Publication Information
ISSN: 2641-6859
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Basal ganglia, Cortex and Pyramidal tract repair through 2 years of Coordination Dynamics Therapy 12 years after hypoxia during birth
Giselher Schalow*
NGOMR: Non-Government-Organized-Medical-Research
Received Date: June 05, 2023; Accepted Date: June 10, 2023; Published Date: June 21, 2023;
*Corresponding author: Giselher Schalow, NGOMR: Non-Government-Organized-Medical-Research. Email: g_schalow@hotmail.com
Summary
The patient Alen suffered at birth an insult and an asphyxia with the consequence of a sustained cerebral hemiplegia on the left side, a parenchyma defect of the basal ganglia and a degeneration of a pyramidal tract. At an age of 12 years coordination dynamics therapy was started.
Most functions were impaired in Alen. He could not use the left hand, which is a disability of 50%. But he could walk and run with deficits. Through 2 years of coordination dynamics therapy, creeping, crawling, walking, and running became nearly normal. He learned to jump in-phase and in anti-phase. Most importantly, he learned left hand functions, including the power and precision grip, and could use them in everyday life. He learned to jump on the paretic left leg through inducing the stepping automatism by a jumping/bumping maneuver and induced in this way a better growth of the paretic leg. The leg length difference reduced from 4cm to 2 cm. He needed no orthosis any more. His cognitive functions improved and he could learn better at school. What main stream medicine did not achieve in 12 years; coordination dynamics therapy succeeded in 2 years. The jumping maneuver is introduced for improving the paretic leg, in some similarity to the Jendrassik maneuver.
Keywords: Human repair-neurophysiology – Electrophysiology – Single-nerve fiber action potentials – Oscillatory firing – Phase and frequency coordination – Coordination dynamics therapy – Basal ganglia repair – Ontogenetic landscape for locomotion – Jendrassik maneuver – co-movement – jumping-bumping maneuver
Introduction
Human repair-neurophysiology is a new discipline with which the human nervous system can be repaired. Since the nervous system is involved in nearly all body functions, the general health can be improved. By combining Human Neurophysiology with the System Theory of Pattern Formation, there is a theoretical basis that through movement-based learning vegetative and cognitive functions can also be repaired by learning transfer.
Based on human repair-neurophysiology [1, 2], a movement-based learning therapy was developed though neural network learning [3], called Coordination Dynamics Therapy (CDT), with which it is possible to improve or repair central nervous system (CNS) functioning after stroke [4], traumatic brain injury [5, 6], spinal cord injury [7-13] (Figure 1A-D), cerebellar injury/atrophy [14,15] (Figure 1E-H), cerebral palsy [16], hypoxic brain injury [17], in Parkinson’s disease [18], spina bifida (myelomeningocele) [19] and scoliosis [20]. Speech had been induced and improved in a patient with severe cerebral palsy [1]. A permanent coma patient could be brought out-of-coma and relearned to speak and move [21,26] and cancer grows could be inhibited through CDT [22, 23] by improving cardio-vascular performance [1, 21] and building of natural killer cells [24]. Urinary bladder functions [1] could be cured in cerebral palsy [1] and spinal cord injury [7,12,13]. There is indication that general health can be improved via CDT to live longer with a better quality of life [25] and euthanasia avoided in organ donation [26]. Spinal muscular atrophy could be stopped [27], incontinence repaired [28, 29] and motor functions in genetic disease (5p-) repaired [30]. The strategies of human repair-neurophysiology are updated and related to genetic disease repair [30].

Figure 1. The spinal cord injury patient Nefeli relearned to walk and became continent again (A-D) [7, 13]. The cerebral palsy girl Sophie with atrophied cerebellum and pons could not stand, walk, run (E, F) or jump and was incontinent. She learned to walk, run (G, H) and jump, became continent and her higher mental functions improved [15]. Anna with a genetic disease (chromosome deletion 5p-), learned to creep (I), hop (K), crawl (L), walk (M) and run (N) [30].
A further repair step through human repair-neurophysiology is to repair an injury of the basal ganglia. With the 12-year-old boy Alen it will be shown on what level of medical research the cerebral cortex, basal ganglia and pyramidal tract can be repaired. Alen had suffered a perinatal insult and an asphyxia with the result of a sustained cerebral hemiplegia, a parenchyma defect of the basal ganglia and a degeneration of the pyramidal tract to the left side. Basal ganglia injuries are supposed to be difficult to repair. But by using probable movements of phylogenetical older species, phylogenetical old motor centers can also be repaired, as will be shown.
In the Method, based on functional anatomy, the deficits of movements and other patterns due to the injury of the basal ganglia and the cortex are explained. It will be shown how CDT is adapted to the repair of these injuries. In the Results, the CNS disorders will be shown at the beginning of therapy and with ongoing CDT. The improvements of CNS functioning are quantified clinically by the repair of movements and other patterns and theoretically with the improvement (lowering) of coordination dynamics values. In the Discussion, the basal ganglia repair of Alen is compared with the repair in cerebellum injury of Sophie (Figure 1E-H) and Dr. Cwienk, the spinal cord injury patients Sten and Nefeli and the genetic disease patient Anna. The jumping-bumping maneuver treatment, which improved the paretic leg of Alen, will be analyzed in detail based on human neurophysiology and compared with the Jendrassik maneuver.
Movement-based learning strategies to repair the human CNS
To repair the human CNS, several strategies are used. First, to repair the always impaired phase and frequency coordination (a principle of CNS organization) through exercising on a special coordination dynamics therapy (CDT) device. Second, to train important automatisms during ontogenetic development like creeping, crawling, up-righting, walking, running and breathing, because genetic support can be expected and repair shows similarities to ontogenetic development. Third, to repair the especially phylogenetic old CNS structures like the spinal cord, the vermis of the cerebellum and the paleostriatum (globus pallidus) of the basal ganglia, movements must be trained, which phylogenetic ancestors like Tiktaalik, may have used for locomotion. Such movements are creeping, salamander crawling, hopping and others. Fourth, to activate and train important regulation circuits, which, for example, are activated during jumping. Jumping helps to repair urinary bladder and other functions. Fifth, those patterns must be trained which activate the CNS most integrative, because only then the whole complexity of the regulation units/circuits are entrained and pathologic neural network organizations cannot shift to another CNS part and escape repair.
The nervous system must recognize which structures or regulation units are deficient, to repair them by error elimination processes. Movement-based learning in combination with instructive training (1-2-3-..), visual (mirror) and auditive (music) feed-back increases the efficiency of treatment.
Basal ganglia and cortex injury due to hypoxia
Alen suffered a brain injury through complications during birth (vasa previa?). After the caesarean delivery he had fluid mixed with blood in the lung and did not breath. When the fluid was removed, he started to breath, but he had suffered hypoxia. Hypoxia is a condition in which the brain or a region of the brain is deprived of adequate oxygen at the brain parenchyma level. Since Alen suffered a tissue loss of the basal ganglia, an injury of the primary cortices and an atrophy of a pyramidal tract, probably the middle cerebral artery transported blood with too little oxygen (Figure 2), because it supplies, among other parts, the primary and sensory cortices, the language areas of Broca and Wernicke (Alen had speech problems), the primary auditory cortex and the basal ganglia. Other nuclei could be slidably damaged, as for example the thalamus, which was not diagnosed by the MRI. Anyhow, those brain tissue parts are most injured, which get the littlest oxygen. The most far away rami striata may lack most oxygen. Since the delivery was a caesarean, a mechanical brain damage is unlikely.
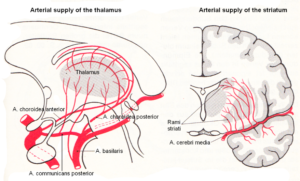
Figure 2. Arterial blood supply of the striatum (and thalamus). The middle cerebral artery supplies with its branches, among other parts, the primary and sensory cortices, the language areas of Broca and Wernicke, the primary auditory cortex, and the basal ganglia. (Van den Berg and Vander Eeken).
Functional anatomy of the cerebral cortex and basal ganglia for understanding the repair
Figures 3 and 4 show the gross anatomy of the basal ganglia and the cortex. A loss of parenchyma of the basal ganglia and an injury of the sensory-motor cortex was diagnosed in Alen and had to be repaired. The basal ganglia are a part of the motor system. The principal nuclei of the basal ganglia are the caudate nucleus, the putamen, and the globus pallidus (Figure 3A). These nuclei are connected to each other and to the motor cortex (Figure 5A) in complex regulatory circuits (Figure 5B). They exert both excitatory and inhibitory effects on the motor cortex. They play an important role in the initiation, maintenance, and modulation of movement patterns. In phylogenetically older species, the older neural centers are primarily responsible for the maintenance of movement patterns and the automatic control of locomotion. Injuries of the basal ganglia, and of other, functionally related nuclei, such as the substantia nigra and the subthalamic nucleus, can produce either an excess or a deficiency of movement-related impulses (the left hand of Alen was not activated), and/or pathological alterations of movement and other patterns.
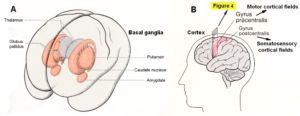
Figure 3. A. Topographical relationship of the basal ganglia (in red) [31]. B. Position of motor and somatosensory cortical fields, displayed in Figure 4.
The hierarchically uppermost center for the control of movement is the cerebral cortex, whose signals are transmitted by the pyramidal pathway (in Alen atrophied to the left body part) to the motor cranial nerve nuclei and to the anterior horn cells of the spinal cord (pyramidal system). The pyramidal and extrapyramidal systems are subunits of a single integrated motor system and, as such, are closely linked to each other, both structurally and functionally (Figure 5) and must repaired integrative.

Figure 4. The cerebral palsy girl Sophie during exercising on a special CDT device. At the same time, the mother (teacher herself) is administering speech therapy to her. In such a very integrative training setting, nearly the whole somatosensory and motor cortical fields (Figure 3B) are activated with their associated fields for repair. - Cortical fields are taken from Penfield W and Rasmussen T, New York, 1950. - This special CDT device for measuring and therapy (int.pat.) is produced by the firm: Giger Engineering, Martin Giger dipl.Ing.ETH/SIA, Herrenweg 1, 4500 Solothurn, Switzerland, www.g-medicals.ch.
As the cerebral cortex developed, the phylogenetically older motor centers (paleostriatum (globus pallidus) and neostriatum (caudate nucleus and putamen)) came increasingly under the control of the new motor system, i.e., the pyramidal system. The cat can still walk without much difficulty after the removal of the cerebral cortex, whereas humans are entirely dependent on an intact pyramidal system. As will be shown in the Results, Alen could not activate on volition the fingers of the left hand. But through co-movement from the rather healthy right hand, he learned within 6 weeks the power grip and the precision grip of the left hand (plegia side), mainly generated by the older motor centers.
When exercising on the special CDT device, including speech therapy (Figure 4), the whole sensory-motor cortex is activated and entrained, including the premotor areas and the association field, and the basal ganglia and other nuclei or structures. But to repair more specifically the basal ganglia, also probable movements of phylogenetic ancestors must be trained. Such movements are creeping, salamander crawling, hopping and similar movements (see below).
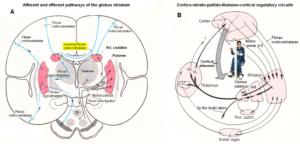
Figure 5. A. Afferent and efferent pathways of the striatum (Ncl. caudatus and Putamen) [32]. B. Cortico-striato-pallido-thalamo-cortical regulatory circuits. CM Centrum medianum, VA Ncl. Ventralis anterior, VL Ncl. Ventralis lateralis [33].
Probable movements to activate, train and repair more specifically the basal ganglia
Tiktaalik and other animals may have used creeping, salamander crawling, hopping and similar kinds of movements for locomotion.
Why is it important to look for repair movements which originate in phylogeny? First, anyway further movements are needed to find and repair the deficiencies of neural structures in different injuries. Second, it seems difficult to repair functions when old CNS structures, including the spinal cord and basal ganglia, are injured and contribute to complex pathologic movement patterns. If, for example, the basal ganglia or the thalamus are damaged, a repair is difficult to achieve by movement-based learning. But maybe if movements of animals of the phylogeny are trained, we may reach more efficiently the injured old brain structures for repair. Gene expression changes may be activated then for further repair of the deficient neural structures.
Tiktaalik roseae is a lobe-finned fish from the late Devonian period, about 375 million years ago, having features akin to those of four-legged animals (tetrapods) (Figures 6) [34-36]. Tiktaalik (Figure 6B) has a possibility of being a representative of the evolutionary transition from fish to amphibians. It and similar animals (Figure 6A) may possibly be the common ancestors of amphibians, reptiles, birds, and mammals. Tiktaalik was gaining structures that could allow it to support itself on solid ground and breath air, a key intermediate step in the transformation of the skull that accompanied the shift to life on land by our distant ancestors.
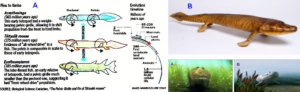
Figure 6. A. Evolutionary transition from fish to tetrapod’s. B. Tiktaalik roseae. Possible movements in shallow water (A) and when coming out of water (B).
The patient Nefeli with an incomplete spinal cord injury at Th10 (Figure 1A-D) could simulate with the arms the symmetrical front fin movements of Tiktaalik (Figure 7C, D) and also the alternating fin movements (Figure 7C, D) and trained in this way trunk stability. Still these movements are not suitable for trunk stability repair, because firstly, the patients do not like those movements, secondly, the movements are not very integrative and thirdly arm and leg movements must be integrated in the movement to activate neural networks across the injury site. The creeping (Figure 10), the propulsion from the arms to the legs (hopping, Figure 8) and the salamander crawling (Figure 10) are more suitable for the repair of spinal cord and brain injuries. May be, also Tiktaalik, Acanthostega or similar animals used these movements for locomotion.

Figure 7. Left. Possible symmetrical front limb movement of Tiktaalik (A, B), simulated for repair by the 10-year-old Nefeli with an incomplete spinal cord injury at the level of Th10 (C, D). Right. Possible rotational body movement of Tiktaalik (A, B), caused by alternately using one front limb for forward locomotion. This front limb movement is simulated by a patient with a spinal cord injury by using alternately the right and left arm (C, D).

Figure 8. Propulsion forward movement from the front to the hind limbs, performed by a patient with an incomplete spinal cord injury Th10 (Nefeli).
Many scientists regard Tiktaalik as the crucial animal between fish and the first tetrapod’s. But numerous track ways seem to show that first tetrapod’s appeared long before Tiktaalik. Track ways were reflecting quadrupedal gait and diagonal walk (Figure 9). A model of Tiktaalik’s skeleton could also produce a print much like the one published (Figure 9) if it is mushed into sand. Different consistencies or angles could produce an even closer match. There is nothing in Tiktaalik’s described anatomy that suggests it did not have a stride.
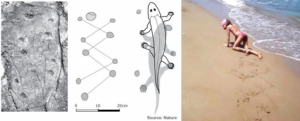
Figure 9. Footprints of tetrapod’s and track way of crawling of a patient with incomplete SCI Th10.
The salamander-crawling (Figure 10) may be a pattern from phylogeny which helps to repair the CNS. How much such salamander-walking is helpful must be seen. This movement pattern includes the trot gait crawling combined with a bending of the trunk and will be anyway helpful to reduce scoliosis and improve trunk performance. As can be seen from Figure 10, the spinal cord injury patient Nefeli could bend the trunk well to one side (Figure 10C) but only little to the other side (Figure 10D) because of scoliosis and spasticity. Emphasizing the bending to the difficult side will reduce the scoliosis. A more efficient reduction of scoliosis will be achieved when exercising the trunk rotation on the special CDT device in the lying position (Figure 4).

