the 0.004% concentration. The inoculum density was adjusted with sterile saline solution (NaCl 0.9%) to McFarland 0.5 corresponding to 108 CFU / mL. Cent (100) μL of bacterial inoculum was added to all wells except the wells in line 11 which contained only the essential oil and Muller-Hinton broth. Line 11 (without inoculum) served as a negative control. Line 12 containing Muller-Hinton broth and bacterial suspension served as a positive control. The microplates were closed and incubated for 18 to 24 hours at 37 ° C [17, 18].
For fungal strains, Sabouraud broth supplemented with tween 80 (0.5%) was used. The same procedure was performed for experiment. The microplates were incubated at 30°C for 48 to 72 hours. Microbial growth is indicated by optical density of culture [17]. The lowest essential oil concentration inhibiting the bacterial growth after 24 hours, incubation was identified as minimum inhibitory concentration (MIC). The treatments were made for three times and mean values were calculated.
Ø Determination of minimum bactericidal concentration (MBC)
One hundred (100) μL were taken from wells without detectable growth after 24 hours of incubation at 37°C and seeding was reducing by spreading on Mueller-Hinton [19]. The lowest concentration at which 99.99% of bacteria cells after 24 hours of incubation was identified as minimum bactericidal concentration (MBC). Experiments were done for three times.
Ø Determination of minimum fungicidal concentration (MFC)
A volume of one hundred (100) μL was collected from wells that did not present detectable growth after incubation at 30°C for 72 hours. It was seeded on Sabouraud Chloramphenicol agar [19].
Ø Determination of ratio MBC/MIC and MFC/MIC
The ratio MBC/MIC and MFC/MIC allowed to determine the bactericidal or bacteriostatic and fungicidal or fungistatic capacities of essential oil on the strains tested. According to Canillac and Mourey, Derwich and Collaborators [20, 21]:
When MBC/MIC> 4: The essential oil is called bacteriostatic;
MBC/MIC≤4: The essential oil has bactericidal property;
MFC/MIC <4: The essential oil has fungicidal effect;
MFC/MIC≤4: The essential oil has fungistatic effect.
Shelf-like test of essential oil
The essential oil concentration which is necessary so that it exerts its power antibacterial in a food matrix must be higher than that applied "in vitro". ¶Burt [2] suggested that the values of the ICM of HE obtained "in vitro" must be affected by a weighting going from 2 to 100, so that they have the same effect in a food matrix.
In this study the fresh ground meat has been separated in two Stomacher bags by 50 g per sachet. The essential oil of Laggera aurita (20 μL) was incorporated in a bag containing ground meat and the second bag containing only ground meat was used as control. The samples were stored in the refrigerator at 4°C for 7 days. Microbiological analysis was carried out 1st day, 4th day and 7th day on ground meat treated with the essential oil and on the control.
Microbiological analysis of minced meat during storage
The bacterial evolution in the samples was monitored during storage in the refrigerator at 4°C for 7 days. Enumeration included total mesophilic aerobic flora (TMAF), total coliforms (TC) and fecal coliforms (CF). It was previously performed on the meat samples before their treatment with the essential oil. The evolution of the bacterial flora was followed on day 1, day 4 and day 7 of conservation.
Enumeration of the total mesophilic aerobic flora
Ten (10) g of ground meat were taken from each sample and introduced into 90 mL of sterile peptone water and then homogenized in a stomacher 400 (England). Decimal dilutions were prepared from the stock solution. One (01) mL of the dilutions were taken and seeded on Petri dishes containing Plate Count Agar (PCA) agar. Incubation was carried out at 37ºC for 72 h followed by enumeration of the number of microorganisms [22].
