Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Behavioural and Electrophysiological Responses of Sweetpotato Weevils to Volatile Compounds from Sweetpotato Plants
Milton O. Anyanga1, 2*, D. Rees2, G.N. Ssemakula1, D. I. Farman2, R.O.M Mwanga3, P.C. Stevenson2, 4
1National Crops Resources Research Institute (NaCRRI), P.O. Box 7084, Kampala, Uganda
2Natural Resources Institute, Chatham Maritime, Kent ME4 4TB, United Kingdom
3International Potato Center (CIP), P.O. Box 22274, Kampala, Uganda
4Royal Botanic Gardens, Kew, Surrey, TW9 3AB, United Kingdom
Received Date: January 17, 2020; Accepted Date: February 05, 2020; Published Date: February 14, 2020
*Corresponding author: Milton O Anyanga, National Crops Resources Research Institute (NaCRRI), P.O. Box 7084, Kampala, Uganda. Email: moanyanga@yahoo.com
Citation: Anyanga MO, Rees D, Ssemakula GN, Farman DI, Mwanga ROM, Stevenson PC (2020). Behavioural and Electrophysiological Responses of Sweet potato Weevils to Volatile Compounds from Sweet potato Plants. Adv Agri Horti and Ento: AAHE-109.
Abstract
The study investigated the diversity and role of chemical signals which control the interactions between SPW and sweet potato plants. Volatile chemicals from the root and shoot of two Ugandan commercial sweet potato varieties New Kawogo resistant to sweetpotato weevils (SPW) and Tanzania, susceptible to SPW were extracted using headspace extraction technique at National Crops Resources Research Institute (NaCRRI). The extract was analysed using GC/MSin an advanced laboratory at the Natural Resources Institute (NRI) in the United Kingdom for comparison before and after sweetpotato weevil infestation. The objective was to determine whether the two varieties differ in their volatile profile and if the volatiles affect the behavioural response of SPW aiding it in host location. There were significant differences in the sweet potato root volatile before and after SPW infestation. Emission of caryophyllene and germacrene D before infestation as recorded from the roots and shoot of Tanzania was associated with location cues for the weevil whereas the production of the same compounds in New Kawogo. Electro-Antennography (EAG) recordings were made from male and female SPW between 0-14 days old for response to decyl-E-2-butenoate.Although there were differences in volatile production in the root and shoot, weevils were not significantly attracted to these two volatile odours implying they do not influence the host locating behaviour of the herbivores. EAG responses were observed to the pheromone, decyl-E-2-butenoate, in male SPWs. However no other response was clear for any of the plant volatiles of either resistant or susceptible varieties. Overall, the differences in the volatile chemistry of New Kawogo and Tanzania did not influence the behaviour of sweetpotato weevils. Therefore, this may not on its own account for the resistance reported in New Kawogo.
Keywords: Resistance; Root volatiles; Shoot; Susceptible
Introduction
Plants produce several volatile organic compounds continuously and disperse them through air. The volatiles can be perceived by insects to indicate a source of food [1]. According to Korada et al. (2010) [2] sweet potato emits different volatile organic chemical compounds that could provide olfactory stimuli to SPW. These compounds comprise a chain of 5-20 carbon atoms with a number of functional groups some of which mediate insect-insect interactions while others promote insect-plant interactions.
The differences in volatile organic compounds among sweet potato clones relate to the variability in susceptibility or resistance to sweet potato weevils. Wang and Kays (2002) [3], for instance, demonstrated that the sweet potato host plant compounds can potentially modify behaviour of the weevil to aid host finding (volatiles), feeding (surface chemicals) and larval development in the roots (internal root chemistry). Production of one or a mixture of these volatile organic compounds from the leaves, root surface or the storage root of sweet potato could influence host location of SPW. The objective of this study was to establish whether there are differences in the volatile chemistry of the shoot and storage roots of resistant and susceptible varieties which can influence sweetpotato weevil behavioural response. The hypothesis tested was sweetpotato location behavior is influenced by sweetpotato volatiles.
Volatile phytochemicals, is therefore, thought to play a critical role in host finding and insect egg laying behaviour. Earlier work in the United States of America by Mullen (1984) [4] on volatile chemicals released from sweet potato showed that adult male and female C. formicarius were differentially attracted to root and leaf volatiles. This suggests that the knowledge of the volatile profile between the aerial plant parts and the storage roots might help in the selection of lines for breeding for volatile assisted resistance to SPW. The objective of this study was to establish whether there are differences in the volatile chemistry of the shoot and storage roots of resistant and susceptible varieties which can influence sweet potato weevil behavioural response.