Figure 10. A, B. Moving of the salamander (Salamander-walking). C, D. Salamander-walking (salamander-crawling) of the 10-year-old Nefeli with a spinal cord injury (Th10). In D the bending is disturbed because of the scoliosis and spasticity.
With respect to the repair of the basal ganglia by movement-based learning, it is unimportant when tetrapod’s appeared. Important is whether there are movements which can repair old brain structures efficiently. When a patient or healthy person moves at beach (Figure 9), different track ways can be mashed into the sand, depending on what movement pattern is performed. The patient Nefeli could creep, crawl, salamander-crawl, bear walk, spider-walk, walk and could run a tiny bit and can mush into sand many very different track ways. The movement pattern of Figure 10 is the here named salamander-crawling. Crawling in pace or trot gait in the forward or backward direction can generate already many different track ways. What movements a nervous system can generate, we only know when the nervous system is available and we can measure it up with basic methods.
The cerebral palsy girl Sophie (Figure 1E-H) was not able to creep. When she learned to exercise by herself on the special CDT device (Figure 4) she suddenly could creep with quite a good performance (Figure 21A,B). Obviously, the creeping (Figure 21) is an automatism. Interesting is, why does the pelvis rotate during the creeping movement? Is there an animal with a similar moving pattern? One possibility is that rotational movements occurred already in the Tiktaalik, Acanthostega or similar animals. Tiktaalik may moved symmetrical with the front fins, moved alternately with one front fin or moved with front and hind fins at different patterns. A repair of trunk stability/performance is necessary in most CNS injuries. But especially in spinal cord injuries between the intumescentia cervicalis and lumbosacralis, the repair/improvement of the trunk stability is important. The possible different trunk movements of Tiktaalik or similar animals may contribute to the repair of the trunk.
In Alen the improvement of trunk movement performance was also important, because he was growing and because of the hemiplegia, his trunk was already deformed and may get further deformed.
Repair of phase and frequency coordination through exercising on special CDT devices
The first and most important movement, to be trained during repair, is the exercising on a special CDT device to repair the coordinated firing of neurons, namely, the phase and frequency coordination. This new repair strategy is based on a new development in human neurophysiology with which it is possible to record the impulse traffic among neurons at the single-neuron level.
Human Neurophysiology
With the single-nerve fiber action potential recording method, single-nerve fiber action potentials can be recorded from sacral nerve roots, running in and out of the spinal cord (Figures 11, 67) [37].
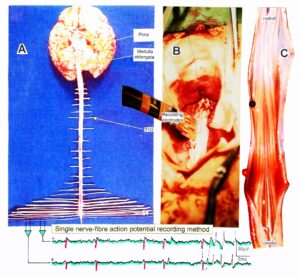
Figure 11. Layout of the recording of single-nerve fiber action potentials to analyze the self-organization of neuronal networks of the human CNS under physiologic and pathophysiologic conditions. A, B, C. By recording with two pairs of platinum wire electrodes (B) from sacral nerve roots (cauda equina, C) containing between 200 and 500 myelinated nerve fibers, records were obtained in which single nerve-fiber action potentials (APs) were identified from motoneurons (main AP phase downwards) and afferents (main AP phase upwards). A. Human CNS with the schematic illustration of the recording layout and an original record of single nerve-fiber action potentials. Note the time calibration of 2ms. B. Intraoperative recording layout (when implanting a bladder stimulator) with two pairs of wire electrodes and one temperature sensor. A thin nerve root is positioned over the platinum wire electrodes. C. Dissection of the human cauda equina. At the caudal end, the filum terminalia and thin nerve roots can be seen. Dissections of the Author apart from the laminectomy in B.
By measuring the conduction times and with the known electrode pair distance of 10 mm, conduction velocity distribution histograms were constructed in which the myelinated nerve fiber groups larger than 4mm could be characterized by group conduction velocity values (Figure 12). After the recording, morphometry was performed. Distributions of nerve fiber diameters were constructed and nerve fiber groups were characterized by the peak values of asymmetrical distributions (Figure 12). By correlating the peak values of the conduction velocity distributions with those of the diameter distributions, obtained for the same root, a classification scheme was constructed for the human peripheral nervous system (Figure 13) [38,39]; the only existing one for human peripheral nerve fibers.
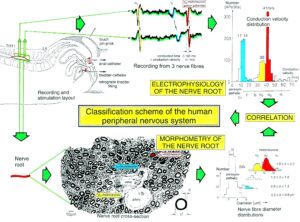
Figure 12. Development of a classification scheme for human peripheral nerve fibers. Conduction velocities (V) and nerve fiber diameters (Æ) of afferent (from receptors) and efferent (motor) nerve fiber groups in normal humans and in patients with a traumatic SCI for 0.5 to 6 years.
This classification and identification scheme represents a solid basis for classifying and identifying nerve fiber groups in the human peripheral nervous system and analyzing CNS functions at the single-neuron level. It became thus possible to record natural impulse patterns simultaneously from identified single afferent and efferent nerve fibers and analyze self-organizing mechanisms of the human CNS under physiologic and pathologic conditions.

Figure 13. Classification scheme for human peripheral nerve fibers. Conduction velocities (V) and nerve fiber diameters (Æ) of afferent and efferent nerve fiber groups in normal humans and in patients with a traumatic spinal cord injury for 0.5 to 6 years. The splitting of the a1-motoneurons into the 3 subgroups, a11, a12, a13, has not yet been confirmed. This is the only existing classification scheme for human peripheral nerve fibers!
The most important finding with the single-nerve fiber action potential recording method was that nerve cells in the human CNS are organizing themselves through “Phase and Frequency Coordination” [40, 41] (Figures 16, 17). In nerve fibers, this phase and frequency coordination can easily be measured, because the three motoneuron types fire for high activation oscillatory [42] and offer in this way a structure to which the timed firing of neurons can be related to. Since the α2-motoneuron oscillations are most stable, firing phases of neurons are related best to the α2-motoneuron firings.
Figure 14 shows schematically the oscillatory firing patterns of the three kinds of motoneurons and the muscle fiber types they innervate.
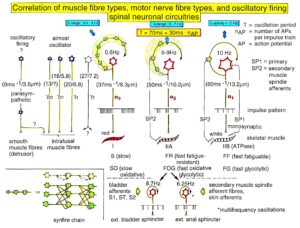
Figure 14. Correlation of muscle fiber types, motor nerve fiber types, and oscillatory firing spinal neuronal networks (oscillators), based on histochemical, morphological, and neurophysiological properties. This figure provides a simplified correlation between muscle fiber, motoneuron and sacral oscillator types. No additional subtypes have been included. The existence of a1-motoneuron (FF) oscillators firing at 10 Hz has been predicted and they have been identified in paraplegics. a = motoneuron, g1, g2 = dynamic and static fusimotors, parasympathetic = parasympathetic preganglionic motoneuron. S1, ST, S2 = stretch, tension and flow receptor afferents.
By comparing CNS functioning in brain-dead humans (where the spinal cord is functioning rather physiologically) and patients with spinal cord injury, injury-induced changes of CNS functioning can be measured and partly repaired. Mainly the phase and frequency coordination of neuron firing becomes impaired following injury. This impaired coordination among neuron firings can efficiently be repaired through exercising on a special CDT device (Figure 4).
The drawing back of the single-nerve fiber action potential recording method is that it is an invasive recording method. But with the surface electromyography (sEMG) [43] one can record non-invasively coordinated firing among motoneurons via their motor units if one records from suitable patients, like incomplete spinal cord injury patients, when a certain muscle is only innervated by a few motoneurons.
In Figure 15, the recordings from motoneurons and motor units are compared. The firing patterns of α1, α2 and α3-motoneurons can easily recorded with the single-nerve fiber action potential recording method but not with the sEMG (Figure 15). From spinal cord injury patients, on the other hand, single-motor unit APs can be easily recorded from α1 motor units but not from α2 and α3 motor units (Figure 15), because their AP amplitude seems to be too small. Clinical sEMG recordings therefore show mainly the activity of α1 motor units. The phase and frequency coordination among neuron firings can be measured in human with the single-nerve fiber action potential recording method (Figure 16) and with surface EMG (Figure 17).
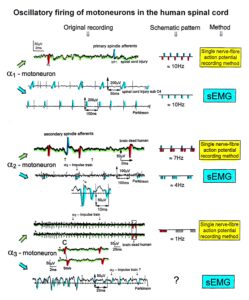
Figure 15. Oscillatory firing patterns of a1, a2, and a3-motoneurons recorded from motoneuron axons with the single-nerve fiber action potential recording method and by surface electromyography (sEMG) from FF, FR, and S-type motor units. The left panel shows original recordings, the middle panel the schematic patterns; the recording methods are indicated on the right side. The recordings were taken from patients with spinal cord injury and Parkinson’s disease and from brain-dead humans.
The neural networks of the human brain, including the cerebral cortex and the basal ganglia, organize themselves by phase and frequency coordination among neuron firings and neural subnetworks as for example the network oscillators of which the motoneuron is a part. This coordination is achieved by the organization tendencies of the network, the descending impulse patterns from the brain and the spatiotemporal afferent impulse patterns from the periphery.
If the premotor spinal oscillators would not coordinate their firing and synchronize their firing for longer periods of time, tremor would occur. Such pathologic synchronization can be observed in patients with Parkinson’s disease [44, 45].
If the neural networks are damaged by trauma, degeneration or malformation, the coordination between neuron firings becomes impaired and has to be repaired by movement-based learning (CDT). Drugs and operations cannot repair neural network functioning.
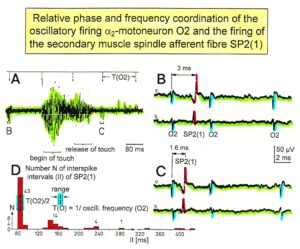
Figure 16. Time relation between the occurrence of the action potentials (APs) of the oscillatory firing a2-motoneuron O2 and the firing of the secondary muscle spindle afferent fiber SP2(1). Brain-dead human HT6. S4 dorsal root recording. A. Overall view of the used sweep piece; only trace “a” shown. Four oscillation cycle periods of the motoneuron O2 are indicated (T(O2)). The APs of the impulse trains can be recognized only partly, because of the slow time base and poor digitalization. One impulse train (dashed arrow) is lost in the touch stimulated activity, which consists of a touch (large overall activity) and a release part (lower overall amplitude). B, C. Sweep pieces from A, time stretched. In B, motoneuron impulse train APs are marked O2, spindle afferent APs are marked SP2(1). Note that the APs of the spindle afferent fiber are not time-locked to the first AP of the impulse train of the rhythmically firing motoneuron (relative phase coordination). D. Occurrence of interspike intervals of the secondary muscle spindle afferent fiber SP2(1). The numbers give the amount of IIs in each distribution peak. The oscillation period of motoneuron O2 (and the range of variation) and the half period are indicated by short dashed lines. Note that the IIs of fiber SP2(1) are very similar to the oscillation period (or the half of it) of a2-motoneuron O2 (relative frequency coordination).
The generation of motor patterns of α1-motoneuron firings with increasing load and the phase and frequency coordination among single-motor unit firings can be recorded with sEMG (Figure 17).
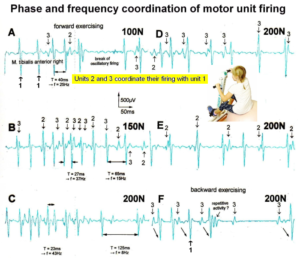
Figure 17. Phase and frequency coordination between oscillatory firing of 3 motor units (FF-type, motor units ‘2’ and ‘3’ are partly marked) during the generation of a motor program when exercising on the special coordination dynamics therapy device at loads increasing from 100 to 200N. Oscillation periods (T) and oscillation frequencies (f [Hz]) of oscillatory firing motor unit 1 (largest motor unit) are partly indicated. ‘C, F’ soleus electrodes shifted to gluteus muscles. In ‘F’, some coordination’s between motor unit ‘3’ and ‘1’ are marked.
Improvement of stability and exactness of phase and frequency coordination to allow specific patterns formation and learning transfer (System Theory of Pattern Formation)
The importance of stable and exact phase and frequency coordination, to allow specific pattern formation and in consequence learning transfer [46] to other patterns, can be understood at the collective variable (X) level (System Theory of Pattern formation [47-49]) and at the neuron level. The behavioural information Finf of the coordination pattern dynamics, characterized by equations of motion of collective variables, dX/dt = Fintr(X) + ∑cinfFinf(X,t), affect the whole coordination pattern dynamics, including stability, rather than only certain coordination patterns. If the behavioural information includes the exercising of extremely coordinated, integrative movements, like exercising on the special CDT device for turning, then the quality of CNS self-organization can be enhanced by improving the exactness of self-organization, namely the precision of phase and frequency coordination between neuron and neural assembly firings. By improving the precision of organization of the intrinsic dynamics Fintr(X), that is, the specific variability of the injured networks, certain patterns do then already reappear. In the 12-year-old patient Alen, the left hand became operational through 6 weeks of coordination dynamics therapy, first time in his life.
Neurons often serve more than one network pattern at the same time by time sharing of neuron firing and, in this way, give rise to learning transfer among the activated patterns. If subnetworks are improved in the organization of one pattern, the organization of the other pattern will also improve. Neurons involved in the organization of breathing and activating intercostal muscles, for example, are also involved in the organization of trunk stability. By reducing the spasticity of the trunk (in patients with Parkinson’s disease), the breathing will also improve. Similarly, sphincteric motoneurons are involved in continence and pelvic floor weight bearing. If during pregnancy the pelvic floor is not trained, sometimes incontinence occurs. This stress incontinence after birth can be repaired by learning transfer from coordinated movements. By mainly exercising on the special CDT device and jumping on springboard, urinary bladder functions can be repaired by learning transfer in otherwise healthy women.
Measuring CNS functioning by the arrhythmicity of exercising (coordination dynamics value)
The impaired phase and frequency coordination at the single neuron level, the assembly level and the macroscopic level can be measured macroscopically when the patient is exercising on a special coordination dynamic therapy device (Figures 28A) on which arms and legs turn with a slightly different frequency (transmission 19 (arms) : 18 (legs)). The phase coordination between arms and legs is imposed by the device. The loss of phase and frequency coordination between arm and leg movements becomes visible and measurable by the arrhythmicity of turning. During a turning cycle, the coordination between arms and legs changes between pace and trot gait and according to the difficulty of the coordination, the turning frequency increases and decreases. This frequency variation (df/dt; f = frequency) can be recorded, quantified and displayed on a computer screen (Figure 51) and is called coordination dynamics value. CNS functioning is therefore measured though pattern change (continuous change from trot gait to pace gait and backwards) according to the System Theory of Pattern Formation.
During the functional reorganization of the injured CNS of patients, the relative phase and frequency coordination of neuron firing has to be trained as exactly as possible by the movement induced afferent impulse patterns from the receptors (learning through feedback information) to restore coordination in the range between 3 and 5 milliseconds (approximate lengths of postsynaptic potentials). The device has therefore to impose the exercising patient a coordination in the millisecond range for the different coordination’s of arm and leg movements between pace gait and trot gait. The easy pace and trot gait coordination’s, but not the difficult intermediate coordination’s, can often be performed easily by the patient. Therefore, the continuous change from the easy to the difficult coordination’s and backwards diagnoses the capability of the CNS to organize easy and difficult organizational states. If the movement states can be easily generated by the neuronal networks of the CNS, then the frequency variation of turning is small during the turning cycle, and if the movement state is difficult to be organized by the patient’s CNS, then the frequency variation is large (the coordination dynamics value is large).
Unique properties of special CDT devices
The special CDT device has three important properties. First, the patient performs coordinated arm, leg and trunk movements when exercising on it. The training of the integrative patterns take care of that the pathologic organization cannot escape from repair by shifting to another part of the CNS and the whole CNS, including the injured parts, is reorganized so that other CNS parts can take function over through plasticity. Second, the device is extremely exact, so that the endplate potentials in the neural networks (approximately 5ms long) overlap, to improve the efficiency of organization. In spinal cord injury, for example, the transmission over the injury site will increase. In basal ganglia, cortex and pyramidal tract repair, more action potentials will reach the motoneurons in the spinal cord for activating arm and leg muscle fibers. Third, the coordination between arm and leg movements changes from pace to trot gait, imposed by the device. The intermediate coordination patterns between pace and trot gait are difficult to generate for the CNS neural networks. If the patients CNS learns to generate these intermediate patterns, imposed by the device, then the neural networks have learned to function better in the deep complexity of CNS organization. The patient’s nervous system learns by turning from the device, to function more physiologically through improving especially the phase and frequency coordination among neuron firings. This phase and frequency coordination can be measured by the single-nerve fiber action potential recording method (Figure 16) invasively and by single-motor unit surface electromyography non-invasively (Figure 17).
Motor learning and problem-solving therapy
Because of basal ganglia and cortex injury and atrophied pyramidal tract in the patient Alen, there are retarded, accelerated or deviant development of motor and other functions. Some functions may not develop at all, while others show only a decrease in variability. Both impaired and healthy parts of the brain mature over time and thus lead to increased complexity, which has direct repercussions on the quality of the learning (trial-and-error-elimination [50]) processes. Processes that in themselves are normal cannot bring about good results because some areas of the brain which are also necessary for the accomplishment of the particular motor function are deficient.
The well-known symptoms and signs of cerebral palsy in the first year of life (poverty of movements, stereotypy of posture and motility, inability to “discover” new motor possibilities, neglect of one extremity, stereotyped extension of the legs during vertical suspension, head-lag during the traction test or during sitting) can all be traced back to a lack of trial-and-error-elimination processes (learning) because of deficient brain structure. In the case of an inability to “discover” new motor possibilities, there may be a disturbance in the chain of events because errors are not recognized (or not eliminated), with the result that the processes stop prematurely [51]. The learning therapy has therefore to be administered continuously over longer times to run through the whole chain of events. Further, learning ‘tricks’ have to be used to enhance the ability to discover new motor possibilities. In the case of Alen, know how has to be used so that his CNS discovers movements of the left not functioning hand.
The System Theory of Pattern Formation of CNS development encompasses all areas of development and is derived from mathematics, physics, human neurophysiology, clinical research, and developmental psychology. The CNS is considered as one neuronal network. A new behavior is generated, which is dependent on the input of all subsystems. This behavior may have a characteristic that could not have been determined by evaluating the contributing behaviors individually [52].
The system repair approach is a “feed-forward system” that is self-correcting ‘en route’ rather than hardwired from the cerebral cortex. It also implies that all factors, subsystems, or structures contributing to the motor behavior (or patterns in general) are important and exert an influence on the outcome [52]. If cortex and basal ganglia are injured in an infant, then many patterns become abnormal or do not exist. The higher centers can no longer control movements or other patterns sufficiently. But through movement-based learning, a self-correction ‘en route’ may partly compensate for the missing contribution of a subsystem in the way that other subsystems take functions over and/or the cerebellum and pons are partly repaired.
The problem-solving learning tries to repair sub-networks that are necessary for functioning and learning. By inducing trial–and-error-elimination processes in subunits of the normal developing nervous system, an optimal development is achieved [50]. To teach the injured CNS to repair itself by trial–and-error-elimination processes, the CNS has to recognize upon CDT which sub-networks, regulation units or sub loops are not functioning properly (or are missing) and to repair them by error elimination, including the possibility that other brain parts partly take functions over, and sub-networks build anew to a limited extent.
Interpersonal coordination and co-movement
To induce learning of movements in the hemiparetic Alen, also interpersonal coordination [47] and co-movement were used. When the therapist performs the movement simultaneously in interpersonal coordination (Figure 23A), she can draw the patient into a better movement pattern.
When a hemiparetic patient performs with both arms, hands, fingers or legs the same movement (co-movement), then the paretic side can learn from the good side the movement pattern. Such learning/pattern transfer can be very fast. In the patient Alen, the CNS learned the power (Figures 29 and 30) and precision grip (Figure 31) immediately, first time in life!
Repair strategies at the neuron membrane and genetic levels
The building of functions/patterns in the 12-year-old Alen, first time in life, make it likely that excitation-neurogenesis coupling [53] contributed, stimulated through CDT.
- Repair depends on learning and memory formation, mediated or supported by epigenetic mechanisms. Epigenetics is the interplay between genes and the environment resulting in phenotype and epigenetic landscape.
- Epigenetic mechanisms, like DNA methylation, are probably sensors for movement-based learning and memory formation and fine modulators of neurogenesis though CDT (Figure 18).
- The epigenome consists of non-coding RNA and chromatin, a proteinaceous matrix surrounding DNA. The dynamic interactions of post-translationally modified chromatin proteins, covalently modified cytosines inside DNA and non-coding RNA define the complex pattern of gene expression beyond the four bases of DNA.
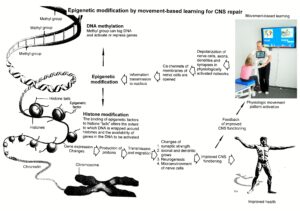
Figure 18. Epigenetic regulation for repair by movement-based learning. CDT-induced stimulation of the pathways that regulate neural network repair is a proven therapeutic and preventive tool. Epigenetic mechanisms, stimulated by physiologic network activation, are likely key players within signaling networks, as DNA methylation, chromatin remodeling and small non-coding RNAs superfamilies’ are required for the fine-tuning and coordination of gene expression during neural network repair by learning.
- The hippocampus plays an essential role in learning and memory. In the hippocampus there exists a specialized form of neural plasticity, which is, the generation of new functional neurons from stem cells occurring throughout life. Adult hippocampal neurogenesis contributes to learning and memory formation.
- New neurons are important for learning and memory formation (besides functional reorganization), i.e., for increasing the rate of repair, for the following reasons:
- The insertion of new neurons helps to store the memory of the same activity that led to the creation of the neuron.
- Activity-dependent neurogenesis enhances the learning of new memories and degradation and clearance of previously stored unwanted memories like spasticity, because the synapses, dendrites and axons can be devoted more fully to the newer memories. The old neurons with large and complex axon and dendritic trees are difficult to change. They can only be changed with sustained effort.
- New neurons seem to improve the accuracy of relearned patterns (from model study [53]). This means that new neurons help to improve phase and frequency coordination of neuron firing and pattern stability.
- The advantage of new neurons seems to be dramatically greater in networks that had been more active and had been required to store more memories [53]. The advantage of neurogenesis for memory storage in heavily active networks is that it provides an increased rate of repair if movement-based learning is administered aggressively and if different movements are trained.
- Specific natural network activity is required for multiple aspects of repair. Specific activity is essential for correct migration of interneurons and it also controls the development and repair of their axons and dendrites. During repair there is a specific requirement of network activity in shaping the cortical integration of specific neural subtypes. Newly build neurons are likely electrically active shortly after their birth and participate in the early network activity that contribute to circuit maturation during repair by CDT.
- Specific activity is required for migration and maturation at several stages of repair. A break in CDT may invalidate the whole chain of repair events. Specific interneuron subtypes require activity for migration and morphological maturation at two distinct stages of development [40]. Newly built neurons may even require specific activity for migration and maturation at several distinct stages of repair. During a break in CDT, the specific activity, required for neuron migration, maturation, and network integration, may not be supplied at one of these stages so that the chain of repair events is severed, the whole repair chain has to be started anew.
- Drug application may undermine repair. Altering the level of neuronal excitability within genetically targeted neurons from drug application, for example antiepileptic drugs may have profound consequences on multiple aspects of the repair of select types of neurons within a population of neurons, as well as their associated gene expression. The pain-killer ‘Contergan’, taken during pregnancy, changed gene expression and the babies were born without arms.
- Excitation-neurogenesis coupling [53]:
- Excitation increases or decreases neuron production directly by excitation-neurogenesis coupling.
- The excitation acts indirectly on the surrounding mature (hippocampal) cells through depolarization-induced release of growth factors.
- Adult neurogenesis is enhanced by excitatory stimuli and involves Ca2+ channels and NMDA receptors.
- The Ca2+ influx pathways are located on the proliferating stem/progenitor cells (NPCs), allowing them to directly sense and process excitatory stimuli. The Ca2+ signal in NPCs leads to rapid induction of a proneural gene expression pattern.
- Integrative coordinated movements have to be trained to allow functional reorganization and new nerve cell integration across very large distances. CDT has to activate injured and uninjured networks to enhance physiologic CNS functioning and learning transfer.
- Conclusion for optimal therapy according to the present stage of knowledge. If there is similarity between development and repair, animal (mice) data also hold in humans and the principles of neurogenesis of the hippocampus also hold in other parts of the brain, albeit to a much lesser extent, then the patient has to be trained at his limits (1) to induce substantial building of new nerve cells [54]. The treatment has to be continuously administered (2) to support all stages of repair at the progenitor level as migration, maturation and integration. The networks, requiring repair, have to be activated specifically (3) to generate repair-friendly, micro-environmental properties in the networks. No drugs should be administered that change neuron excitability (4). The exercises have to include coordinated arm, leg and trunk movements (if possible) to improve the impaired phase and frequency coordination for CNS self-organization (5). The performed movements have to be as integrative as possible to reconnect distant brain parts and to induce learning transfer.
This short introduction to the theory of coordination dynamics therapy may help to understand the substantial progress achieved in the patient Alen.
Results
CNS functioning at the beginning of coordination dynamics therapy
Location of the injury
The patient Alen suffered a perinatal insult with asphyxia. He sustained a cerebral hemiplegia on the left side, a parenchyma defect of the basal ganglia and a degeneration of the pyramidal tract. Figure 19A, B shows the MRI of the 9-year-old Alen. The insult with asphyxia changed the brain. Rather normal ventricles are shown in Figure 19C-E for comparison. Since there is no MRI available from after birth, it is not clear how much a subependymal germinal matrix bleeding is involved in the changes.
Alen was born through a cesarian. He could not breathe. When the fluid, mixed with blood, was removed from the lung, he started to breathe. The time period of apnea is unknown. At an age of 9 years, a parenchyma loss of the basal ganglia was diagnosed and a Wallerian degeneration of the pyramidal tract at the site of the pons found, indicating a degeneration of the lateral corticospinal tract and the anterior corticospinal tract (Figure 20A). The loss of the parenchyma of the right basal ganglia was probably caused by post-ischemia and/or post-hemorrhage following a subependymal germinal matrix bleeding with pressure onto the close pyramidal tract. Brain injuries could therefore be caused at different sites of the brain (Figure 20).
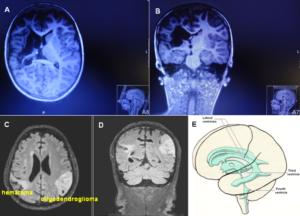
Figure 19. A, B. MRI (with movement artifacts) of the 9-year-old Alen: Strongly changed brain and ventricles, parenchyma loss of the basal ganglia and a Wallerian degeneration of the pyramidal tract and the anterior corticospinal tract. C, D. For comparison, MRI of a cancer patient, ventricles are somehow normal [23]. E. Picture of the ventricles (taken from [31]).
An injury involving the cerebral cortex, causes weakness of part of the body of the opposite side. Hemiparesis is seen in the face (not in Alen) and hand more frequently than elsewhere, because these parts of the body have a large cortical representation (Figure 4 and 20B). The typical finding is a predominantly distal paresis of the arm, most serious functional consequence of which is an impairment of fine motor control of hand and finger. The weakness is incomplete (paresis rather than plegia), and is flaccid, rather than spastic, because the accessory (nonpyramidal) motor pathways are largely spared.
If with hemorrhage or ischemia the internal capsule is involved (Figure 20), there will be a contralateral spastic hemiplegia. In the patient Alen, no real spastic could be found so far. He could not use the left hand and had to manage everyday life with one hand. School medicine did not try to repair or failed to repair the left-hand functions during his life.
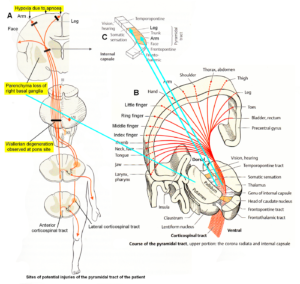
Figure 20. Sites of injuries of the CNS of the patient Alen. The Wallerian degeneration of the right pyramidal tract has consequences also on the ‘good’ right side through the anterior corticospinal tract. Figures partly taken from [31].
Sensitivity in the left hand
By examining touch and pain, it was found that there was full sensitivity in the left hand and fingers. He could distinguish between warm and cold. Therefore, the thalamus was not or only little damaged through the hypoxia, because the thalamus ‘tells’ you that you have a feeling. In comparison to the blood supply of the basal ganglia, the thalamus may obtain more oxygen with the blood than the basal ganglia because of the artery communicans posterior (Figure 2). But when exercising strongly, Alen showed cold sweating in the left hand, indicating also an impairment of the vegetative nervous system.
The next question was, whether the sensory cortical fields were damaged? Since with closed eyes, Alen could clearly say where he was touched and he had the same feeling than on the right hand, the sensory cortical fields (Figure 4) were not or only little injured. Since, apart from the pyramidal system, the spinal cord was not injured, the intrinsic apparatus of the spinal cord was probably functioning and could be used for repair of the left-hand functions.
Deficits of motor functions
When starting CDT, the 12-year-old Alen could not creep in a physiologic pattern (Figure 21c). Rather physiologic trot gait creeping patterns are shown in Figure 10ab. The patient Nefeli with a spinal cord injury (SCI) and the patient Sophie with an atrophy of the cerebellum and pons are creeping in interpersonal coordination. Apart from the spasticity of the pelvis (lifted pelvis), Nefeli creeps physiologically. Sophie creeps with deficits. During moving she overstretches (Figure 21a) and overswings the legs (Figure 21b). The creeping of Alen is worse than that of Sophie. His legs are swinging about and the pelvis is not moving on the ground (Figure 21c). This very poor creeping performance may indicate an injury preferentially of the paleostriatum (globus pallidus), because over the course of phylogeny, phylogenetically old species (Tiktaalik 375 million years ago, phylogeny between fish and Ichthyostega) (Figure 6), may also moved in a creeping way or performed salamander crawling (Figure 10).