Enumeration of total and thermotolerants coliforms
Ten (10) g of ground meat were taken from each sample and introduced into 90 mL of sterile peptone water and then homogenized with a stomacher. Decimal dilutions were prepared from the stock solution. One (01) mL of the dilutions was taken and seeded on Petri dishes containing the Eosin Blue Methylene Agar. Petri dishes were incubated at 37ºC for 24 h for total coliforms and at 44ºC for 24 h for fecal coliforms. The total coliforms were counted according to the method of the international standard ISO 4832 [23] while the thermotolerants coliforms were counted according to AFNOR NF V 08-060 [24].
Statistical analysis
The data were collected on Excel and analyzed using XLSTAT software version 7.5.2. Variance analysis (ANOVA) was used and Fisher's Least Significant Difference (LSD) was used for comparisons of means in case of significant difference. The difference between means was significant when p value < 0.05. The values were estimated with a 95% confidence interval.
Results and discussion
Yield and organoleptic characteristics of essential oil from Laggera aurita
The essential oil yield of leaves and flowers from Laggera aurita obtained by hydro-distillation was 0.22%. This yield is lower than those found (0.3%) by Samate [25] in Burkina Faso, on a sample collected during the flowering period. It is higher than those obtained by Kabera and collaborators [26] in Benin, which was 0.008% on the same plant. The difference in yield may be due to geographical origin, ecological climatic factors such as temperature and humidity, links of soil, plant organ, level of growth, harvest period, conservation plant material, extraction time and method [27, 28, 29].
The organoleptic characteristics of essential oil are summarized in (Table 1).
Color
Yellow gold
Appearance
Fluid, clear, transparent, shiny
Odor
Strong, aromatic, characteristic of the plant
The essential oil from Laggera aurita was golden yellow in color, fluid, clear, transparent and shiny with a strong, aromatic and characteristic smell of the plant. It can become cloudy by lowering the temperature.
Biological properties of essential oil from Laggera aurita
Antioxidant activity
Ø Capacity of inhibition of the radical DPPH
The antioxidant activity of Laggera aurita essential oil was determined and compared to the reference antioxidants as ascorbic acid, BHT and quercetin. (Figure 1) showed the variation of the percentage of inhibition following to quantity of essential oil and standards.
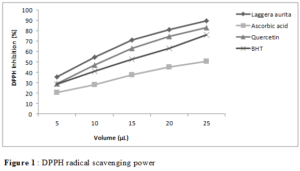
The percentage of inhibition of free radical was inversely proportional to the increase of essential oil and standards quantities. Statistical analysis showed a significant difference (p <0.0001) between percentage of inhibition of essential oil and standards.
The concentration needed to reduce the DPPH free radical at 50% was determined from the linear regression equations. The different values of 50% inhibitory concentration (IC50) were exhibited in regressions equations and presented in (Table 2).
Essential oil and standards
Regression equation
R2
IC50 (µL)
Laggera aurita
Y = 2.4546x + 24.059
0.9884
10.562 ± 0.331
Ascorbic acid (0.005 M)
Y = 1.7179x + 12.080
0.9894
22.098 ± 0.128
Quercetin (0.05 M)
Y = 2.9372x + 14.316
0.9967
12.142 ± 0.234
BHT (0.05 M)
Y = 2.083x + 22.891
0.9908
13.013 ± 0.053
Laggera aurita essential oil was found to have greater antioxidant power than standards as ascorbic acid, BHT and quercetin (Table 2). In fact, the 50% inhibitory concentration (IC50) of Laggera aurita was 10.562 ± 0.331 μL compared to those of ascorbic acid, BHT and quercetin, which were respectively 22.098 ± 0.128 μL; 13.013 ± 0.053 μL and 12.142 ± 0.234 μL.
Ø Reducing power of essential oil of Laggera aurita
The diagram showing the variation of the reducing power according to the volume of essential oil and the standard has been recorded in (figure 2).