Materials and Methods
Clean roots of the resistant variety New Kawogo and a susceptible variety Tanzania were harvested from a field planted at the National Crops Resources Research Institute (NaCRRI), Namulonge, Uganda (00° 31 30N, 32° 36 54 E). The shoots were obtained from the vines planted in pots in the screen house at NaCRRI. Volatiles was extracted from both infested and non-infested shoots and storage roots using headspace extraction technique separately for 24 hours at NaCRRI and analysed using GC/MSin an advanced laboratory at NRI. Volatile components emitted through polythene bags (oven bags, Sainsbury’) were trapped at the outlet on to Porapak-Q (Waters Corp, U.S.A), an adsorbent polymer in a glass Pasteur pipette by drawing polished air, filtered through charcoal, at a flow rate of 1.0 L min-1 across the enclosed shoot and root samples respectively. Compounds were desorbed by eluting the adsorbent with 1.5 ml of methylene chloride into a drum vial that was later sealed, labelled and refrigerated in preparation for analysis.
Volatile Analysis
Samples were analysed at NRI, University of Greenwich with the technical assistance of Dudley Farman. Gas Chromatography-Mass Spectroscopy (GC-MS) was carried out on a Perkin-Elmer GC Auto system 2000 fitted to a Perkin-Elmer Mass Spectrometer QMass-910. The GC was fitted with a 25 mm. 0.18 mm ID, DB-5 fused silica capillary column. The GC oven temperature was programmed from 40°C (4 min) to 150°C at 6°C/min, and final temperature maintained for 15 min. The individual volatile compounds separated were identified by comparison of their mass spectra with National Institute of Science and Technology (NIST) spectral libraries.
Behavioural Responses of Sweet Potato Weevils to Caryophyllene and Germacrene D
Behavioural response was conducted through dual-choice olfactometer. The rationale was to determine whether the compounds incite a response from SPW. A synthetic form of caryophyllene and germacrene D prominent in the volatile metabolome of sweet potato plants were purchased from Boots, Nottingham, UK. Caryophyllene was made up in a solution of 1mg/ml solution of caryophyllene in hexane prepared by weighing 5mg of caryophyllene and making a solution in 5 ml of hexane. A series of dilution was made to test a lowest concentration of 10ng/µl solutions and germacrene-D prepared in ylangylang oil extract was used in the studies. Both compounds were tested with male and female weevils.
Twenty SPW adults aged 14 days were used individually for the experiment were deprived of food for one day before the start of the bioassay. A vacuum pump was used to generate an air volume of 1.0 L min-1 adjusted using airflow meter on a silicon tube connected to the pump to pass air through an air inlet chamber fitted with a charcoal filter column. A 30 µl aliquot of each compound was dispensed on to filter paper using a pipette and the carrier solvent was allowed to evaporate. Carbon filtered air was drawn from the chamber using a vacuum pump passing through a charcoal dust filled column at a flow rate of 1.0 L min-1. This process delivers the odour towards the entry point for the insect and enabled the test insect to choose to move towards one or the other odour source. Single insect introduction to the arena was done to control for insects attracted by aggregation pheromones. The weevil movement was monitored for their response to the stimulus. The movement of the weevils was observed from the time they were introduced into olfactometer chamber. The position of weevils was recorded after 30 minutes with fresh insect and data collected 5 times and the experiment replicated five times with a fourteen day old weevils of either sex in each experiment. The numbers of weevils that responded and those that did not respond were pooled and the response of the weevils was expressed by an attraction index defined by the relationship; attractive index (a. i) = n/N where; n= number of weevils in the arm or outside the buffer zone orienting towards the direction of the odour and N= total number of weevils introduced into the olfactometer arena. This index was converted into percentage by multiplying with 100. The data was transformed using arc sine.
Response of Sweet Potato Weevils to Natural Sweet Potato Volatiles
The bioassay was conducted using a dual-choice olfactometer already described above. The set up was connected to the olfactometer chamber and lunch boxes on either end with Teflon tubes. The other ends of the plastic boxes were connected to a charcoal dust filter to clean incoming air. This was designed to ensure any record of attraction to an odour represented a commitment to seek that source of food and was not a measure of potential random exploratory behaviour. Roots and leaves were used as odour sources in experiments investigating the possible attraction or repellency of both weevil sexes for one of the two host plants leaves and storage roots. The youngest shoot or the roots for both Tanzania and New Kawogo were used for the bioassay. The response of males and females to sweetpotato volatiles was tested in two (2) experiments in a combination to elucidate whether there was Cylas preference for: i) shoot volatiles from Tanzania or New Kawogo compared to a blank control; ii) and sweetpotato root volatiles versus sweetpotato shoot volatiles. Each experiment was replicated five times with either male or female adult weevils at a time to see if they respond to the odour in the same way. The data obtained was pooled and transformed using arc sine as in the section above.