Figure 21. a, b. The patients Nefeli (SCI) and Sophie (atrophy of cerebellum and pons) during creeping in interpersonal coordination in antiphase. Sophie is overstretching (a) and overswinging the legs (b) in comparison to Nefeli. She had not fully learned so far to control the inertia and centrifugal forces of leg movement. She cannot stop leg movement in time. The spinocerebellum (vermis) had not been repaired sufficiently so far. c. The boy Alen with injured basal ganglia, caused by hypoxia, during trying to creep. The performance of the pattern is very pathologic in comparison to that of Nefeli. Strong overshoot (dysmetria) of the left leg can be seen (marked). The left marked hand is also activated pathologically due to the atrophy of the pyramidal tract.
At the beginning of therapy, Alen was able to crawl in pace gait coordination (one body side against the other) in quite a good coordination (Figure 22). But he could not crawl continuously. The maintenance of the pace gait crawling performance was inexistent. This means, the automatic control of the pace gait crawling was impaired.

Figure 22. The patient Alen during in-phase crawling. At the start of pace gait crawling, the left hand is positioned rather physiologically (A). Then he loses the hand pattern. Only one finger is activated (B). And then the left hand is not included in the crawling pattern anymore (C). But he has no spasticity in the hand, like in stroke. He only cannot hold the starting pattern and the hand and the fingers are not activated.
The trot gait crawling was continuously in Alen. Only there was no coordination between arm and leg movements and the left hand was not activated (Figure 23B). For comparison, Figure 23A shows a rather coordinated trot gait crawling of a cerebral palsy girl in interpersonal coordination with a physiotherapist.

Figure 23. A. Trot gate crawling of a cerebral palsy girl in interpersonal coordination with the therapist. The crawling performance of the therapist is not optimal. The right arm is leading with respect to the left knee. The crawling performance of the girl is also not optimal; the knees are too much apart. B. The trot gait crawling of the patient Alen is continuously, but the coordination between arms and legs is between the pace and trot gait pattern (pathologic).
Alen could walk speedily (Figure 24). The stride length was quite large and the positioning of the hands were not normal. Especially the posture of the left hand was pathologic, due to the atrophy of the right pyramidal tract. At the beginning of walking (Figure 24A), the performance was best. But after 3 to 5 steps the performance got worse (Figure 24B, C). The maintenance of the starting pattern was not possible. This means, the automatic control of the walking was impaired, due to the injury of the basal ganglia and the left hand was not activated properly because of the atrophy of the pyramidal tract.

Figure 24. The patient Alen during walking. The performance is suboptimal.
The patient could also run speedily (Figure 25). As for walking, the running stride length was quite large (Figure 25A) and the positioning of the hands pathologic. Again, at the beginning of running, the pattern was best (Figure 25B) and deteriorate with ongoing running steps (Figure 25C,D,A). Therefore, also during running, one could see that the automatic control of locomotion was impaired due to the injury of the basal ganglia.

Figure 25. The patient Alen during running. At the beginning the running performance is quite good (B), but he could not maintain the good performance (A).
Also, during jumping in anti-phase at a fixed place, he started with a quite good performance, apart from the hand positioning (Figure 26A). But then he lost the jumping pattern after 3 to 5 jumping steps. The pattern stability was low during jumping. Alen had also some problems to initiate (to start) the jumping pattern. After approximately three weeks of CDT, he had no problems any more with the initiation of the jumping pattern and the jumping in series quickly improved with therapy (Figure 26B).
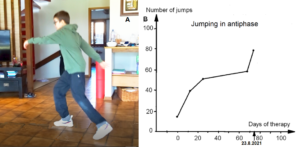
Figure 26. Jumping in antiphase of the patient Alen (A). With therapy the jumping improved quickly (B).
The speech of Alen was quite good. He spoke German, Serbian and English. The Author could communicate with him in German or English. He was attending a special language school for pupils with impaired speech. Accidently a good school choice because the other healthy pupils accepted him fully since they also had a problem (the speech).
Alen could write with the right hand (Figure 27). Probably he was right-handed. In the 5th class, he had not learned so far, the continuous writing.