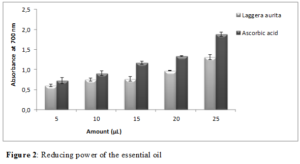
The results obtained by the FRAP test showed that the reduction of ferric ions was related to the amount of essential oil and that the capacity of essential oil to reduce iron was lower than those of ascorbic acid. The evaluation of the antioxidant activity of Laggera aurita essential oil by the DPPH and FRAP test revealed its antioxidant properties. The biological activities of essential oil of Laggera aurita could be attributed to its high concentration of α-cadinol and ˥-cadinol [30]. Previous studies have shown that oxygenated monoterpenes such as thymol, carvacrol and α-terpineol where mainly responsible for antioxidant potential of essential oils [31]. Monoterpenes, such as β-caryophyllene, also have free radical scavenging activity [32].
Antimicrobial activity
Inhibiting capacity of essential oil of Laggera aurita
The average inhibition diameters due to Laggera aurita essential oil as well as their interpretation according to the criteria set by Negreiros and Collaborators [16] were mentioned in (Table 3).
Bacterial strains
Diameters of inhibition (mm)
Sensitivity of strains
Escherichia coli ATCC8739
19.167 ± 1.546
Very sensitive
Salmonella paratyphi
13.667 ± 1.247
Sensitive
Salmonella typhi
14.000 ± 1.633
Sensitive
Shigella dysenteria
12.500 ± 1.871
Sensitive
Pseudomonas aeruginosa ATCC9027
14.833 ± 2.014
Sensitive
Bacillus cereus
50.000 ± 2.143
Extremely Sensitive
Staphylococcus aureus ATCC25923
47.667 ± 1.027
Extremely Sensitive
Listeria monocytogenes
48.667 ± 1.472
Extremely Sensitive
Clostridium perfringens
30.167 ± 1.472
Extremely Sensitive
Fungal strains
Candida albicans
9.667 ± 0.17 1
Sensitive
Aspergillus niger
14.833 ± 0.224
Sensitive
The results in (Table 3) showed that essential oil has antimicrobial activity. The importance of the action of the essential oil of Laggera aurita varied according to the microorganism tested.
The essential oil of Laggera aurita showed antimicrobial activity on all strains tested. The largest diameter was obtained on B. cereus (50 mm) followed by L. monocytogenes (48.667 mm) and S. aureus (47.667 mm). The smallest diameter was observed with C. albicans (9.667 mm). The antibacterial activity of the essential oil of Laggera aurita was depending to the Gram of bacteria. In fact, Gram-positive bacteria were more sensitive to Gram-negative. These results corroborate with those of Shahwar and collaborators [33] who showed that Gram-positive bacteria were more sensitive to Laggera aurita essential oil than Gram-negative ones.
For the sensitivity of microbial strains to the essential oil, B. cereus, S. aureus ATCC25923, L. monocytogenes and C. perfringens were extremely sensitive E. coli ATCC8739 was highly sensitive. S. paratyphi, S. typhi, S. dysenteria, P. aeruginosa ATCC9027, C. albicans, and A. niger were sensitive to the essential oil.
The antimicrobial activity could be explained by the presence of phenolic hydroxyl groups able to form hydrogen bonds with the active sites of the targeted cell enzymes [21]. The low sensitivity of Gram-negative bacteria to essential oil of Laggera aurita could be related to the complexity of the cell envelope of microorganisms that have a double membrane, different to simple membrane structure of Gram-positive bacteria [2]. The biological activity of an essential oil was related to its chemical composition, the functional groups of the major compounds (alcohols, phenols and aldehydes) and the synergistic effects between the components [34].
Ø Minimum concentrations of essential oil of Laggera aurita
The results of the determination of minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) were reported in (Table 4).