Electrophysiological Responses
Electroantennography (EAG) analysis of the electrophysiological responses was used to determine sweet potato weevil response to specific odours. The SPW used for Electroantennography (EAG) recording were C. puncticollis, and maintained in a controlled environment room at 22-250C with a 14L:8D cycle on sweet potato roots purchased within the UK (Asda, UK).
GC-EAG recordings were carried out by M. Fernandez-Grandon at NRI on behalf of the author using an integrated unit (INR-02; Syntech, Germany) consisting of electrode holders, micromanipulators and amplifier. This was connected to one detector of an Agilent 6890 gas chromatograph to digitise the output and data were collected and processed using EZChrom Elite v3.0 software (Agilent). Electrodes were glass capillary tubes (o. d. 1.50 mm, i. d. 1.17 mm) pulled to a fine point and filled with electrolyte (0.1 M potassium chloride with 1% polyvinylpyrrolidine to reduce evaporation). These were placed over silver wires in the electrode holders. The unit had built-in amplification x10.
Gas Chromatography Equipment (GC) Used For EAG
A 6890N Agilent GC was used for the separation of compounds for EAG. Samples were injected into a wax polar 50-2, 50/20 column. Oven temperature was set to 80ºC starting temperature ramping by 20ºC every 2 minutes until reaching the 250ºC. In total the run lasted 17 minutes. These oven temperatures were selected over longer runs which may provide greater separation to ensure that the response of SPW did not deteriorate dramatically during the run and allow more replicates to be completed. At the start of each day of testing standards of acetates and hydrocarbons were run through the equipment to ensure that separation was consistent and that no contamination was present on the column. A clean, continuous airflow was humidified through distilled water and delivered the odours over the antenna at a rate of ~150 ml/min.
EAG Recordings from Male and Female SPW
EAG recordings were made from male and female SPW between 0-14 days old. Individuals were chilled in ice before having the head and one antenna removed using fine dissection scissors. Anaesthetisation with CO2 was experimented with in early trials, though was not found to confer any additional advantage in the preparation. The most distal segment of the antenna was removed and the proximal end was placed in the indifferent electrode. The distal end was then gently fitted into the recording electrode ensuring a complete circuit with the olfactory receptors exposed.
A consistent response of the SPW to decyl-E-2-butenoate from male antenna was detected. The response ascertained with this pheromone was used as an internal positive control to test the volatiles from sweet potato resistant and susceptible varieties. Entrainment samples from three varieties were tested but results were presented for two varieties and these and were: # 01 Tanzania and #03 New Kawogo with # 01 Tanzania representing susceptible variety and # 03 New Kawogo an example of the SPW- resistant sweet potato variety. The numbers represented the sample codes tested during entrainment.
Results
Volatile Components of the Storage Roots of Tanzania and New Kawogo
Uninfested storage roots of Tanzania and New Kawogo emitted volatile chemicals in the absence of weevils. The roots of Tanzania emitted quantitatively fewer compounds than the roots of New Kawogo before weevil infestation. The chemical profile of the volatiles differed between the storage roots of the two sweetpotato varieties and indicated that carophyllene, α-farnesene, germacrene B and 4-methyl-1,5-heptadiene were produced exclusively by the roots of New Kawogo and not by the roots of Tanzania prior to infestation.
The emission of volatiles from the storage roots of Tanzania increased six fold after the weevils were introduced to feed on the roots for 24 hours. Four volatile compounds: α-elemen, β-caryophyllene, germacrene D and germacrene B was more emitted by the storage roots of Tanzania (susceptible) compared to New Kawogo (Figure 1).
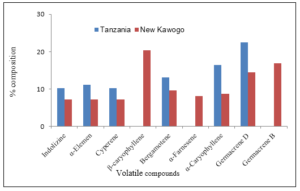
Figure 1: Volatile chemical compounds produced by the storage roots of Tanzania and New Kawogo before infestation.