Figure 27. Writing of the patient Alen, attending the 5th class.
The patient Alen was able to exercise on a special CDT device in the sitting and standing position (Figure 28). For turning in the sitting position (A), for measuring the coordination dynamics values, the left hand had to be fixed, because it would slip from the handle after 10 to 20 turns. Astonishing is that he could exercise in the standing position quite well (B), despite the deficits of the left arm and hand. He could only put little weight on the left hand and arm, because of the atrophied pyramidal tract due to the injury of the right cerebrum. Probably the not crossing extrapyramidal tract of the left side contributed to the performance of the left arm and hand, even though the basal ganglia were also injured. The hand grip power of the left hand was nearly zero, therefore the mother supported him.
A possible motor program for exercising in the sitting or standing position would probably be like the one of a Parkinson’s disease patient (Figure 28C), because Parkinson’s disease patients have also impairment of basal ganglia regulatory circuits (Figure 5B), because of the degeneration of a nigrostriatal projection.
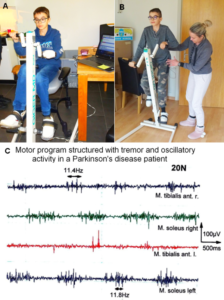
Figure 28. A, B. The patient during exercising on the special CDT device in the sitting (A) and standing position (B). In the sitting position, his CNS was measured to obtain the coordination dynamics value. The left hand had to be fixed, otherwise it would slip from the handle. C. sEMG motor programs of a patient with Parkinson’s disease during the exercise on the special coordination dynamics therapy device at a low load of 20N. The motor program muscle bursts are structured by rhythmicity (no rhythmic structure occurs in normal motor bursts). Rhythmic activity at a frequency of 11.4 and 11.8Hz is indicated.
Impaired coordination among motor and other neurons, leading to oscillatory firing (Figure 14), synchronized oscillatory firing of FR and FF-type motor units, tremor and other dysfunctions, will have consequences for the general organization of CNS neural networks and can be repaired [55,56]. In the motor program bursts, shown in Figure 28C, rhythmic activity can be identified, which cannot be seen in the rather physiologic motor pattern. The highlighted rhythmic firing at 11.4 and 11.8Hz may indicate oscillatory firing of FF-type motor units innervated by α1-motoneurons.
The main disability of Alen was, that he could not move on volition the left hand and fingers because of the cortex damage and the atrophy of the pyramidal tract. Losing one hand function is a disability of 50% (in Switzerland).
Strategy for the brain repair
Because the brain-injured patient Alen had big problems to perform creeping and crawling, but could perform the upright movements walking, running, jumping, and exercising on the special CDT device in the standing position quite well, the brain injury was located more in the phylogenetically old structures like the paleostriatum (globus pallidus) and the neostriatum (caudate nucleus and putamen), apart from the injury of the right cerebrum.
To repair/re-organize the cerebrum, walking, running, jumping, and exercising on the special CDT device in the standing position must be trained with the inclusion of the left arm and hand as much as possible. The repair of the finger functions will be most difficult, because the neural networks activating them are very complex which means very difficult to repair. For the repair of the basal ganglia, creeping and crawling must be trained in a continuous way with good coordination performance between arms and legs. Further, movements have to be trained, which phylogenetically older species may have used for locomotion (Method).
He can crawl in pace gait and in trot gait, but with poor coordination. He has especially big problems with the left hand, but the hand is not spastic. He can walk and jump in anti-phase with poor performance. When jumping in anti-phase, he can hold the pattern for approximately 3 jumps. Then he is losing the pattern (very poor pattern stability).
The 12-year-old Alen has a good prognosis to catch up with normal pupils, if coordination dynamics therapy is administered to him at his limits for several years. The most important repair steps are to improve the cognitive functions through learning transfer from movements and to get the left hand and finger functions working.
Brain repair through 6 weeks of Coordination Dynamics Therapy
Through 6 weeks of CDT, Alen’s motor functions improved and he could hold longer times the left hand on the leaver when exercising on the special CDT device. But he was still not using the left hand in every-day life. Therefore, the Author started to work with know-how on the left hand to get it functioning.
Power grip
To induce the power grip pattern, the Author used the co-movement strategy. Alen was asked to take with the right good hand the glass with no water in and should simulate the water drinking. Of course, there was no problem. Then he was asked, to hold strongly with the right one glass and take with the left had the second glass (Figure 29A) and bring it to the mouth for drinking (Figure 29B,C) and put it back then to the table (Figure 29D). Unbelievable, he could manage through strong concentration. And then he was asked, by the mother, to perform the drinking performance with the left bad hand without holding with the right good hand the glass (Figure 29E-H). Unbelievable, he could manage. The power grip worked a bit.

Figure 29. A-D. The patient Alen learned the power grip. A-D. Through co-movement from the right good hand to the left bad hand, he could initiate the power grip in the left bad hand. E-H. After realizing that the left hand can do the drinking performance, he became able to use only the left bad hand for the drinking pattern.
The next step was the reality test. Is Alen able to perform the drinking in reality? Mother, Alen and the Author were going to the kitchen to see whether Alen could drink by himself with both hands. Alen opened the tap with the right good hand (of course no problem), took the glass with the bad left hand, filled the glass with water (Figure 30A), did drink water in the normal way (Figure 30B), and pore the rest of water to the basin (Figure 30C). After finishing the drinking, he was crying for joy, because first time in life, he managed to drink water in the normal way (Figure 30D). Before he had to open the tap with the right hand, take a glass with the right hand, get water into the glass, drink, put the glass back with the right hand and close the tap with the right hand.

Figure 30. After 6 weeks of CDT, the patient Alen with a Cortex and a basal ganglia injury learned at an age of 12 to drink with both hands (A-C), first time in life. Ale is crying for joy because he succeeded (D).
Precision grip
After being very successful with the learning of the power grip, the question arose, is Alen able to generate the precision grip, first time in life. Again, the co-movement strategy was used. First, he took a glass ball with the right good hand, which was of course working. Then, when holding one glass ball with the right good hand, he became able to take a second glass ball with the bad left hand (Figure 31A), bumping both balls together (Figure 31B) and put them back to the table. Then he was asked by the mother, to take a glass ball with the left hand only and he succeeded (Figure 31C,D). Even though, the right good hand was not in the precision grip pattern, still the fingers are a bit in that position (Figure 31C,D).

Figure 31. Through co-movement, the patient Alen became able to generate the precision grip pattern. Note, the positioning of the left wrist joint is plantar flexed (A-C). In D the wrist joint is in a quite well position. Note further, with the right good hand, he took the ball with two fingers (A,) and with the bad left hand he used 3 fingers for the precision grip. Motor units could be activated coordinately in finger muscles (E).
That Alen learned the power grip and precision grip patterns through 6 weeks of CDT is a dimensional step forward (reduction of disability by approximately 30%). Still much more had to be learned by him through repair. As can be seen from Figure 30C,D and Figure 31, the fingers and the wrist of the left hand are often not activated in the right way. Also the power was missing. Anyway, some motor units were activated in the fingers in a very important physiologic pattern (Figure 39). Alen became able to eat a bit with both hands, but the pattern of the left hand was pathologic.
Training of other arm, leg and trunk movements to improve symmetrical grows of the body
Alen could exercise on the special CDT device, but only having the hands in the prone and not in the supination position, because he could not rotate arm and hand. Other brain-injured patients also have that problem. But when exercising on the special CDT device, he slowly learned to shift the hand positioning from pronation to supination.
To train the left hand and to play at the same time, Alen was allowed to play sometimes with a video game, where he had to use the left hand and fingers. He had to hold the handle and use the thumb (Figure 32). For efficient repair, the exercising on the special device is much more efficient.

Figure 32. The patient Alen during playing a video game in which he has to use the left arm and hand.
To improve the functioning of the left side and to induce growing of the left body side, in comparison to the right side, Alen had also to train the jumping on one leg (Figure 33). He easily managed to jump on the good right leg (A), but he succeeded with the left bad leg only one jump so far (B). It can be seen from Figure 33 that he could easily jump with the right leg, that means, no special concentration was needed. But when jumping with the left leg, he had to concentrate very much. He looked down to realize visually the task (jumping) to be managed. The visual feedback is very efficient to train and learn a task. It was reported that a visualization of a task is very important for relearning movements [60]. For movement control, Alen also used a mirror. To force himself to jump with the left leg, Alen clenched the right hand to a fist, to get more power to the left leg (B). The left hand could probably not contribute in that way.

Figure 33. The patient could easily jump continuously with the right leg (A), bad managed with the left bad leg only one jump (B). Note the clenched fist of the right hand in B. When trying to jump with the poor left leg, he looked down for realizing and controlling the jumping (visual feedback).
Because Alen could not crawl properly and was not able to use the left hand properly (Figures 22,23), the crawling position was trained with the left hand in the physiologic position (Figure 34A-E). To get an absolute simultaneous afferent input to both hands for co-activation, sometimes the hands were put on top of each other (Figure 34F, G). In Figure 34H, the first step to a push up movement pattern is trained.

Figure 34. The mother during training with Alen the crawling position: Backward moving (A), forward (B), up (C), down (D), up without hand support (E), shift to the right side (F), shift to the left (G). In H, the first step to the push up movement is trained.
Neuron as a coincidence detector with respect to coordinated afferent input
How is it possible that Alen learned the precisian grip within 6 weeks, what he did not learn in the 12 years of his life? A regeneration of neurons in the pyramidal tract is unlikely, because with a regeneration growing speed of approximately 1mm/day [7] and a growing distance of 200 to 300mm, more than 200 days would be needed already for the growing of axons from the cortex to reach the cervical motoneurons for hand functions. Further time would be needed for the reorganization of the cortex and the synapse formation at the motoneurons. Therefore, the repair of hand function was coming from a functional reorganization and not from a structural repair so far.
Through exercising on a special CDT device, the coordinated firing of neurons is improved in the whole CNS, and especially in Alen in the cortico-striato-pallido-thalamo-cortical regulatory circuits (Figure 5B) and among the damaged or atrophied pyramidal tract neurons. Existing connections were reorganized in the way to make them functional for power and precision grip and other functions.
When offering the thalamus of both brain sides simultaneous coordinated afferent input in the range of a few milliseconds from the left hand and right hand (Figure 34F, G) (co-movement), then the neurons become even more activated for repair because neurons are coincidence detectors and excitation threshold are reached earlier (Figure 35). They become now able to activate pyramidal tract neurons and in turn the motoneurons to activate motor units in hand muscles, including those for the precision grip (Figure 31E).
Alen’s teacher at school reported, that Alen could through treatment concentrate longer times during the lessons. This means, the whole brain optimized its functioning including those of the cognitive functions.
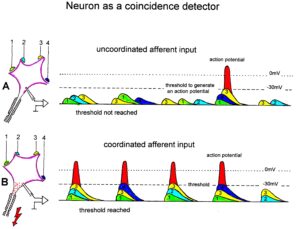
Figure 35: Neuron operating as a coincidence or coordination detector. A. Afferent input is reaching rather uncoordinated the cell soma. Only sometimes an action potential is generated, because the threshold of action potential generation is mostly not achieved. B. The action potentials in fibers 1 through 4 are reaching time-coordinated the dendrites or the cell soma. The postsynaptic potentials add up and the threshold is achieved at approximately –30mV, and action potentials are generated time-coordinated at the axon hillock. In the real CNS mostly, many more smaller postsynaptic potentials will contribute to the generation of an action potential and passive conduction from the dendrites to the cell soma has to be taken into account. Coordinated afferent input may thus induce or enhance (coordinated) communication between neuronal network parts following CNS injury.
CNS repair through 3 months of CDT
Improvement of hand and finger functions on the paretic (left) side
Within 6 weeks of CDT, Alen learned to use the left hand for the power grip (Figure 29), the precision grip (Figure 31) and to use the learned functions for drinking water with both hands (Figure 30). But his nervous system could not generate other left-hand functions. Especially he was not able to rotate the left hand.
The rotational movements of the left (bad) hand were trained now on the special CDT device by changing from the prone to the supination pattern. The left hand was fixed to the leaver. Such rotational hand movement pattern training is more efficient than just trying to rotate the hand, because such movement training is more integrative. After 10 weeks of CDT, he learned a bit to rotate the left hand. He became able to turn a bit in the supination pattern on the special CDT device without fixing the hand (Figure 36). The consequence of this new movement was that he could exercise better on the special CDT device in the standing position in the way that he could hold tighter the left leaver and the left wrist positioning was more physiologic, as can be seen when comparing the wrist positioning of Figure 37 with Figure 28B.

Figure 36. Patient Alen during the training rotational wrist and arm movements on the special CDT device, when the left (bad) hand was not fixed (arrows). The right good hand had no problems to turn in the supination position (B).

Figure 37. Patient Alen during exercising in the standing position. Note the more physiologic left wrist positioning in comparison to that in Figure 28B (marked with an arrow).
When exercising on the special CDT device, the holding of the leavers was similar to the power grip pattern. But more functions of hand and fingers were needed for everyday life. The next step of hand repair was to get more finger functions.
Alen was not able so far to force the left fingers apart. With the right hand he could. To make the patients CNS to learn this finger movement, again specific knowhow was used. The mother was holding both hands of Alen together (Figure 38A) and he tried then to put the fingers apart of both hands (Figure 38B). Unbelievable, it worked continuously. Alen was now able to open and close the fingers, apart from the thumb. The absolute simultaneous afferent input from both hands induced the pattern in the left (bad) hand. Therefore, the co-movement between the right and left hand, the simultaneous afferent input, the strong concentration, and the visualization of the task started/organized the pattern in the left hand.
When the mother did not push the two hands together, Alen could not activate/generate the pattern in the left hand (Figure 38C, D). Therefore, so far, after 10 weeks of CDT, the co-movement and the concentration alone were an insufficient stimulus to activate these left finger functions; the simultaneous afferent input of the touch from both hands was needed additionally.

Figure 38. When the mother holed both hands together, Alen was able to force the fingers apart and together of both hands (A, B). When having the hands apart for opening and closing the fingers (C, D), co-movement, concentration and visualization of the task were insufficient to activate the pattern in the left hand.
The consequences of the improvement of the left hand and left finger functions was that the patient Alen became able to eat pasta with both hands in a rather physiologic way (Figure 39).
Plasticity
Approximately 75 years ago, Sperry transposed the nerve supply of flexor and extensor muscles in the rat [57] and in the monkey [58]: the monkey relearned the task, the rat did not. In Sperry’s experiment on monkeys, their learning to flex or extend the elbow in one situation did not necessarily become generalized to other performances. This indicates that the neural readjustment was not localized solely in the spinal centers, but involved reorganization at the supraspinal levels, including the red nucleus [59]. Surprisingly few trials were required for poliomyelitis patients to use transposed tendons successfully. The visualization of the task seemed to be the prime aid to the patients [60].

Figure 39. Patient Alen during mixing the pasta (A) and turning the pasta in the spoon (B).
As shown here, repair through plasticity can be enhanced more strongly, when using additionally to the visualization of the task, concentration, co-movement and simultaneous afferent input from the fingers in this case. May be other nuclei than the red nucleus, including the repair of the basal ganglia, contributed to the tremendous speed of hand repair.
Training of left leg functions
To visualize the hemiparesis of the patient with respect to the leg, Alen was exercising without trousers and shirt (Figure 40). When standing, one could see that the proximal leg muscles were little or not atrophied, but the lower leg muscles were (Figure 40A). The circumference around the calves, when sitting, were 21.5cm on the left and 28.3cm on the right side. But when jumping with the mother in anti-phase (Figure 40C-D), the gastrocnemius muscle was activated (Figure 40B, arrow) in the phase when jumping upwards. Because of the atrophy of the lower leg muscles, the muscular strength was reduced. It was hard work for him to jump with the mother and he complained to be exhausted.
This jumping in antiphase is a very good movement pattern for training especially leg muscles, to stimulate the growing of the left leg (which was 4cm shorter), to train the coordination between arms and legs and to train the left-hand functions of Alen.

Figure 40. A. The left calf muscles were strongly atrophied. B-C. During jumping in anti-phase with the mother, the gastrocnemius muscle was activated in the lift-off phase (B, arrow) and not so much in other jumping phases (C, D).
Orthopedics intended to stop the growing of the right good leg by putting transiently plates into the knee, so that the lengths of the legs would not differ so much in the future anymore (see below). The strategy of CDT is different. The growth of the left bad leg must be stimulated and not the growth of the right leg stopped. The Author believes in CDT because it is natural and not only the legs are growing when getting older.
A one year and 10 months old patient with spina bifida and brain injury learned within 2 years of CDT to creep, crawl, stand and walk a few steps. With the therapy also hip joints were build [19].
Further improvement of hand functions
When fixing the two thumbs together, he was able to move the fingers of both hands apart (Figure 41A, B). A tiny bit he became able to wash his hands with soap (Figure 41C).
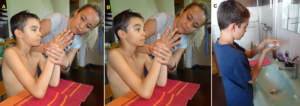
Figure 41. A, B. Opening and closing of the fingers, including the thumb. C. First try in life to wash the hand with both hands and soap.
It is not sufficient that hands and fingers can perform certain movement patterns, also power is needed. On the special CDT device, Alen trained also to generate power, when training at higher loads (Newton). So far, he succeeded to train up to 50N. A healthy pupil could approximately exercise against 100N. To see the increase of power with respect to CNS repair, the grip force was measured (Figure 42A). Within 8 weeks the grip force of the left bad hand increased from around zero to 2.5kp and of the good right hand from 10 to 11kp (Figure 43). The power improvement in the left (bad) arm as can be seen from the size of the biceps muscle (Figure 42B). Alen was proud of the increase of the biceps because even a girl at school noticed it. With therapy continuation, the hand grip force further increased (Figure 43).

Figure 42. A. Measuring of hand grip force. B. The increase of size of the biceps muscle in Alen can clearly be seen. Even at school it was noticed.
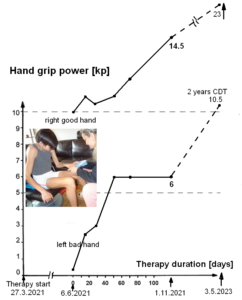
Figure 43. Development of hand grip power of the 12-year-old patient Alen (and 14-year-old Alen; dashed line). Note the strong improvement of the left and right hand. In the picture of hand grip performance, the activation of the biceps of the now 14-year-old patient can be seen.
Pattern variability and forefoot training by running backwards
Because of basal ganglia and cortex injury and atrophied pyramidal tract in Alen, there were retarded, accelerated or deviant development of motor and other functions.
Characteristic for normal development is variability in motor performance [50]. But the impaired nervous system is not able to attain such variability in motor performance because the structure of the CNS is deficient in enabling the infant to use various modes of operation for single performance. When there is serious damage to the CNS, it is even possible that the usual modes cannot be developed at all; the brain has to resort to unusual modes. Distorted motor patterns arise, which fall outside the range of the normal variability and which are stereotyped, due to the deficiency of the “hardware.”
At the beginning of therapy, Alen was only able to run and walk with a large stride length (Figure 25), possibly because of a mild cerebellum injury due to hypoxia. Rather quickly he learned to reduce the stride length. The question was now, how much did the nervous system of Alen could improve his CNS functioning with respect to the pattern variability of running. As can be seen from Figure 44, Alen learned to run backwards with large and small steps. This means, the pattern variability of the neural networks for running improved strongly. His CNS functioning improved strongly.