Microorganisms
Minimal concentration (%)
Bacterial strains
MIC
MBC
Escherichia coli ATCC8739
0.065±0.009c
0.312±0.000b
Salmonella paratyphi
0.416±0.102c
4.167±0.123a
Salmonella typhi
0.521±0.128c
5.000±0.000a
Shigella dysenteria
0.416±0.124c
4.167±0.029a
Bacillus cereus
1.667±0.089ab
4.167±0.120a
Pseudomonas aeruginosa ATCC9027
0.130±0.037c
0.521±0.132b
Staphylococcus aureus ATCC25923
0.416±0.108c
0.416±0.141b
Listeria monocytogenes
0.130±0.036c
0.833±0.095b
Clostridium perfringens
0.260± 0.074c
1.250±0.000b
Fungal strains
MIC
MFC
Candida albicans
2.083 ±0.139a
5.000±0.000a
Aspergillus niger
1.250± 0.000b
1.250±0.000b
Values in the same column with the same superscript letters are not significantly different (p <0.05).
MICs ranged from 0.065% to 2.083%. The highest MIC of essential oil was obtained on C. albicans (2.083%) and the lowest MIC on E. coli (0.065%). MBCs ranged from 0.312% to 5%. The highest concentration was observed on S. typhi and C. albicans and the lowest on E. coli. The essential oil of Laggera aurita showed a significant inhibitory effect on all the bacteria tested.
Ø Ratio MBC/MIC and MFC/MIC of essential oil from Laggera aurita
The results of ratio MBC/MIC and MFC / MIC were presented in (Table 5).
Bacterial strains
MBC/MIC
Sensitivity of strains
Escherichia coli ATCC8739
4
Bactericidal
Salmonella paratyphi
10
Bacteriostatic
Salmonella typhi
9
Bacteriostatic
Shigella dysenteria
10
Bacteriostatic
Pseudomonas aeruginosa ATCC9027
4
Bactericidal
Bacillus cereus
2
Bactericidal
Staphylococcus aureus ATCC25923
1
Bactericidal
Listeria monocytogenes
6
Bacteriostatic
Clostridium perfringens
4
Bactericidal
Fungal strains
MFC/MIC
Candida albicans
2
Fungicidal
Aspergillus niger
1
Fungicidal
The ratio MBC/MIC and MFC/MIC revealed differents activities of essential oil of L. aurita as bactericidal or bacteriostatic and fungicidal or fungistatic properties on eleven (11) strains tested. The essential oil of Laggera aurita showed bactericidal effect against B.cereus, P. aeruginosa, S. aureus and C. perfringens. It effect was bacteriostatic against E. coli, S. paratyphi, S. typhi, S. dysenteria and L. monocytogenes. This essential oil indicated also fungicidal on C. albicans and A. niger.
Effect of essential oil on prolonging shelf-life of food matrix
Initial microbial load of ground meat
Microbial control was done to know it initial contain of microorganisms, before testing the capacity of essential oil to prolong shelf-life of ground meat. The control concerned total aerobic mesophilic flora, total and thermotolerants coliforms. The initial count of microorganisms in the meat was given in the Figure 3.
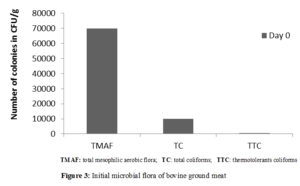
The results showed means values as 7.104 CFU/g, 104 CFU/g and 5.102 CFU/g respectively for total initial aerobic mesophilic flora (TMAF), total coliforms (TC) and thermotolerants coliforms (TTC). The high concentration of TMAF could reduce shelf-life of meat. The presence of coliforms indicates recent fecal contamination that may be explained by the lack of good hygienic practices throughout the meat production chain.
Effect of essential oil on total mesophilic aerobic flora (TMAF)
The evolution of total mesophilic aerobic flora was monitored for untreated ground meat (control) as well as meat incorporated with essential oil. The results obtained were presented in (Figure 4).