Emission of α-caryophyllene was also lower in New Kawogo than Tanzania. Another interesting observation was that volatile emissions by New Kawogo roots were quantitatively lower in infested roots compared to uninfested root and the compound, 3-hexen-1-ol, acetate, (Z) was only emitted by Tanzania and not by New Kawogo after the roots were infested with sweet potato weevils (Figure 2).
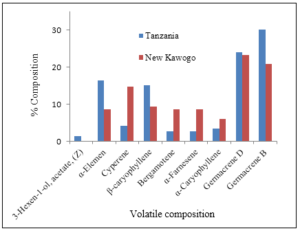
Figure 2: Volatile compounds produced by storage roots of Tanzania and New Kawogo 24 hours after infestation by weevils.
Volatile Components of the Aerial Parts of New Kawogo and Tanzania
The volatile emissions from undamaged shoots of New Kawogo and Tanzania showed that overall similar compounds were emitted by both varieties. However, the results showed that the aerial parts of Tanzania emitted α-caryophyllene which was not produced by the aerial part of New Kawogo before infestation (Figure 3).
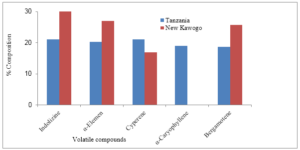
Figure 3: Mean percentage volatile chemical compound composition produced by the aerial parts of Tanzania and New Kawogo before weevil infestation.
After sweet potato weevil infestations, the emission of α-caryophyllene and germacrene D increased in the above ground shoots of Tanzania whereas in New Kawogo these two volatile compounds were not emitted. The emission of the rest of the compounds did not change before and after sweet potato weevil infestation (Figure 4).

Figure 4: Mean percentage volatile chemical compound composition produced by the aerial parts of Tanzania and New Kawogo after weevil infestation.
Emission of α-caryophyllene and germacrene D before infestation as recorded from the roots and shoot of Tanzania seems to be associated with location cues for the weevil whereas the production of the same compounds in New Kawogo are triggered after the weevils feeding. This emission could be a defense response either as an attempt to deter further damage by the weevils or provide alert to other plants of the potential problems.
Response of the Weevils To α-caryophyllene and ylangylang Oil
There was no significant difference in the response of SPW to sweet potato volatiles in the linear flow olfactometer experiment. Fifty two percent of the weevils were lured by α-caryophyllene while germacrene D trapped 60.2% of the weevils during the study (Figure 5).
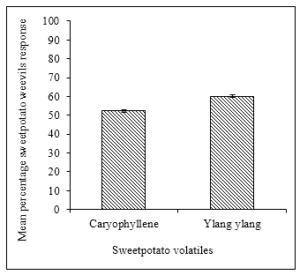
Figure 5: Mean percentage attraction of sweet potato weevils to caryophyllene and ylangylang (n=20, replicated 5 times).
Response of the Weevils to Sweet Potato Leaf Odours
The attraction of male SPW to New Kawogo leaf odour was significantly (P≤0.04) greater than the attraction of female weevils to the same odour. Similarly significantly more (P ≤ 0.05) males (41%) were also lured to leaves of Tanzania compared to females (23%) (Figure 6). However, there was no significant differences (P>0.05) in the number of each sex attracted to the leaves indicating the insects were responding equally to the stimulus in the leaves of both New Kawogo and Tanzania .
Forty seven percent of the male weevils were lured to the odour compared to only 16% female weevils that responded to the volatile (Figure 6).
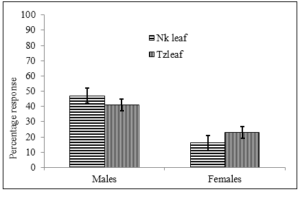
Figure 6: Mean attraction of male and female sweet potato weevil to sweet potato leaf odours (n=20, replicated 5 times) of New Kawogo (NK) and Tanzania (Tz).
Response of the Weevils to Sweet Potato Root Odours
The responses of SPW to root volatiles were not as profound as their responses to above ground plant parts. The data suggested that males were less able to recognize root volatiles compared to females although the difference was not significant (P ≥ 0.05). For instance, New Kawogo root volatiles attracted 2% males while 8% females responded to the volatiles and were attracted to the root of New Kawogo in the olfactometer study (Figure 7). The response changed when the root of Tanzania was used. The root volatiles from Tanzania attracted 6% males and 15% females respectively (Figure 7). The response of female weevils to the root volatiles of both New Kawogo and Tanzania was surprisingly low and not significantly different.