Figure 44. The patient Alen during running backwards with small (A) and large steps (B). Mother and father are motivating him. During this backward running, he is running on the forefoot and training in this way the forefoot muscles and the neural networks activating them.
The importance of backward walking and running is that the patient trains forefoot muscles and the neural networks activating them. With the forefoot walking and running, the foot arch can be built on the long-term, which is often impaired in children with CNS injury.
In Alen the left leg was 4cm shorter than the right one. Therefore, he was wearing in Figure 44 still orthoses, to adapt partly the difference in leg length, to run properly and “discover” more quickly the new motor possibility running backwards. Generally, it was fascinating how quickly Alen in general “discovered” with professional help new motor possibilities of arms and legs.
CNS repair through 4 months of CDT
With 4 months of therapy, the 12-year-old Alen, with diagnosed persistent hemiparesis on the left side, learned to brush the teeth with the left hand (Figure 45).

Figure 45. Alen during brushing the teeth with the left hand.
Importantly, Alen became able to move a bit the bad left foot from right to left (Figure 46).

Figure 46. Right-left movement of the left bad leg. The arrow indicates the movement direction.
This became possible, because Alen trained for one month walking (Figure 47), running (Figure 48) and jumping without orthosis (Figure 49). The orthosis hindered the CNS to learn more left foot movements. When walking and running in the forward and backward direction on the forefoot without orthosis, the CNS of Alen had the chance to learn more foot movements. The jumping in anti-phase will have contributed to the foot learning process.

Figure 47. Walking on forefoot in the forward (A) and backward direction (B) without orthosis.

Figure 48. Patient Alen running on forefoot in interpersonal coordination with the mother. Note the atrophied left triceps.

Figure 49. Jumping of the patient in anti-phase with the father without orthosis.
Correction of leg length discrepancy
Because of the hemiplegia from birth, the left bad leg of the 12-year-old Alen was with 88.5cm 4cm shorter than the right good leg (92.5cm). Orthopedic surgery suggested a hemiepiphysiodesis of the good right leg to stop its grows and correct the leg length discrepancy.
Epiphysiodesis is a pediatric orthopedic surgery that aims at or stopping the bone growth naturally occurring through the grows plate also known as the physeal plate. Temporary hemiepiphysiodesis works through arresting or inhibiting the physeal growth at one hemi-side of the growth plate. In consequence the other hemi-side is allowed to grow normally and unhindered.
The strategy of CDT was, to stimulate the grows of the too short left leg to catch-up with the length of the right leg. To stimulate the growth of the too short left leg, Alen trained especially the turning on the special CDT device (Figure 28), jumped in antiphase (Figure 49) and tried to jump with the left leg only, which was not possible so far, probably because of too little muscle power.
CNS repair through 6 months of CDT
The diminished muscular strength and impaired fine motor control on the left paretic side and the other (rather healthy) side further improved. The hand grip force of the left hand did not increase further over 6kp. The right-hand grip power increased up to 14.5kp (Figure 43). The strong increase of also the right-hand grip power can be understood, because also the right side was impaired by the Wallerian degeneration at the pons side via the not crossing anterior corticospinal tract (Figure 20A). Even though the power grip force of the left hand did not increase further so far, the precision grip improved. Alen became able to throw a small ball downwards and catch it again with the left hand when coming up (Figure 50B). He learned a bit to coordinate the precision grip with arm movement, which is an important functional improvement. The power and the coordination of the muscles of the left leg improved in the way that Alen became able to jump one time with the left leg (Figure 50A). This jumping with only the left leg may help that the left leg grows faster than the right leg to reduce the 4cm length difference between the legs. The mother refused a hemiepiphysiodesis for her son.
That the left-hand playing with the ball (Figure 50B) became possible was found by Alen himself. To “discover” new motor possibilities of the left and right side increased. Often Alen was angry that he had to train so much. But when he learned a new pattern, then he was using it in different motor patterns.

Figure 50 A. Alen during trying to jump a few times with the left leg only. B. The patient during throwing the blue ball down and catching it again. The small ball is marked with a double arrow. As can be seen, Alen is too much standing on the right leg. More training on specially the left side/leg (A) is necessary.
The low-load coordination dynamics (CD) values (Figure 51) did only improve (decrease) by 10%, probably because of the large neural network reorganization changes taking place.
Normally, when exercising on the special CDT device, the CD values get better. But on the other hand, with every bit of reorganization, the CD values get worse because of neural network changes. So far, the increase and decrease of the CD values canceled mainly each other out. When the main reorganizations for repair will have been done, then the CD values will improve (decrease) more.
Therefore, probably the main brain repair so far came from large reorganizations of the neural networks.

Figure 51. Low-load (32N) coordination dynamics in the forward (A) and backward (B) direction of the patient Alen. For higher loads (55N) (C, D) the coordination dynamics values are much worse (increase from 8.4 to 27.3 and from 6.9 to 26.9). E, F. Nearly ideal values from the Author for comparison (CDvalue37N = 2.4 and 162N = 6.0).
Figure 51shows the CD values for low load (A, B) and higher load (C, D). For higher load the CD values get worse (higher). In comparison to the CD values of the Author, the patients’ values are much worse (higher) for low and for high load. A lot of further therapy is needed to improve the functioning of the patients’ CNS. The dreaming goal is that Alen can attend a normal school.
CNS repair through 2 years of CDT
Even though the therapy became suboptimal, because he had to go to school, still some further progress was achieved.
Improvement of the paretic leg
One problem could be improved, namely the functioning of the left paretic leg. It was so far not possible to jump continuously with the left leg to get more muscle power and make it growing faster to reduce the leg length difference from 4cm to 2 cm and not to need an orthosis any more.
It seemed so far that he could not jump with the left leg because of missing muscle power of the atrophied muscles (Figure 52B,C). But when putting the right good forefoot on top of the left paretic forefoot, as the Author showed him (Figure 52A), he was after 5 trials able to jump 12 times continuously with the paretic leg (Figure 52B,C). This means that the main cause for not being able to jump with the paretic leg, were not the atrophied muscles (Figure 52C) but the insufficient activation from the brain, which could be compensated partly by support from the other side (Figure 52D). Through pushing the left forefoot, exactly coordinated with the good right forefoot, and activating in this way the stepping automatism and co-movement, the missing activation from the brain was partly compensated by the activation of the stepping automatism. It may also be that through pushing the forefoot, several movement patterns are activated.
Being able with this co-movement bumping/jumping maneuver to train the jumping with only the left paretic leg, the brain could specifically be activated for brain repair through the movement-induced afferent input from the left paretic leg. When jumping in-phase with both legs, his weight was mainly on the right good leg and the atrophied leg could not specifically be trained. The brain used already the input from the foot optimally, because the left paretic foot was hypersensitive. In consequence the muscle power/size increased and the growth of the too short left leg was specifically activated. The circumference around the calves, when sitting, were 21.5cm on the left and 28.3cm on the right side at the beginning of therapy and reached 24.2cm and 34.1cm respectively after 2 years of therapy. More specific jumping training is needed.

Figure 52. Jumping with left atrophied leg of the 14-year-old hemiparetic patient Alen (B,C) with basal ganglia, cortex and pyramidal tract injury. The continuous jumping is only possible when the right leg is pushing the forefoot of the left leg and inducing the jumping automatism. In A the Author is showing the patient how he has to jump and bump the forefoot to induce the jumping/bumping maneuver. After a few trials, the patient jumped continuously 10 times. Note the atrophied left leg. The patient is jumping with socks because of hypersensitivity of the left foot. D. The missing activation from the brain through the pyramidal tract of the paretic leg is partly compensated by the pyramidal tract of the good right side through the jumping pattern generated in the spinal cord intrinsic neural networks of the intumescentia lumbosacralis.
The further improvement of the paretic leg had the clinical consequence that Alen could learn to ride a normal bicycle (Figure 53A). Also, the training on the special CDT device in the standing position became easier (Figure 53B). Still the main problem was that he slipped to easily from the pedal. He preferred therefore to cycle on even ground. Fixing the paretic foot to the pedal, he was afraid of in case of falling. That he learned to ride a normal bicycle was psychologically important; one step nearer to the healthy children. For training the CNS, the normal cycling is helpful. He just has to train slowly to manage also uneven ground.

Figure 53. A. Alen during riding a normal bicycle. He seems to have no problem with the left paretic hand, but the left paretic foot is a bit too much forward (marked with arrow). B. When exercising in the standing position on a special CDT device, he had also some problems with the left foot, but he could use some support.
Now the jumping/bumping maneuver is tackled to see its importance for Alen and similar patients. The Author had the Jendrassik maneuver in mind when he was looking for a maneuver to improve the movement performance of the paretic leg.
The Jendrassik maneuver enhances the monosynaptic muscle stretch reflex. This one-leg jumping/bumping maneuver activates the stepping automatism and or jumping automatism.
Stepping automatism
The neural network for the pattern ‘newborn stepping’ is mainly located in the intumescentia lumbosacralis, because in cervical spinal cord injury the stepping automatism can be induced again by heel strike or bumping the forefoot. During physiologic development the stepping automatism network is brought under supraspinal control and is changed into the walking pattern. The change from newborn-stepping to walking can be seen in Figure 54. In A and B the newborn Juliane steps automatically. Characteristic is the high lifting of the knees. In C the 4-month-old healthy Jürgen performs a pattern intermediate between newborn stepping and walking and in D the now 8-months-old Jürgen nearly walks.

Figure 54. Development from newborn-stepping to walking. A,B. Automatic stepping in a newborn infant. A. The 5-day-old healthy infant, Juliane, performing primary automatic stepping; slight backward posture. The heel of the right foot touched the ground first. B. Infant Juliane, 8-day-old, performing automatic stepping. C,D. Supported walking of the healthy 5-months-old (C) and 8-months-old old “Jürgen” (D). In C the walking resembles automatic stepping, because of the strong lifting of the left knee. The toes of the right foot are plantar flexed, which is not physiologic.
The movement-based learning therapy (CDT) partly recapitulates the development. In the young patient Kadri with a cervical spinal cord injury (approximately 95% injury), the stepping automatism could be induced again by heel strike and sometimes by bumping the forefoot when administering to her supported treadmill walking (Figure 55) [12]. When the stepping automatism was activated, the leg moved by itself for one step. For continuous stepping, the stepping automatism had to be activated again and again. Hard work for the therapists.

Figure 55. Supported treadmill walking (Author on the left side) of a patient with a spinal cord injury C5/6 (injury 95%). The stepping automatism was sometimes induced by heel strike or bumping the fore foot (arrow), as could be felt by the Author and the father (right). With training, the stepping automatism could be induced more often, but the injury was too severe to reach the volitional walking through CDT.
In the patient Alen, the stepping automatism seemed to be activated in the paretic leg in similarity to the activation of the stepping automatism in the spinal cord injury patient Kadri. But he activated the stepping automatism with the other good leg (forefoot) by himself. Since the input to both feet were activated exactly at the same time, the stepping automatism was supported by the exactly coordinated co-movement of both legs and the exactly coordinated movement-induced afferent input. This, by the jumping maneuver induced, continuous jumping was even fascinating for the patient Alen and he started immediately to train it. Again, it was first time in his life that he could jump with the left leg.
Improvement of the coordination dynamics support the motor improvements on the paretic side
Through improving the muscle power and the innervation especially on the left paretic side, the patient Alen became able to turn on the special CDT device up to 100N (Figure 56) and training better neural network learning in the deeper complexity of CNS organization.

Figure 56. Coordination dynamics measurements of the patient Alen with a hemi-paresis with increasing load from 20 to 100N. With increasing load from approximately 20 to 100N, the coordination dynamics value increases (gets worse) from 8.2 to 52. More repair is needed for 100N, that means in the deep complexity of neural network organization.
To see better the neural network learning with ongoing therapy, the coordination dynamics values are drawn in Figure 57 with ongoing therapy. The improvement of CNS functioning can be seen best for the turning load of 50N.
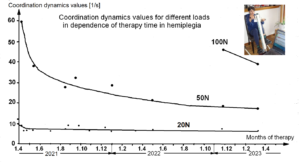
Figure 57. Improvement of coordination dynamics values for increasing load from 20N to 100N with ongoing coordination dynamics therapy of the patient Alen with hemiplegia, basal ganglia, and pyramidal tract injury. He did not need any fixation for the left hand at all loads. The values are the mean of forward and backward exercising.
In conclusion, the patient Alen learned to use the left hand, his paretic leg grew longer so that he needed no orthosis anymore and his cognitive functions improved.
Discussion
General state of the treatment and the educational systems
It is shown that the patient Alen with a so-called persistent hemiplegia for 12 years could learn functions on the paretic side. Most importantly he learned to a certain extent to use the left plegic hand in everyday life through suboptimal coordination dynamics therapy (CDT) for 2 years. It seems possible that his motor and cognitive functions can substantially further be improved with further optimal CDT.
A drawing back in therapy was that the educational and therapy systems were out-of-date and members of these systems were not interested to update their knowledge. If the patient Alen could leave out school for one year for an optimal CDT, his cognitive functions would improve further and he could learn afterwards much better and could attend may be a normal school or a school between disabled and normal. So far, he attended a school for disabled. In Switzerland disabled children get support, but the parents cannot choose the best therapy for their child. They are forced to use the out-of-date therapy system. Since the repair progress is not coming from the offered treatment (neurology, rehabilitation, physiotherapy), the parents are not using the offered treatments and, in this way, it is cheaper for the system on the short-term, but not on the long term and anyway it is not ethical.
Basal ganglia repair
The improvement of creeping, walking, running, and hand functions indicate repairs of the basal ganglia through functional reorganization, because the basal ganglia play an important role in initiation, maintenance and modulation of these movement patterns. Alen learned to initiate the power and precision grip and learned to initiate finger functions. He learned to maintain these patterns better and longer and learned to variate the stride length of walking and running. The regulation circuits were therefore partly repaired through reorganization of their neural networks, especially the cortico-striato-pallido-thalamo-cortical regulatory circuit.
Comparison of basal ganglia repair with cerebellum repair
Cerebellar injuries manifest themselves with disturbances of movement and balance. Dysmetria, the inability to stop a directed movement on time, typical for cerebro-cerebellum injury (Figure 21A, B; Sophie), was at the beginning of CDT also observed in Alen (Figure 21C and 25A). But quickly Alen could repair his dysmetria, whereas Sophie with the cerebellum atrophy had not completely solved the dysmetria over 6 years of CDT. Possibly Alen had with the hypoxia only a mild injury of the cerebellum.
Apart from the hemiparesis, Alen with the basal ganglia injury had no problems with the protection automatisms when falling during walking or running. Sophie with the cerebellum atrophy learned through CDT the protection automatisms in one years’ time [15] and the patient Dr. Cwienk, who lost 80% of the cerebellum in a traumatic injury [14], after 20 years of CDT. Sophie learned to keep balance, but Dr. Cwienk could not overcome the balance problem in 20 years. Sophie and Dr. Cwienk had no trunk stability problems, because they could exercise on the special CDT device in the standing position like Alen (Figure 58). Alen had no principal problems with balance, protection automatisms and trunk stability, but with the hemiparesis.
The severance of a hypoxic injury depends strongly on the duration of the hypoxia. A 25-year-old woman suffered a severe hypoxic brain injury in an accident and had big problems with standing, walking and balance and could not improve them substantially within 6 months of CDT.

Figure 58. Exercising on the special CDT device in the standing position of the 7-year-old Sophie with cerebellum and pons atrophy, the 60-year-old patient Dr. Cwienk with traumatic cerebellum, pons, and cerebrum injury and the 14-year-old Alen with cortex, basal ganglia, and pyramidal tract injury. Note that when no balance is needed, Sophie and Dr. Cwienk can perform this coordinated arm and leg movement quite nicely, but the girl with more elegance. Alen had some problems with the paretic side. The left paretic hand is in a suboptimal position.
Comparison of basal ganglia repair with spinal cord and cerebellum injury repair
Alen learned to ride a normal bicycle within 2 years (Figure 59A). The spinal cord injury patient Nefeli (Figure 1A-D) learned also to ride a normal bicycle, following a partial regeneration of the spinal cord, through 3 years of CDT (Figure 59B). The cerebellum injury patient Sophie (Figure 1E-H) started to ride a bicycle through 6 years of CDT (Figure 59C).