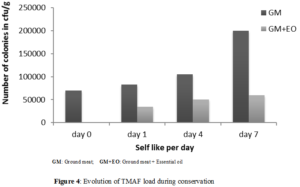
(Figure 4) showed the evolution of TMAF during storage. Thus, on Day 1, microbial load was 8.3x104 CFU/g for untreated ground meat, 3.5.104 CFU/g for Laggera aurita GM + EO. On day 7, microbial load was 2.105 CFU/g for untreated ground meat, 6.104 CFU/g for Laggera aurita GM+EO. An increase in microbial load was noted during storage for the control. This increase was greater in untreated ground meat than in that sample containing essential oil. The reduction of microorganisms number of in minced meat containing essential oil was 40% at 1st day and 54% at 7th day. This reduction could be explained by the inhibitory effect of essential oil on bacterial strains. The increase in microbial load in the meat incorporated with essential oil during storage could be related to the bacteriostatic effect of essential oil on some bacterial strains, as well as to the loss of the activity of certain compounds bioactive during conservation. This increase could also be explained by the high fat content of microorganisms that could significantly reduce action of essential oils in ground meat by forming a protective layer of fat around the bacteria. The lipid fraction in the food can absorb the antimicrobial agent by decreasing its concentration and effectiveness [2]. The presence of macromolecules (lipids or proteins) in the food matrix could protect bacteria to the action of the essential oils [35]. The bioactivity of the essential oil could be reduced by some components of food (greases, carbohydrates, proteins, water, salt, antioxydants, condoms, other additives) and pH [36].
Effect of essential oil on total coliform
The evolution of the number of total coliforms in untreated ground meat and in that incorporated by the Laggera aurita essential oil was recorded in (Figure 5).
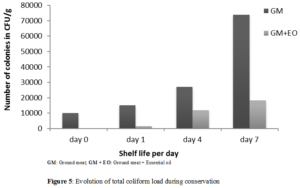
The number of total coliforms at Day 1 was 1.5 x 104 CFU/g for untreated ground meat; 1.4.103CFU/g for ground meat soaked in Laggera aurita essential oil. After seven days of storage (Day 7), this number was 7.4x104CFU/g for untreated ground meat and 1.83.104CFU/g for ground meat with oil essential of Laggera aurita.
An increase in total coliforms during conservation has been observed. This increase was higher in untreated ground meat than sample containing essential oil. This reflects an important antibacterial activity of essential oil of Laggera aurita. The reduction of microorganisms in ground meat containing essential oil was 82% at 1st day and 60% at 7th day. The evolution of bacterial load in meat supplemented with essential oil could be explained by the fact that a high fat content can significantly reduce the action of essential oils in meat products [2]. The presence of macromolecules in the food matrix could also protect bacteria from the action of essential oils [35]. Some bioactive molecules may also lose their activity during storage, which will allow the bacteria to repair the damage and be able to proliferate.
Effect of the essential oil on the evolution of the thermotolerants coliforms load
The evolution of the number of fecal coliforms during refrigeration has been presented in (Figure 6).
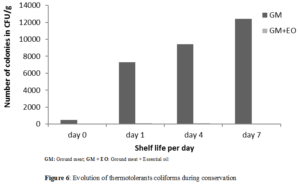
For thermotolerant coliform evolution, the microbial ranged from 7.3.103 CFU/g (Day 1) to 1.24.104 CFU/g (Day 7) for the untreated sample. This number varied from 102 CFU/g (Day 1) to 8.101 CFU/g (Day 7) for the sample treated with Laggera aurita essential oil. Unlike untreated minced meat, a 98% reduction in the number of fecal coliforms in ground meat incorporated with essential oil during storage. The almost complete inhibition of the number of fecal coliforms could be explained by the bactericidal effect of this essential oil on E. coli. The ability of hydrophobic antibacterial components of the essential oil to diffuse into the fat of the food matrix may inhibit and thus prevent their contact with bacteria in hydrophilic regions in foods [37]. Thus the proportion of contact between the bioactive substances and the germs could explain the persistence of thermotolerants coliforms in the sample treated with the essential oil of Laggera aurita.