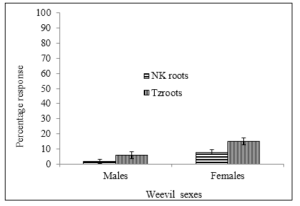
Figure 7: Mean attraction of male and female sweet potato weevil to sweet potato root odours (n=20, replicated 5 times) of New Kawogo (NK) and Tanzania (Tz).
Electrophysiological Responses
The only antennal response observed with any consistency was that of the pheromone sample (decyl-E-2-butenoate) with a peak produced in all replicates at ~9.8 minutes (Figures 8, 9, 10, 11, 12 and 13). Due to the diverse chemical content of the entrainment samples it would be difficult to ascertain the response of individuals to specific compounds without repetition. Although responses in the antenna were occasionally observed to plant volatile compounds, repetitions have shown these not to be consistent with the release of compounds from the column and are more likely to be false positives, which are not uncommon in EAG.
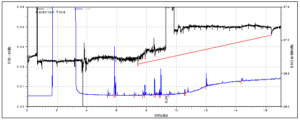 Figure 8: Chromatogram of Tanzania volatiles plus pheromone and EAG response using a male C. puncticollis antenna with the tip cut and the second antenna removed.
Figure 8: Chromatogram of Tanzania volatiles plus pheromone and EAG response using a male C. puncticollis antenna with the tip cut and the second antenna removed.
Around 54 replicates were completed with the SPW using concentrated samples, males and females, antenna-only preparations and SPWs of different ages. The most useful of these are probably the series of trials included in which male SPW are tested against the volatile blends and the pheromone as an internal positive control in which the only responses were recorded to the pheromone, which can be seen around 9.8 minutes, are included. Three replicates are completed for each of the volatile blends.
Discussions
The study showed that there were differences in the sweet potato root volatile both before and after weevil infestation. This suggests that weevil feeding may induce biochemical processes that trigger the production volatiles such as sesquiterpenes first reported by Uritani et al., (1975). Volatile compounds such as, α-elemen, β-caryophyllene, germacrene B and germacrene D were produced in greater quantity by Tanzania root after infestation compared to the New Kawogo. If these compounds were active then such chemical activity could influence host location and therefore susceptibility to herbivory.
Olfactometer study showed that although more C. puncticollis females oriented towards the roots of Tanzania than New Kawogo, this orientation was not significantly different. In other words the weevils did not differentiate between the volatiles from the roots of Tanzania and that New Kawogo in the olfactometer chamber. According to Lewis, et al. (1991) [5] when female insect receive stimuli, they learn to recognise the volatile odours associated with the plants. This finding was supported with the report of Korada, et al. (2010) [6] that volatile compounds in susceptible sweet potato varieties play a significant role in plant recognition by C. formicarius.
The roots of New Kawogo (the resistant variety) produced carophyllene, α-farnesene and germacrene B which were not produced by Tanzania (the susceptible variety) but only before it was infested by sweet potato weevils implying these compounds are produced for a physiological need that is not responding to herbivory although may still be repellent or attractive. Paul and Tomlinson (1999) [7] reported that leaves normally release small quantities of volatile chemicals for different physiological roles but upon wounding by external agent, many more volatiles are released. These induced volatiles may either attract natural enemies of the insect or induce alert responses in neighbouring plants.
Emission of caryophyllene and germacrene D before infestation as recorded from the roots and shoot of Tanzania seems to be associated with location cues for the weevil whereas the production of the same compounds in New Kawogo are triggered after the weevils feeding. It is possible that this response could alert a natural enemy of sweet potato weevil. A large number of chemical compounds have been implicated in signaling to herbivores, predators and parasitoids. Similar work by Rasman, et al. (2005) [8] found that root worm larvae (Diabrotica virgifera virgifera) feeding on maize roots induced (E)‑β‑caryophyllene production in the roots and attracted entomoparasitic soil nematodes to orientate toward damaged plant roots in tests with a sand‑filled olfactometer. This appears to relate with the release of caryophyllene by New Kawogo after weevil infestation in this study.