Figure 59. The patient Alen, with cortex, basal ganglia, and pyramidal tract repair, during quite normal cycling (A). The spinal cord injury patient Nefeli during cycling on a normal bicycle with difficulties, following a regeneration of the spinal cord (B). Her legs slipped easily from the pedals. Even without having the feet on the pedals, she had no balance problems. Note that Nefeli had to concentrate very much to keep the feet on the pedals, seven years after the SCI injury, whereas Alen did not have to concentrate so much (A). The patient Sophie with a cerebellum and pons malformation started to learn to ride a bicycle following 6 years of CDT (C). Because of primary balance problems she had to concentrate very much. She had the hands on the brakes because of uneven ground.
With a partial repair of the hemiparesis, Alen learned comparable easily to ride a bicycle and he will use it with further treatment in everyday life. For the spinal cord injury patient Nefeli, 12.5 years-old, it was difficult to learn to ride a bicycle [7]. Despite the partial regeneration, she had not sufficient power in the legs. But she had no problems with the balance. Since the parents did not continue the bicycle training, she did not use it further and the rehabilitation system is anyway out-of-date.
The patient Sophie with cerebellum atrophy (Figure 1E-H) [15] started to learn to ride a bicycle like small children. It will be difficult for her to learn cycling, because of primary balance problems due to cerebellum and pons damage. The girl Anna with genetic disease (Figure 1I-N) must get first further better before to think about learning to ride a bicycle. The cognitive functions are the big problem.
Basal ganglia and cerebellum repair with respect to the ontogenetic landscape for locomotion
A physiologic landscape for locomotion [61] is shown in Figure 60. Because of basal ganglia and cerebrum injury and the degeneration of the pyramidal tract, Alen’s landscape for locomotion would be completely different and cannot be analyzed because of missing data.
CDT partly recapitulates the development. But the learning process is hampered by the deficiency of the basal ganglia, cerebrum injury and pyramidal tract, on which the learning process also depends. To teach the injured CNS to repair itself by trial-and-error elimination processes, the CNS has, in similarity to the development, to recognize through CDT which subnetworks, regulation units, subloops or tracts are not functioning properly (or are missing) and to repair them by error elimination, including the possibility that other brain parts take functions partly over and subnetworks and tract fibers built anew to a limited extent.
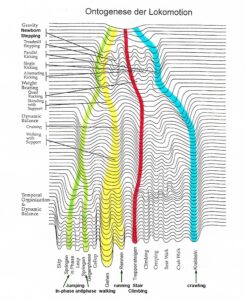
Figure 60. Ontogenetic landscape for locomotion. The evolution of the attractor layout (System Theory of Pattern Formation) for the different movements. With permission of Esther Thelen (†) [61].
In cerebellum injury the ontogenetic landscape would also be different from the normal one. At the beginning of CDT, at an age of 5.5 years, Sophie could only crawl in antiphase to cover distances at home by herself, whereas healthy children at that age can creep, crawl, upright, walk, run, and jump. Because of missing dynamic balance, she relearned the jumping on springboard late and the running very late. The functioning of the cerebellum and pons was pathologic and too slow to be able to generate in time the patterns jumping and running, according to the ontogenetic landscape. When she eventually learned the running (Figure 1H), her dynamic balance had improved strongly.
Comparison of coordination dynamics value improvement of brain and basal ganglia injury with incomplete spinal cord injury
Figure 57 shows the improvement of the coordination dynamics values for increasing loads of the patient Alen with brain, basal ganglia, and pyramidal tract injury. A patient with an incomplete spinal cord injury showed a similar improvement of the coordination dynamics values for different loads (Figure 61). Only this 19-year-old patient Sten was able to turn also at 150N.
This similarity indicates that the main repair was coming from neural network repair and reorganization and not from growing of pyramidal tract fibers. The brain is the genius organ in human for repair and adaptation.
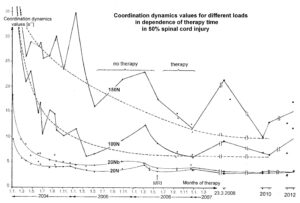
Figure 61. Relation of coordination dynamics values to therapy length for increasing load between 20 and 150N for the patient Sten with a spinal cord injury of approximately 50%. The loads for forward exercising (dots, 20N, 100N, 150N) are marked at the curves (20Nb = backward exercising (crosses) at 20N). Note that with no therapy the coordination dynamics values got worse and upon administering therapy again the values improved again even 2 years after the accident. After stopping therapy, the coordination dynamics values for 100 and 150N increased again (23.3.2008). When the patient trained himself intensively (including running and jumping), he further improved his high-load CD values (8.9. and 23.9.2010).
Jendrassik maneuver, co-movement, jumping maneuver and interpersonal co-ordination
It was shown that in the patient Alen with a brain, basal ganglia and pyramidal tract injury, the co-movement and jumping maneuver were very successful to repair the hemiplegia. The development of function in the left hand was very important for everyday life. More information of the different maneuvers for repair will be given.
Interpersonal co-ordination [47] is taken place, when the therapist is moving in coordination with the patient to pull the patient into a better movement performance. When the therapist moves a bit in front of the patient, then she is in his/her field of vision and the visual input helps to improve the patient’s movement performance. The sound from touching the ground can also help. The marching of soldiers is an example for interpersonal coordination. In movement therapy, the crawling in interpersonal coordination is a typical training (Figure 23A). This interpersonal coordination works also during climbing staircases (Figure 62A), running (Figure 62B) and other movements.
When the Author climbed with the patient Anna, with genetic disease (5p-), the staircases continuously, Anna had no or only little balance problems when the interpersonal coordination was good. She needed not to touch the wall for support. When the interpersonal coordination was less good, she touched the wall for balance support. When she claimed the staircases by herself, she needed the support from the wall or rail. In Figure 62, the Author was holding the hand of Anna for safety and to induce a bit of co-movement by moving her arm in coordination with the leg movements. For running the same strategy was used, namely interpersonal coordination, and a bit of co-movement. In the patient Alen the interpersonal coordination was used seldomly because he could walk and run quite well and had no balance problems.

Figure 62. The Author during climbing staircases and running in interpersonal coordination with Anna, a patient with genetic disease (chromosome deletion, 5p-). The Author is holding her hand for support in case of falling and to coordinate the left arm with the legs to induce co-movement. Note that Anna concentrates automatically on the legs of the Author for optimizing the interpersonal coordination to improve her pattern of climbing staircases, which is no automatism. During running, which is an automatism, she needed not to look at the legs of the Author.
Co-movement is induced, when one arm or leg is brought in coordination with the other arm or leg and induces/improves in this way the movement pattern performance of the other (paretic) arm or leg. In the patient Alen, this co-movement was used extensively (Figures 29-31,38), because it was very successful. The co-movement is especially strong if legs, feet, and hands touch each other and the spinal cord is getting absolutely coordinated movement-induced afferent input especially from the skin, but also from other receptors like muscle spindles and Golgi tendon organs. The visualization of the task improves the success. When Alen started CDT, he had no function in the left hand. But on the special CDT device, he could hold at the beginning the left leaver for a few turns, before slipping from it, through co-movement. For turning continuously, the left hand was fixed (Figure 28A). Later, when hand functions developed and improved with therapy, no left-hand fixation was needed any more (Figure 12B).
The Jendrassik maneuver is a medical maneuver wherein the patient clenches the teeth, flexes both sets of fingers into a hook-like form, and interlocks those sets of fingers together. The tendon below the patient’ response is compared with the reflex result of the same action when the maneuver is not in use. The Jendrassik maneuver is known for long (Figure 63) and works on the principle of facilitation of monosynaptic reflexes.
Figure 63. The Jendrassik maneuver was first observed in the late 19th century by the physician Ernö Jendrassik. It facilitates monosynaptic reflexes.
The jumping/bumping maneuver is a completely different phenomenon. Patterns in the intrinsic apparatus of the spinal cord are specifically activated. The partly missing supraspinal control for continuous jumping with one leg is partly compensated by the induction of the stepping automatisms (Figure 52). Whereas the Jendrassik maneuver is for diagnostic, the jumping/bumping maneuver is for treatment, for repairing leg functions in hemiplegia, including a partial repair of pyramidal tract functions. The maneuver is clear. The patient puts the forefoot of the good leg on top of the forefoot of the paretic leg and tries to jump with both legs. If the maneuver works, the paretic leg can, as in Alen, jump continuously. To understand the mechanism of the of the jumping/bumping maneuver, human neurophysiology is needed. It involves the activation of the automatic stepping automatism. But normally the stepping automatism is present after birth (Figure 54, newborn stepping) and partially reappears after spinal cord (Figure 55) or pyramidal tract injury (Alen, Figure 20A).
Jumping/bumping maneuver explained on the basis of human neurophysiology
It is tried now to analyze the jumping/bumping maneuver based on human neurophysiology. Especially it is looked for temporal facilitation. Is it possible to understand this maneuver?
Based on the classification scheme for human peripheral nerve fibers (Figure 12), it is possible to identify myelinated nerve fibers by group conduction velocity and group nerve fiber diameter (Figure 13) and calculate conduction times from legs and arms to the spinal cord. When knowing processing times of the human CNS of patterns and pattern change, the jumping maneuver can be analyzed. But conduction times to the spinal cord can only be calculated if the nerve fibers do not taper or taper only little. Because in good medical text books for medical students nothing can be read about tapering or pictures seem to indicate that there is tapering of human peripheral nerve fibers [31], some details about tapering of human peripheral nerve fibers are given here. It will be measured that human peripheral myelinated nerve fibers taper only very little or not at all.
Tapering of nerve fibers
Consequently, a nerve fiber with a diameter of 10μm would reduce to approximately to 9.8μm at 1m away from the cell soma if there is no branching in between.
To identify nerve fiber groups in the cauda equina nerve roots and to improve the classification scheme of the human PNS, we need to compare the nerve fiber diameter distributions of peripheral nerves of a known nerve fiber group composition (e.g. skin, muscle or autonomic nerves) with the distribution of the cauda equina nerve roots. Such a comparison is only justified if there is no (or only little) tapering of nerve fibers, which means that the nerve fibers have the same (or nearly the same) diameter both close to and distant from the cell soma. The tapering of nerve fibers is especially of interest in human neurophysiology since afferent and efferent nerve fibers can exceed 1m in length.
The morphometric measurements indicate that there is only very little tapering of human nerve fibers. Consequently, it seems justified to compare nerve fiber distributions of peripheral nerves with those of the nerve roots. Secondly, the classification scheme of the human PNS holds close to the CNS as well as at a distant from it, and thirdly, conduction velocities (saltatory conduction) measured in the cauda equina can be compared with those measured from peripheral nerves.
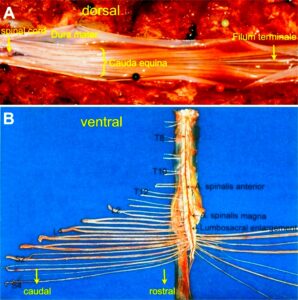
Figure 64. Schematic layout for measuring the tapering of human peripheral nerve fibers. After the opening of the spinal canal (A) and removing the lower spinal cord with the cauda equina nerve roots and the dura mater (B), rostral and caudal nerve root samples of 1 to 2cm length were removed from identified dissected and fixed nerve roots (mounting in some similarity to B).
The cauda equina nerve roots are suitable for measuring tapering because in the long caudal sacral nerve roots, there is only little or no branching which would reduce the nerve fiber diameter. Figure 64 shows where the samples for morphometry were taken from. Figure 65 shows the cross-section for morphometry and Figure 66 shows the morphometry in detail. It can be seen from Figure 66 that the diameter distributions are very similar at 3cm and 15cm distance from the spinal cord.
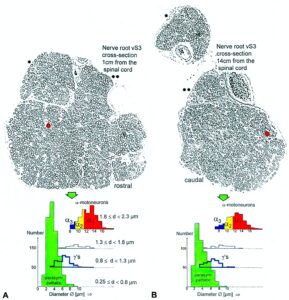
Figure 65. A. Rostral; B. Caudal. Comparison of the nerve fiber diameter distributions. After embedding, cutting, and staining (thionin acridine-orange), the cross-sections were photographed (magnification x 1000), and mean nerve fiber diameter and myelin sheath thickness were measured of the whole cross-section or parts of it. Nerve fiber diameter distribution histograms from rostral and caudal cross-sections for the 4 myelin sheath thickness ranges d and the rostral and caudal distributions were compared with respect to tapering. Probably fasciculation of the vS3 root is marked by one or two stars. An identified single fiber in the rostral and caudal cross-sections is marked by red. If only a few ventral root afferents are present in the vS3 root, then the fiber diameter distribution of the myelin sheath thickness range 0.25 ≤ d < 0.8 µm represents mainly parasympathetic fibers.
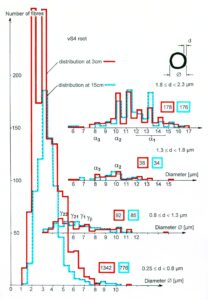
Figure 66. Nerve fiber diameter distributions for 4 myelin sheath thickness ranges d of the rostral (solid line) and the caudal (dashed line) cross-section of a vS4 root. Nerve fiber groups are partly marked at the corresponding distribution ranges or peaks. Figures in the solid and dashed line rectangles give fiber numbers from the fiber distributions of certain myelin sheath thickness ranges rostrally and caudally.
Tapering of afferents, g-motoneurons and preganglionic parasympathetic and sympathetic fibers
The rostral and caudal nerve-fiber diameter distributions of the same root (Figure 66) and other cases seem to indicate, that the g-motoneurons and the preganglionic parasympathetic fibers also taper only little or not at all. The tapering of the preganglionic sympathetic fibers leaving the spinal cord via the TH10 to L2 roots is more difficult to measure since these roots are much thicker than the lower sacral roots in which the parasympathetic fibers are contained. Since however, the preganglionic sympathetic and parasympathetic root fibers had very similar nerve-fiber diameter distributions rostral and caudal (Figure 67), it seems that also the preganglionic sympathetic fibers (contained even more caudally in the ramus albeus), taper only very little or not at all. The much thinner and unmyelinated postganglionic sympathetic fibers contained in the ramus griseus have not been measured here.
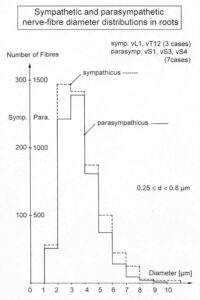
Figure 67. The sum of sympathetic (dashed line) and parasympathetic (solid line) efferent nerve-fiber diameter distributions of several roots indicated in the upper right Figure. Myelin sheath thickness between 0.25 µm and 0.8 µm. Note that the sympathetic and parasympathetic distributions are very similar. Sympathicus (symp): vL1, vL2 (3 cases); Parasympathicus (para): vS1, vS3, vS4 (7cases).
Visually it has been shown here that human peripheral nerve fibers do not or only little taper. For the actual measurements see Table 1 of [62].
For analyzing the jumping/bumping maneuver, the processing time of pattern generation or pattern change must be measured. This has been done by the Author, also based on measurements of single-nerve fiber action potentials.
Organization time for pattern change
For jumping on a springboard (Figure 68Aa) the equation of motion of the collective variable φ (angle between the legs) could be solved in the HKB model [63] and an attractor layout constructed for the jumping in in-phase and in anti-phase (Figure 68Ab). By way of analogy an attractor layout for bladder continence and protection reaction (Figure 68Bb) may be drawn. As can be seen from Figure 68Bb, the enhancement of the continence safety (deepening of the potential well) needed approximately 6ms and the change from the pattern continence to protection reaction needed approximately 100ms. The short time to improve continence pattern stability (6ms) and the much longer time for pattern change from continence to protection pattern (100ms) were obtained from latency differences of the response of premotor spinal continence oscillators following touch and pin-prick (Figure 68Ba). It can be seen from Figure 69A that in nine out of ten cases, the latency of the oscillation period shortening following touch stimulation of the perianal skin was shorter in duration than the oscillation period (»100ms), represented by the dotted line. The shortest latency was approx. 10ms, when measuring from the afferent volley running in direction of the spinal cord. The latency of the first reduction of the oscillation period, measured from the skin touch stimulation artefact, was longer or shorter than the duration of the oscillation period (Figure 69B). The shortest latency was approx. 30ms. The difference of the latency, i.e., the time from the stimulation artefact (touching of the skin) to the moment when the skin afferent volley passed the recording electrodes, was approx. 15ms.
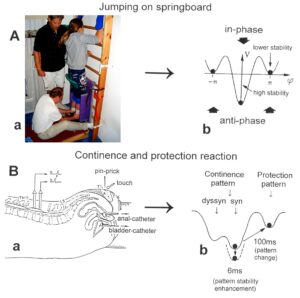
Figure 68. Attractor layout for jumping on springboard in comparison to that of continence and protection. Exercising of a 20-year-old female patient with a severe spinal cord injury at C5/6 levels on the special CDT device in the prone position. Such very coordinated turning with arms and legs is stimulating autonomic functions like bladder, intestine (peristaltic), and blood circulation functions by improving the phase and frequency coordination of the networks driving those autonomic functions.
According to the group conduction velocities of skin afferents (T1 skin afferents (PC) » 44 m/s at 36 °C) (Figure 13), most of the time was lost in the touch receptors and the thin nerve fibers connected to them. With a conduction distance of 0.3 m, a conduction time of tcond = s/v = 300 mm/ 44mm(ms)-1 = 7ms results. The latency to the reduction of the first oscillation period duration following pin-pricking the sites 1 to 10 (see Figure 69K) was mostly longer than the oscillation period itself, irrespective of whether it was measured from the skin afferent volley (Figure 69E) or from the stimulation artefact (Figure 69F). Since not every pin-prick was strong enough to generate pain (stimulation of pain receptors), it is concluded that the latency from the pin-pricking to the first reduction of the oscillation period was longer than the oscillation period. By comparing the latencies to the first reduction of the oscillation period following repetitive touch and pin-prick stimulation of sites 1 to 10 (Figure 69K), it turns out that following touch stimulation the delay is shorter than the oscillation period, whereas it is longer following pin-prick stimulation. It seems therefore that touching reinforced the sustained stretch reflex of the anal sphincter while pin-prick did not.
The first shortening of the oscillation period in comparison to two preceding periods is plotted in Figure 69D,C (touch) and in Figure 69H,G (pin-prick). The reduction of the oscillation period for touch stimulation was between 8 and 28ms and between 5 and 40ms for pin-pricking. The pin-prick stimulation generated a larger transient reduction of the oscillation period. When correlating the latency of shortening to the extent of shortening for pin-pricking (Figure 69H) it seems as if the shorter latency correlated with a smaller reduction of the oscillation period (more touch-like), and the long latencies correlated with the greater reduction of the oscillation period (more pain-like). A similar relation was not found following touch stimulation (Figure 69D). It is most likely that the painful pin-prick stimulation transiently replaced the sustained stretch reflex of the anal sphincter by a protective reaction of the anal sphincter against pain application; the time needed for the reorganization of the oscillatory firing neuronal network was at least one oscillation period long. The relation between the sites of pin-prick (Figure 69G) or touch stimulation (Figure 69C) and the oscillation period shortening shows no clear correlation. The dotted and the dashed lines may show similar latencies. Perhaps the protective reaction against pin-pricking of the anal canal and the pelvic floor is highest at sites 3 and 8 at the border of the anal reflex region (Figure 69K).
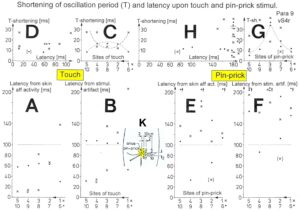
Figure 69. Shortening of the oscillation period T of the a3-motoneuron (perhaps an a2-motoneuron upon touch and pin-prick stimulation). A, B. Latency of the shortening of the oscillation period measured from the skin afferent activity (A) and from the stimulation artifact (B) with successive touching of sites 1 to 5 (x) and 6 to 10 (·) as indicated in Fig.163B. Note that the delay is often shorter than the oscillation period. D,C. Shortening of the oscillation period (T-shortening), with respect to two preceding oscillation periods, in relation to the latency of shortening (D) and in relation to successive touching of sites 1 to 10 (C). E.-H. Same description as for A-D, only with respect to pin-pricking. Note that touch stimulation induced a shorter latency for the reduction of the oscillation period than did pin-pricking.
By comparing the latencies from the moment when the skin afferent volley passed the recording electrodes to the first reduction of the oscillation period following repetitive touch and pin-prick stimulation of the sites 1 to 10, it appears that the delay following touch stimulation (≈ 10ms) is much shorter than for pin-prick stimulation (≈ 110ms). It seems therefore that touching reinforced the sustained stretch reflex of the anal sphincter while pin-prick stimulated the protection reaction against pin-prick (pain). It can be evaluated how much time is needed for the reinforcement of the sustained stretch reflex and for the pattern change from the continence state (sustained stretch reflex) to the protection reaction. With a conduction distance of 0.1 m (distance between the recording electrodes and spinal cord), a conduction time for the fastest skin afferents (group conduction velocity of T1 skin afferents (Parcinian Corpuscle) » 44 m/s at 36 °C) of tcond = s/v = 100 mm/ 44 mm(ms)-1 = 2.3ms is resulting. The conduction time of the action potentials of the motoneurons to cover the distance from the spinal cord to the recording electrodes is resulting also to approximately 2.3ms (the group conduction velocities of the α2 and α3-motoneurons are 37 and 50m/s respectively). The processing time to reinforce the continence pattern of the anal sphincter was therefore approximately 5.4ms (10ms-4.6ms = 5.4ms). If we assume a synaptic transmission time of 2.5ms, the processing time in the spinal cord is sufficient for the transmission time of two synapses. The reinforcement of the sustained stretch reflex was may be just a di-synaptic pathway.
The conduction times to and from the spinal cord of the protection reaction are approximately 10ms (7.7 + 2.3), because the fastest pain afferent action potentials needed a bit more time than the touch afferent action potentials (group conduction velocity of the fastest pain afferents = 13m/s; tcond = s/v = 100 mm/ 13 mm(ms)-1 = 7.7ms). The processing time in the CNS to change from the sustained continence pattern to the protection reaction pattern is therefore approximately 100ms. This time would be enough to involve 40 synapse transmissions. For a reorganization of the neuronal networks for pattern change, conduction times of intra-spinal axons and dendrites (electrotonic conduction, passive conduction) would also have to be considered. But it is obvious that the pain application induced a completely different CNS response.
That pin-pricking resulted in a longer response time of motoneurons than touching of the perianal skin is not obvious and cannot be explained based on the reflex theory. Being a stronger stimulus, pin pricking (touch plus pain afferent activity, Figure 70) would be expected to have the same or slightly shorter rather than a much longer response time than touching of the perianal skin (touch afferent activity only, Figure 70).
It is concluded that the change from one network state to another one, restricted to the spinal cord (measurement in a paraplegic), needed approximately 100ms.
With respect to movement learning and learning transfer, strong pain must be avoided during exercise because a certain pattern such as jumping on a springboard must be trained (and neuronal networks entrained) and not a combination of a movement pattern and a protection reaction.
These measurements and estimations show that it is possible to analyze integrative patterns of the human CNS with natural impulse patterns of single-nerve fibers recorded from sacral nerve roots. Natural impulse pattern can explain integrative functions of the human CNS.
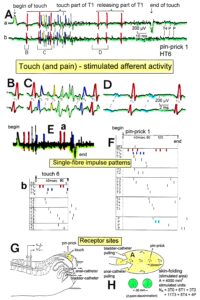
Figure 70. Touch and pain activity stimulated by pin-pricking (A) and touching (Ea) S5 or Co dermatomes and recording extracellularly from a dorsal coccygeal root (brain-dead human HT6). T1, T2, T3, T4, P = mark action potentials (APs) from single touch and pain fibers. Subscripts 1, 2, 3 mark single fibers. --- A. Whole sweep following pin-prick 1 at a slow time base. The large upward artifact on trace ‘a’ marks electronically the beginning of the pin-prick/touch. The large downward artifact on trace ‘a’ marks the end of the pin-prick. Note that 2 intervals of high activity of large APs occurred, one after the beginning of the pin-prick with 1 AP in front, and a second before the end of the pin-prick; potentials with small amplitude follow potentials of large amplitude. Time intervals B, C and D are time-expanded. --- B, C, D. Time expanded sweep pieces of ‘A’. Identified APs are indicated. Note that the APs from the T11 touch unit can be safely identified by the waveforms in B, C, D. --- Eb, F. AP occurrence patterns of single touch and pain fibers following short touch 6 and pin-prick 1. No pain afferents are stimulated upon touch 6. Upon pin-prick 1, the single-fiber AP activity of the different touch and pain groups is identified by the AP waveforms on traces ‘a’ and ‘b’, and by the conduction times. The single touch afferents of the T1 group are marked with subscripts. One active secondary muscle spindle afferent fiber (SP2) could always be identified in F. Note that for pin-prick 1, touch and pain afferents are stimulated whereas for touch 6 only touch afferents. --- G. Recording and stimulation arrangement for simultaneous recording of several single touch and pain units. A = area stimulated by skin folding, drawn in H in more detail. T11, T16 = suggested touch points of the T11 and T16-units. --- H. Drawing of the very approximate skin area stimulated by skin folding. T11-6 = suggested focal T1 touch points. Two-point discrimination indicated for the sake of comparison. NA = number of stimulated units in the dorsal coccygeal root. Skin tractions evoked by anal and bladder-catheter pulling are indicated by the large open arrows.
Analyzation of the jumping/bumping maneuver and its organization, based on human neurophysiology
After having found that there is no or only little tapering [62] and that the pattern formation time is in the range of 100ms [2] (page 342), the jumping/ bumping maneuver can be analyzed.
The stepping automatism in newborn is continuous, it is the newborn stepping (Figure 54A,B). The re-appearing stepping automatism in patients with spinal cord injury is not continuous. Pushing the heal or bumping the forefoot induces one step on the pushed side, which does not activate the stepping movement on the other side. The re-appearance of the stepping automatism following spinal cord injury shows similarity to the occurrence of the micturition automatism of the sacral micturition center following spinal cord injury. The micturition pattern is mostly pathologic and needs repair [28, 29]. And this is what is done here with the not used stepping automatism in the patient Alen. The re-appearing stepping automatism, of which the patient does not know of, is used for repair and to bring it under supraspinal control as the micturition automatism repair. But now to the temporal facilitation of the on-leg-jumping through the training of the jumping/bumping maneuver.
Figure 71 shows the brain and spinal cord. The neural networks for generating the stepping automatism are mainly located in the intumescentia lumbo-sacralis but involve nearly the whole intrinsic networks of the spinal cord, because without trunk stability a stepping/walking is difficult to perform.