The results of the preservation tests confirm those of the antimicrobial activity observed previously by this essential oil. These results corroborate with several works that have dealt with the application of essential oils to foods in order to reduce the microbial flora and increase its shelf life. Thus, the studies of Caillet and Lacroix [38], showed that the incorporation of the essential oil in ground meat contributed to the maintenance of microbiological quality and the reduction of fat oxidation beyond its normal storage life.
The improvement in food safety is due to the inhibition of pathogenic microbial growth and reduction of biogenic amines, mainly in meat and meat and dairy products, as a consequence of the inhibited growth of spoilage microorganisms. The extension of food products shelf-life results from enzymatic reduction, mainly due to their antioxidant activity. The essential oils effectiveness is attributed to the presence of phenolic natural compounds and they are an important and healthy alternative to synthetic preservatives and chemical additives [39].
Conclusion
The essential oil of Laggera aurita was extracted with a good yield. It has presented organoleptic and biological properties very appreciable. Conservation test results showed that Laggera aurita essential oil could be used as a natural preservative in the food industry. The essential oil of Laggera aurita is a potential natural antimicrobial with its immediate effect in food industry.
Conflict of interest: Authors have declared no conflict of interest.
References
- Guiraut, J. P. (2003). Microbiologie alimentaire. Ed. DUNOD, Paris.110 -112.
- Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods. A Review. International Journal of Food Microbiology, 94, 223 –253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022.
- Nessrien, M. N. Y., & Mohamed, A. T. (2007). Antioxidant and antimicrobial effects of marjoram and thyme in coated refrigerated semi fried mullet fish fillets. World Journal Dairy & Food Science, 2 (1), 01–09. ISSN 1817-308X.
- Nakahara, K., Alzoreky N.S., Yoshihashi T., Nguyen H. T. T., (2003a). Chemical composition and antifungal activity of essential oil from Cymbopogon nardus (Citronella Grass). Japan Agricultural Research Quarterly, 37(4), 249–252. Record ID:JP2005000711.
- Rashid, A., Qureshi, M. Z., Raza, S. A., William, J., Arshad, M. (2010). Quantitative determination of antioxidant potential of Artemisia persica. Bucuresti – Chimie (serie Noua), 19(1), 23–30. ebscohost.com.
- Goni P., Lopez P., Sanchez-Jarabo C., Nerin C. (2009). Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chemistry 116(4):982-989. DOI: 10.1016/j.foodchem.2009.03.058.
- Dung, N. T., Kim, J. M., Kang, S. C. (2008). Chemical composition, antimicrobial and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb.) Merr and Perry buds. Food and Chemical Toxicology, 46, 3632–3639
- Gachkar, L., Yadegari, D., Rezaei, M. B., Taghizadeh, M., Astaneh, S. A., & Rasooli, I. (2007). Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chemistry, 102 (3), 898–904. DOI: 10.1016/j.foodchem.2006.06.035.
- Rasooli, I., Fakoor, M.H., Yadegarinia; D., Gachkar, L., Allameh, A., Rezaei, M. B. (2008). Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum essential oils. International Journal of Food Microbiology, 122(1) 135–139. DOI:10.1016/j.ijfoodmicro.2007.11.048.
- Baser, K.H.C., Buchbauer, G. (2010). Handbook of essential oils: Science, Technology, and Applications. Ed. Taylor and Francis Group, LLC. United States of America. 994p.
- Joshi, L., Gupta B., Prakash, R. (2010). Chemical synthesis of poly(5-carboxyindole) and poly(5- carboxyindole)/carboxylated multiwall carbon nanotube nanocomposite. Thin Solid Films, 519, 218–222.DOI: 10.1016/j.tsf.2010.07.123.