Although there were differences in volatile production in the root and shoot, weevils were not significantly attracted to these two volatile odours implying they do not influence the host locating behaviour of the herbivores. The response observed between caryophyllene and ylangylang oil could have been a result of other chemical odours not sweet potato volatiles themselves. Electrophysiological responses were observed to the pheromone, decyl-E-2-butenoate, in male SPWs. However no other response was clear for any of the plant volatiles of either resistant or susceptible varieties. Plant volatiles appear be a response to alert a natural enemy of sweetpotato weevil for the presence of a prey. Terpenes such as germacrene D, (Z,E)-α-farnesene, (E,E)-α-farnesene which are similar to the ones identified in sweetpotato roots after infestation have been reported as constituents of the alarm pheromone released when pea aphids are attacked by a predator, triggering escape responses in the aphid colony [9, 10] This could play a similar role in alerting natural enemies of the presence of SPW since the response of weevils to volatile components was not detected by EAG suggesting they are not involved in attracting SPW, C. puncticollis in the plants but reported luring C. formicarius to the plants by Korada, et al. (2013) [6].
Elsewhere, sex pheromone were found effective in reducing C. formicarius populations in mass trapping trials in Taiwan [11], Vietnam [12] and Cuba [13]. Unfortunately in Uganda, mass trapping using these sex pheromone traps did not lead to a reduction in weevil damage to roots [14].
However the absence of a positive control made testing of females unreliable. It is also worth noting that the amplitude of the responses may indicate a problem with the equipment used. The EAG response to pheromone while present is of low amplitude which may suggest that a failure to identify any volatile components from the plant is an issue of sensitivity rather than indicating that a volatile component does not play role in sweet potato weevil behavioural response. Overall, New Kawogo and Tanzania traces looked appeared very similar in the EAG indicating that the differences in the volatile chemistry of New Kawogo and Tanzania do not to influence the behaviour of sweet potato weevils and does not account for the resistance in New Kawogo. This therefore contrasts with the original hypothesis that sweet potato volatile odours influence host location, oviposition and feeding of C. puncticollis and C. brunneus.
Funding
- This study was funded by McKnight Foundation Collaborative
- Crop Research project no. 08-019 on sweet potato breeding.
Conflict of Authors: The authors declare no competing financial interest.
Acknowledgments: The authors thank M. Fernandez-Grandon (Natural Resources Institute (NRI)) for GC-EAG recordings. Technical support by J. Nanteza and M. Mwondha are acknowledged.
References
- Metcalf RL (1987) Plant volatiles as insect attractants. Critical RevPlantSci 5: 251-301.
- Korada RR, Naskar SK, Prasad Prasuna AL, Jyothi KN (2010) Differential volatile emissions from sweetpotato plant: mechanism of resistance in sweetpotato for Cylasformicarius (Fab). Current Science 99: 1597-1601.
- Wang Y, Kays SJ (2002) Sweetpotato volatile chemistry in relation to sweetpotato weevil behaviour. Journal of American Society of Horticultural Sciences 127: 656-662.
- Mullen MA (1984) Influence of sweet potato weevil infestation on the yields of twelve sweet potato lines. J. Agric. Entomol. 1: 227-230.
- Lewis WJ, Tumlinson JH, Krasnoff S (1991) Chemically mediated associative learning: An important function in the foraging behaviour of Microplitiscroceipes (Cresson). Journal of chemical Ecology 17: 309-1325.
- Korada RR, Naskar SK, Bhaktavatsalam N, Prasad AR, Sinha K, et al. (2013) Plant volatile organic compounds as chemical markers to identify resistance in sweetpotato against weevil Cylasformicarius. Current Science 105: 1247-1253.
- Pare PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant physiology 121: 325-332.
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, et al. (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434: 732-737.
- Francis F, Vandermoten S, Verheggen F, Lognay G, Haubruge E (2005) Is the (E)-betafarnesene only volatile terpenoid in aphids? Journal of Applied Entomology 129: 6-11.
- Pickett JA, Griffiths DC (1980) Composition of aphid alarm pheromones. Journal of Chemical Ecology 6: 349-359.
- Hwang JS, Hung CC (1991) Evaluation of the effect of integrated control of sweet potato weevil, Cylasformicarius Fab. With sex pheromone and insecticide. Chinese Journal of Entomology.
- Braun AR, van de Flieart E (1997) Implementation of IPM for sweet potato in Vietnam and Indonesia. Annual Progress Report. Lima, Peru: International Potato Center.
- Alcazar J, Cisneros F, Morales A (1997) Large-scale implementation of IPM for sweet potato weevil in Cuba: a collaborative effort. Program Report 1995-1996. Lima, Peru: International Potato Center
- Smit NEJM, Downham MCA, Laboke PO, Hall DR, Odongo B (2001) Mass-trapping male Cylas spp. with sex pheromones: a potential IPM Component in sweetpotato production in Uganda. Crop Protection 20: 643-651.