Figure 71. The human CNS with the sacral and pontine micturition centers. A difficult dissection (by the Author) to have the brain and the spinal cord together. To make the length and thickness of the roots visible, the roots are turned away from the cord.
When touching or pin-pricking the perianal skin, the fastest skin afferents need approximately 15ms (Figure 70A; time from the begin of touch to the first action potential) to reach the spinal cord. But the distance to the touching/bumping area of the forefoot is approximately 1m more far away. The fastest skin afferent action potentials need approximately 25ms more time to reach the spinal cord because of the longer distance (v=s/t; t=s/V= 1m/40m/s=25ms). The conduction velocity of the fastest skin afferents is taken from Figure 13 and because the nerve fibers do not taper, this calculation is justified. The time for the bumping information to reach the spinal cord equals altogether 40ms (15ms + 25ms = 40ms). Including also more slowly conducting skin afferents (Figure 13), the time interval from bumping the forefoot to reach the spinal cord is approximately 50ms.
The organization time to build up the stepping automatism in the neural networks of the spinal cord is in the range of 100ms (Figures 68,69). Therefore, when bumping the forefoot, the jumping automatism can be organized in the neural networks in the intrinsic apparatus of the spinal cord in 150ms. But the system is more complex since the jumping pattern is organized in the neural networks of the good side which is injured a bit by the not crossing anterior corticospinal tract may. On the other hand, an enhancement of a pattern needs only 6ms (Figure 68). Anyhow, the improvement of neural network organization with the bumping/jumping maneuver in the spinal cord does not need more than 150ms. Since the jumping frequency is approximately 1Hz, the jumping/bumping maneuver acts every 1000ms. There would be sufficient time to realize the continuous jumping through bump by bump in the paretic leg. There are two possibilities, namely that the continuous jumping of the paretic is generated bump by bump or by a continuous jumping pattern organized from the other good side.
Since the pathologic neural network organization is very complex. It seems that only the nervous system itself can repair it when forced. The jumping/bumping maneuver helps the intrinsic neural networks of the spinal cord to recognize the deficiency or absence of stepping, walking, and jumping patterns to repair the paretic leg for fully functioning and healthy growing.
Figure 72A shows some connection of the intrinsic apparatus of the cord in which movement patterns are partly organized. But the jumping pattern is organized in the neural networks and not just that some action potentials are running along certain pathways. The networks for jumping of the good and paretic side cooperate with one another in exact coordination. When jumping with both legs, especially when they touch each other, the networks of both legs cooperate and coordinate. The only problem is that too much weight is on the good leg, as in stroke patients. To get more weight onto the paretic leg, the jumping-bumping maneuver is helpful.
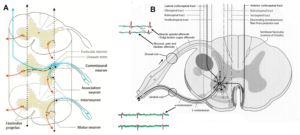
Figure 72. A. Intrinsic neurons and polysynaptic connections in the spinal cord. B. Synapses of the descending motor tracts onto anterior horn neurons. Figures partly taken from [31].
In Figure 72B, both legs get innervation from the lateral corticospinal tract (pyramidal tract) and the anterior corticospinal tract. In Alen, the good side is innervated by the not injured pyramidal tract and the injured anterior corticospinal tract. The paretic side of the spinal cord is innervated by the injured pyramidal tract and the probably not injured anterior corticospinal tract. Since the innervation from the pyramidal tract is larger than those from the anterior corticospinal tract, the not injured pyramidal tract decides what is the good and the paretic side. And this holds probably also for the neural networks.
In Figure 43 also the hand grip power of the good hand improved strongly, most likely because of a partial repair of the injured anterior corticospinal tract. Coordination dynamics therapy improves the whole body.
Why has this jumping/bumping maneuver not realized earlier at times when the Jendrassik maneuver was found. Probably the neurologists had always been on the diagnostic side and too little on therapy side. And by drug therapy and operations one cannot repair the nervous system.
Why human-based learning for repair
A healthy person can also feel the enhanced input from the periphery when performing the jumping/bumping maneuver (Figure 52). The enhanced input from hand and fingers from right and left can be felt when moving them together (Figure 38A,B). But only with an injury these maneuvers become important for repair. The progress in understanding the human nervous system is coming from measurements and treatment of patients with nervous system injuries. Like in animal research, one learns about nervous system functioning when measuring the changes following specific injuries. The newborn stepping and its induction (Figure 54A,B) is well known and used for examining the nervous system after birth.
One could think, why the Author is emphasizing movement therapy by learning. It seems that learning is the only possibility to repair the human nervous system. The Author started the nervous system repair from the existing clinical approach. Carlsson and Sundin started urinary bladder repair by a nerve anastomosis [64]. The Author followed this nervous system repair strategy when trying to improve the anastomosis from the lower intercostal nerves to cauda equina nerve roots [65-68] by analyzing in detail intercostal nerves, nerve roots and mismatch. But the disadvantage of such a nerve anastomosis is that it is a destructive operation and spinal cord injury patients should not lose any further nervous system functions. And when he read that a repair by learning is best when there is no operation with a reconnection of nerves, he started with the movement therapy. In one of the papers [68], more measurements like in Figure 70 can be found.
An important problem in the future of human repair-neurophysiology is with what movements can the genetics efficiently be used to repair the injured, malformed, or degenerating CNS including genetic diseases [30].
Epilogue
Ethics
After an initial financial support of this research project to repair the human CNS by the “Deutsche Forschungsgemeinschaft” (DFG), further support was refused because of professional and ethical doubts. Despite the support by the former German president Richard von Weizsäcker, the DFG did not change its mind and stated that the Author cannot apply any more for funding of this research project. Also, the “Swiss National fond” and the “Max Planck Institution” refused support. For the impossibility to get support for a research project to repair the human nervous system see Epilogue of [1]. Because of the impossibility to get official funding for this research project, the Author went on with the research, mainly on personally saved money for 38 years to reach the present level.
When the Author wanted to work on brain-dead humans at the University of Greifswald (at that time part of the German Democratic Republic, or Russian occupational zone (Figure 79, red)), mainly at the neurosurgery department [37], an ethical committee was founded and this committee decided that it is justified to work on brain-dead humans for repairing the human CNS. For developing the “single-nerve fiber action potential recording method”, the Author was supposed to get a professorship in Dresden (Figure 74, after being re-built). But after 10 years, with the fall of the Berlin wall, everything changed, the bad and the good things.
The ethics in research are important. But it is not only important what research on human is justified, but also what research is allowed to leave out. Here an important example. Through coordination dynamics therapy it is possible to avoid euthanasia in very severe brain injury [26] and to live longer with a better quality of life for 10 to 20 years [25]. If every hundreds person (10-2) on earth (world population = 8x1012) would have the mental discipline to train hard and live longer for 10 years (10-1), then every year a few million lives could be saved when assuming a life time of 100 years (8×1012×10-2×10-1= 8 million). That means, qualified research on human has tremendous consequences and is not a theoretical game.
Even though it happened that a decision for brain-dead was wrong in England, because before removing the organs for transplantation the patient awoke up from death, decisions of brain-dead are save. The problem occurs before. In light brain injury, the brain repairs itself spontaneously. In severe and very severe brain injury, the brain needs efficient treatment to become repaired as much as possible. But in extreme severe brain injury, the coma patient drifts in direction of brain-death without optimal therapy. Since the universities are 30 years out-of-date with respect to human repair-neurophysiology, the universities have not the knowledge to treat such patients efficiently and no money can be earned with movement-based brain repair. Letting the coma patient drift into brain death, organs can be removed for transplantation and money can be earned [26].
When the Author was animal physiologist, he published in basic journals [69-71]. But when he changed to human repair-neurophysiology, the Nature Journal refused two times to publish the new human recording technique, the “single-nerve fiber action potential recording method”, by desk decisions. The Author got the manuscripts back without reviewing and any statement as if humans are not a part of nature.
At an international conference in physiology, the Author had a poster in the muscle spindle group [72] of 20 to 30 participants. When the Author tried to relate his human muscle spindle data with those of animal muscle spindle data with respect to the vegetative innervation of spindles (translational medicine), the animal physiologists refused a correlation and a friend told him afterwards that he behaved wrongly. But an editor of a journal, visiting the conference, was interested to inform the readers of his journal about this research project [73-75].
There are several reasons that universities are not interested in this research project. One reason is that the discipline electrophysiology has been given up because in animal research very much has been done alrady by electrophysiology [76] and the step to integrative neurophysiology and human neurophysiology has not been tried. The study physiology, for example, at University College London does not include human neurophysiology and the discipline neurology includes mainly only diagnostic. With drugs and operations, the brain cannot be repaired. The neurologists of older times (Desmedt, Hagbarth, Vallbo, Torebjörk, Johannson, Rinne, Falck and others), who were fascinated if some basic research could be done on humans, are not working any more. When the Author criticized the low level of neurologic research at an official meeting, where many professors were present at Zürich University, the professors were yelling so that one could not hear the professional arguments of the Author and then he was put one a black list, so that he could not get an official job in Switzerland anymore. A well-known rehabilitation clinic in Switzerland even did put pressure on the editor of the Journal Electromyography and Neurophysiology not to publish from the Author anymore. Prof. Roselle got angry and send the Author a copy of the letter. A neurologist put pressure on the organizer of a conference in North-Italy, not to let the Author participate. The organizer got angry and told the Author about. At a recent conference about brain repair in children, an official female member of the committee got interested in this research project and was punished for it.
If expert knowledge/education is not of interest anymore, the spiritual surrounding for repair is missing and the patients are smartphone addicted, then the theoretical and clinical progress in CNS repair through movement-based learning has no chance.
War children (Kriegskinder)
Nightmares can partly be cured through CDT treatment of scoliosis and in cerebral palsy.
However, the CNS of children cannot only suffer damage from a traumatic brain injury, infection, or malformation, but also from the fallout of conflict zones, especially in the absence of parents. They can suffer nightmares which may last for the rest of their lives. Figure 73 shows Butscha in Ukraine and Figure 74 shows the ruins of Dresden following the bombing in 1945. Such situation may induce nightmares in those ones who survived.