- Singh, G., Maurya, S., De Lampasona, M. P., Catalan, C. (2006). Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food Control, 17, 745–752. DOI: 10.1016/j.foodcont.2005.03.010.
- Lennette, H. E., Bellows, A., Hausler, J. W., shadomy, H. J. (1987). Manual of Clinical microbiology, 4th 336-359. Number ID: 971432431. ISBN-13: 978-0914826699.
- Rhayour, K. (2002). Study of mechanism of bactericidal action of essential oils on Esherichia coli, Bacillus subtilis, Mycobacterium phlei and Mycobacterium fortuitum. Thesis of PhD. University of Sidi Mohamed Ben Abdellah, Marocco. 170p. https://www.imist.ma/index.php/content/?id=200&Itemid=52&lang=fr.
- Wilkinson, J. M. (2006). Methods for testing the antimicrobial activity of extracts. Modern Phytomedicine : Turning Medicinal Plants into Drugs. WILEY-VCH, 405p. https://doi.org/10.1002/9783527609987.ch8.
- Negreiros, M. O., Pawlowski, A., Soares, G. L. G., Motta, A. S., & Frazzon, A. P. (2016). In vitro antimicrobial activity of essential oils from Heterothalamus Less. (Asteraceae) against clinically relevant bacterial and fungal species. Brazilian Journal of Biosciences, 14(1), 26–31. PUBMED : 27590861.
- Yu, J., Lei, J., Yu, H., Cai, X., Zou, G. (2004). Chemical composition and antimicrobial activity of the essential oil. Phytochemistry, 65, 881–884. DOI:1016/j.phytochem.2004.02.005.
- Obame, E. L. C. (2009). Study of phytochemical antimicrobial and activities of some medicinales aromatiques African plants. Thesis of University of Ouagadougou. 227p. http://www.beep.ird.fr/collect/uouaga/index/assoc/M11006.dir/M11006.pdf.
- Ouedraogo, I. (2012). Anti-oxydant et antimicrobial activité of essentials oils of Cymbopogon citratus (DC) Stapf, Citrus sinensis (L.) Obsbeck and Zingiber officinale Roscoe for food preservation. Master Degree. University of Ouagadougou. 94p.
- Canillac, N., Mourey, A. (2001). Antibacterial activity of the essential oil of Picea excelsa on Listeria, Staphylococcus aureus and coliform bacteria. Journal of Food Microbiology, 18, 261 – 268. https://doi.org/10.1006/fmic.2000.0397.
- Derwich, E., Benziane, Z., & Boukir, A. (2010). GC/MS Analysis and antibacterial activity of the essential oil of Mentha pulegium grown in Morocco. Reseach Journal of Agriculture & Biological Sciences, 6(3), 191–198. Record Number:
- Norme Internationale ISO 4833 (2003). Food Microbiology-Flat Method horizontale for microorganisms counting .6p. Technic of colonies counting at 30°C. 9p.
- Norme Internationale ISO 4832 (2006). Food Microbiology-Flat Method horizontale for coliforms counting .6p.
- Norme AFNOR NF V 08-060, 2009. Numeration of fecal coliforms by cell counting at 44°C, 10 p.
- Samate, A. D. (2001). Chemical composition of essentials oils extracted from aromatiques plants of southern area of Burkina Faso: Valorisation. Thesis of PhD. University of Ouagadougou. 264p.
- Kabera, J., Koumaglo, K. H., Ntezurubanza, L., Ingabire, M. G., & Kamagaju, L. (2006). Caractérisation des huiles essentielles d’Hyptis spicigera, Pluchea ovalis (Pers.) DC.et Laggera aurita (L.F.) Benth. Ex. C.B. Clarke, plantes aromatiques tropicales. Série Sciences Exactes, Naturelles et Appliquées. Etudes Rwandaises, 10, 7–18. PI: 242384002. ISSN 1014-4874.
- Viljoen, A. M., Denirci , B., Baser , K. H. C., Potgieter, C. J., Edwards, T. J. (2006). Micro distillation and essential oil chemistry- a useful tool for detecting hybridisation in Plectranthus (lamiaceae). South African Journal of Botany, 72, 99–104. https://doi.org/10.1016/j.sajb.2005.05.003.
- Marzoukia, H., Elaissib, A., Khaldic, A., Bouzidd, S., Falconierie, D., Marongiu, B., Pirasa, A., Porcedda, S. (2009). Seasonal and geographical variation of Laurus nobilis essential oil from Tunisia. The Open Natural Products Journal, 2, 86–91. DOI: 10.2174/1874848100902010086.
- Aprotosoaie, A.C., Spac, A.D., Hancianu, M., Miron, A., Tanasescu, V.F., Dorneanu, V., Stanescu, U. (2010). The chemical profile of essential oils obtained from fennel fruits (Foeniculum vulgare). FARMACIA, 58(1), 46–54. Reference ID: 1433431.
- Chen, L., Yang, X., Jiao, H., Zhao, B. (2003). Tea catechins protects against lead-induced ROS formation, mitochondrial dysfunction, and calcium dysregulation in pc 12 cells. Chemical Research in Toxicology, 16 (9), 1155 – 1161. DOI:1021/tx0340605.
- Bicas, J. L., Neri-Numa, I. A., Ruiz, A. L. T. G., De Carvalho, J. E., & Pastore, G. M. (2011). Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food and Chemical Toxicology, 49, 1610 –1615. https://doi.org/10.1016/j.fct.2011.04.012.
- Dar, M. Y., Shah, W. A., Rather, M. A., Qurishi, Y., Hamid, A., Qurishi, M.C.A. (2011). Chemical composition, in vitro cytotoxic and antioxidant activities of the essential oil and major constituents of Cymbopogon jawarancusa (Kashmir). Food Chemistry, 129, 1606–1611. DOI: 10.1016/j.foodchem.2011.06.016.
- Shahwar, D., Ahmad, N., Khan, M. A., & Ullah, S. (2012). Chemical composition and biological activities of the essential oil of Laggera aurita Linn ( DC .) grown in Pakistan. Research Article [ Arastuma Makalesi ] 37 (July), 329–335. DOI: 10.5505/tjb.2012.04706.
- Oussalah, M., Caillet, S., Saucier, L., Lacroix, M. (2006). Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Science, 73, 236–244. DOI: 1016/j.meatsci.2005.11.019.
- Mejholm, O., Dalgaard, P. (2002). Antibacterial and antifungal activity of the essential oils of Thymus revolutus Celak from Turkey. Letters in Applied Microbiology, 34, 27 – 31. PMID:11849488.
- Gutierrez, J., Barry-Ryan, C., and Bourke, P. (2008). The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. International Journal of Food Microbiology, 124(1):91-7. doi: 10.1016/j.ijfoodmicro.2008.02.028. PMID: 18378032.
- Gill, A. O., Delaquis, P., Russo, P., Holley, R. A. (2002). Evaluation of antilisterial action of cilantro oil on vacuum packed ham. International Journal of Food Microbiol, 73, 83–92. DOI: 10.1016/S0168-1605(01)00712-7 ·
- Caillet, S., Lacroix, M. (2007). Les huiles essentielles: leurs propriétés antimicrobienne et leurs applications potentielles en alimentaires laboratoire de recherche en science appliquée à l’alimentation. INRS- Institues Armand- Frappier, (RESALA), 8p. https://e-dition.net/des-huiles-essentielles-aux-proprietes-antimicrobiennes.
- Laranjo, , Fernández-Léon, A. M., Potes, M. E., Agulheiro-Santos A. C. and Elias M. (2017). Use of essential oils in food preservation. Antimicrobial research novel bioknowledge and educational programs (A. Mendez-vilas, Ed.). https://www.researchgate.net/publication/320729063.