Figure 73. Road in Butscha, Ukraine, 2022.

Figure 74. The Bombing of Dresden took place in the final months of the Second World War, when Hitler-Germany had lost already the war. In four raids between 13 and 15 February 1945 the bombing and the resulting firestorm destroyed over 6.5 km2 of the city. An estimated 25,000 people were killed. There was no army in Dresden. A pilot of the United States Air Force refused to bomb Heidelberg, because he had studied in Heidelberg. Dresden was a cultural landmark and is sometimes referred to as “Florence on the Elbe”. Culture is only protected by those ones who have culture.
During the detriment of a country also the culture is lost. The Authors father, an artist, made paintings of Jesus Christ for a church in Leipzig (Figure 75), which was bombed by USA like Dresden. Like in Dresden also churches and their paintings were destroyed.

Figure 75. Paintings of Edmund Schalow, burned in a church in Leipzig at the end of World War II. Sorry for the poor quality.
When the Stalin and the communists took Berlin, a Russian soldier damaged a picture of the Authors father, which could be restored (Figure 76).

Figure 76. A painting of Edmund Schalow (Authors father). An American general saw a picture in a museum in Austria and took a picture/postcard of it to my father in Berlin after the war for getting a real painting of it. Probably by concentrating on music, he wanted to get mentally rid of the detriment they have done in Germany. The general did not take the picture. At those times, artists mostly painted what customers asked for, to urn money for the family.
The Author experienced the ruined Germany when he walked through Berlin like the child in Figure 77. The streets were cleaned by women (Trümmerfrauen). The bricks in the ruins seem to have been turned several times by bombing.

Figure 77. Ruins in Berlin after World War II. (Scharoun was a famous German architect).
To imagine the criminality done in the war in Ukraine, a comparison is needed to other wars, as the first and second world war. This is not especially the duty of medical research, but first medicine is always involved in wars, second the public in many countries is avoiding such comparisons, third as Kant argues “if justice perishes, it is no longer worth living on earth” (Figure 78), fourth the Author’s families, from the father’s side (Stettin) and mothers’ side (Regentin), were displaced persons (Figure 79), of which nobody wants to speak about and fifth the Author is a contemporary witness.

Figure 78. Nobody wants to speak about the German refugees. "Those who know the truth and fail to call it by its name become the truth's main enemy." (Julius Rupp, Wer die Wahrheit kennt und nicht benennt, ist der größte Feind der Wahrheit).

Figure 79. Top. Parcellation of Germany according to the Potsdam conference. Germany was divided into 10 parts. Below. Real refugees from east Prussia, partly photographed by Russian pilots.
In Figure 80 some data of World War II are summarized with respect to Germany. There were 15 million German refugees, 3.5 million were killed on their way. The most well-known case was the sinking of the “Gustloff” (Figure 81). 300 000 women were raped, abased, or killed. Seven million Germans starved on hunger or disease in between 1946-1947. Eisenhower forced German soldiers to dig diches in wet ground (Rheinwiesen) to stay in and stopped the red cross to bring them food. One million German soldiers died in the hands of the USA after the war according to the statement of three persons of Figure 80.
Expulsion/refugees took place not only in the former east Germany (east Prussia, West Prussia, Silesia, east Pomerania (where the Authors father family is from), but also in middle Germany, as for example, around Dresden (Figure 74). Eisenhower, the head of US air force, gave the order for bombing. When refugees were running over the bridges of the river Elbe to escape, they were attacked by USA fighter pilots. In the war in Ukraine, it is distinguished whether military objects are attacked or refugees. Eisenhower’s ancestors had to leave Baden-Württemberg (Germany) in 1792. It could well be that Eisenhower had other motivations for bombing than to win the war.
My mother and we three children were refugees in the middle of Germany to escape from the bombing and were attacked by Russian soldiers at “Schönwalde” close to Berlin. I will not forget when Russian soldiers were shooting at us.
It is not only important to know about the killing of refugees by the winner of World War II, but to what extent USA supported Stalin and the communism with war material. The Nazis did not think that USA would support Stalin and the communism with lorries and tanks. In the Ukraine war it is demonstrated how important the delivery of tanks, munition and other war material is. If it would be known how much war material the USA gave to the communism under Stalin to beat Hitler-Germany, then one could estimate how much war material the Ukraine needs to survive against Russia.
It is therefore important that the USA is lay open all documents of World War II, what had not happened till now. One cannot understand why the Australian Julian Assange is treated that hardly. Is the USA afraid that a whistleblower may open the important documents of World War II?

Figure 80. Some data about the end of World War II. From “Junge Freiheit”, 6.5.2022, Page 9.
Many refugees, especially from east Prussia, their children and grandchildren are still suffering on the loss of their home country and culture. On the graves they sometimes have the former home country (Figure 82). When the Author drives with the car through the former East Germany (Figures 78 and 79), he suffers by seeing the familiar old houses, small streets surrounded by tries, the old rails of the train, the lakes, and hills, what also parents and grandparents talked about, when sitting in the evenings at the warm oven (Kachelofen).

Figure 81. The sinking of the Titanic in comparison with the sinking of the Gustloff. Mainly women, children and injured soldiers lost their lives in the cold water with the sinking of the Gustloff. In the media it is reported that most civilians died when the Titanic thank, what is wrong.

Figure 82. A grave of refugees from east Prussia in Berlin (Moltkefriedhof). These refugees wanted to keep their home country on the way to a better world.
Since the Author has been a theoretical nuclear physicist [77], he must state the following. The physicists Heisenberg (see also collected works of Heisenberg, especially section C, volume 5, page 164), Einstein and Bohr had the agreement not to tell politicians about the possibilities of nuclear forces with respect to build an atomic bomb. But Einstein convinced Roosevelt in a letter to build the (first) atomic bomb. Following Hiroshima and Nagasaki, he felt sorry for that. The German Heisenberg did not build an atomic bomb for Hitler. After the war, Adenauer did not use the knowledge of Heisenberg to build nuclear power plants and Heisenberg (the father of Quantum Mechanics) was disappointed because his expert knowledge was not used. Who knows how the development of nuclear power plants would have gone with his knowledge? Not only the Author made the experience that expert knowledge (for example human repair-neurophysiology) is not of interest anymore.
In Sweden there was some doubts in the quality of Einstein. Heisenberg argued that his team would have also reached the formula E=h●ν. The Author’s opinion was that to bring physics into an elegant form, there is also a lot of knowledge necessary. But a mathematician of Zürich university argued that Einstein’s wife was a mathematician. It may well be that she developed that elegant formulae but did not get the reputation for it.
References
- Schalow G (2013) Human Neurophysiology: Development and Repair of the Human Central Nervous System. Nova Science Publishers, Inc, Hauppauge NY, USA, 734.
- Schalow G (2015) Repair of the Human Brain and Spinal Cord. Nova Science Publishers, Inc, Hauppauge NY, USA, 525.
- Schalow G (2015) Neural network learning in human. Nova Science Publishers, Inc, Hauppauge NY, USA, 324.
- Schalow G (2002) Stroke recovery induced by coordination dynamic therapy and quantified by the coordination dynamic recording method. Electromyogr. Clin. Neurophysiol. 42:85-104.
- Schalow G (2002) Improvement after traumatic brain injury achieved by coordination dynamic therapy. Electromyogr. Clin. Neurophysiol. 42:195-203.
- Schalow G and Jaigma P (2006): Improvement in severe traumatic brain injury induced by coordination dynamics therapy in comparison to physiologic CNS development. Electromyogr. Clin. Neurophysiol. 46:195-209.
- Schalow G (2019) Regeneration of the human spinal cord via coordination dynamics therapy. Peertechz Publications, 97. eBook.
- Schalow G (2009) Partial cure achieved in a patient with near-complete cervical spinal cord injury (95% injury) after 3 years of coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 49:199-221.
- Schalow G (2002) Recovery from spinal cord injury achieved by 3 months of coordination dynamic therapy. Electromyogr. Clin. Neurophysiol. 42:367-376.
- Schalow G (2003) Partial cure of spinal cord injury achieved by 6 to 13 months of coordination dynamic therapy. Electromyogr. Clin. Neurophysiol. 43:281-292.
- Schalow G, Jaigma P, Belle VK (2009) Near-total functional recovery achieved in partial spinal cord injury (50% injury) after 3 years of coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 49:67-91.
- Schalow G (2010) Cure of urinary bladder functions in severe (95%) motoric complete cervical spinal cord injury in human. Electromyogr. Clin. Neurophysiol. 50:155-179.
- Schalow G (2021) CNS Repair in a Girl with a Spinal Cord Injury. Adv. Pub. Health Com. Trop. Med. 121:201-226.
- Schalow G (2006) Cerebellar injury improvement achieved by coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 46:433-439.
- Schalow G (2021) Cure-like brain-repair in a girl with atrophied cerebellum and pons through Coordination Dynamics Therapy. Adv Pub Health Com Trop Med 123:1-47.
- Schalow G, Jaigma P (2005) Cerebral palsy improvement achieved by coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 45:433-445.
- Schalow G (2006) Hypoxic brain injury improvement induced by coordination dynamics therapy in comparison to CNS development. Electromyogr. Clin. Neurophysiol. 46:171-183.
- Schalow G, Pääsuke M, Ereline J and Gapeyeva H (2004) Improvement in Parkinson’s disease patients achieved by coordination dynamics therapy. Electromyogr. Clin. Neurophysiol. 44:67-73.
- Schalow G and Nyffeler T (2001) Koordinationsdynamik-Therapie: Myelomeningozele (Spina bifida). Physiotherapie.
- Schalow G, Nyffeler T (2000) Koordinatiosdynamik-Therapie: Skoliose. Physiotherapy.
- Schalow G (2019) Permanent coma patient re-learned to speak via Coordination Dynamics Therapy. Arch. Clin. Med. Case Rep. 3:33-50.
- Schalow G (2017) Breast cancer growth inhibition via Coordination Dynamics Therapy. In: “Horizons in Cancer Research. Volume 68”. Editor: Hiroto S. Watanabe. Nova Science Publishers, Inc, Hauppauge NY, USA. 125-151.
- Schalow G (2020) Anaplastic oligodendroglioma WHO III brain cancer-patient recovered following operation, radiation and chemotherapy through Coordination Dynamics Therapy, which is also a Covid-19 treatment without ventilator. Int. J. Med. Clin. Imaging 5:165-210.
- Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, et al. (2014) Muscle dysfunction in cancer patients. Ann. Oncol. 25:947-958.
- Schalow G (2020) To live longer with a better quality of life through coordination dynamics therapy especially in patients with severe brain injury and brain-cancer. Int. J. Med. Clin. Imaging 5:118-155.
- Schalow G (2021) Euthanasia in organ donation can be avoided through Coordination dynamics therapy. Adv Pub Health, Com Trop Med: APCTM-132. 3:1-34.
- Schalow G (2022) Spinal muscular atrophy repair through Coordination dynamics therapy and Translation of frog neuromuscular innervation pattern changes caused by neurotrophins to human. Med.: AOASM-160 2022:1-81.
- Schalow G (2022) Continence Repair Through Coordination Dynamics Therapy. Adv Pub Health Com Trop Med: APCTM-163.
- Schalow G (2023) Continence Repair through Neural Network Learning. Int J Med Clin Imaging, 8:433-476.
- Schalow G (2023) Genetic disease repair (5p-) through Coordination Dynamics Therapy I. Int J Med Clin Imaging 9:477-611.
- Baehr M and Frotscher M (2005) Duus‘Topical Diagnosis in Neurology. Thieme Verlag, Stuttgart. (a very good book for medical neuroscience since it correlates structure and function in human nervous system injuries; only human neurophysiology is missing).
- Kahle W (1984) Nervensystem und Sinnesorgane. Thieme Verlag, Stuttgart.
- Schmidt RF und Thews G (1976) Physiologie des Menschen. Springer-Verlag, Berlin.
- Daeschler, E.B, Shubin, N.H. and Jenkins, F.A. (2006). A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature 440:757-763.
- Shubin, Neil (2008). Your inner fish: A journey into the 3.5-billion-year history of the human body. University of Chicago Press, New York.
- Shubin, N.H., Daeschler E.B. and Jenkins, F.H. (2014). Pelvic girdle and fin of Tiktaalik roseae. Proceedings of the National Academy of Sciences 111:893-899.
- Schalow G, Lang G (1987) Recording of Single Unit Potentials in Human Spinal Nerve Roots: a New Diagnostic Tool. Acta Neurochir. 86:25-29.
- Schalow G (2009) The classification and identification of human somatic and parasympathetic nerve fibres including urinary bladder afferents is preserved following spinal cord injury. Electromyogr. Neurophysiol. 49:263-286.
- Schalow G (2020) Classification and Identification of Human Peripheral Nerve Fibers by Conduction Velocity, Nerve Fiber Diameter and Natural Firing Patterns with Consequences for CNS Repair and Covid-19 Infection Treatment. Int. J. Med. Clin. Imaging 5:231-314.
- Schalow G (2005) Phase and frequency coordination between neuron firing as an integrative mechanism of human CNS self-organization. Electromyogr. Clin. Neurophysiol., 45:369-383.
- Schalow G (2021) Phase and frequency coordination improvement among neuron firing for improved CNS self-organization and neural repair in Parkinson and spinal cord injury. Int J Med Clin Imaging 6:350-425.
- Schalow G (1993) Spinal oscillators in man under normal and pathologic conditions. Clin. Neurophysiol. 33:409-426.
- Schalow G (2006) Surface EMG- and coordination dynamics measurements-assisted cerebellar diagnosis in a patient with cerebellar injury. Electromyogr. Clin. Neurophysiol. 46:371-384.
- Schalow G (2005) Tremor in Parkinson’s disease patients can be induced by uncontrolled activation and uninhibited synchronization of α2-motoneuron firing to which α1-motoneuron firing synchronizes. Electromyogr. Clin. Neurophysiol. 45:393-406.
- Schalow G (2021) Phase and frequency coordination improvement among neuron firing for improved CNS self-organization and neural repair in Parkinson and spinal cord injury. Int J Med Clin Imaging 6:350-425.
- Schalow G (2010) Scientific basis for learning transfer from movements to urinary bladder functions for bladder repair in patients with spinal cord injury. Electromyogr. Clin. Neurophysiol. 50:339-395.
- Kelso JAS (1995) Dynamic Patterns. The Self-Organization of Brain and Behavior. MIT Press, Cambridge.
- Schöner G, Zanone PG and Kelso JAS (1992) Learning as change of coordination dynamics: Theory and experiment. Journal of Motor Behavior 24:29-48.
- Zanone PG and Kelso JAS (1992) Evolution of behavioral attractors with learning: Nonequilibrium phase transition. Journal of Experimental Psychology: Human perception and Performance, 18:403-421.
- Popper KR (1972). Objective knowledge: An evolutionary approach. London: O.U.P.
- Touwen B (1987) Variability and stereotypy in normal and deviant development. In: Care of handicapped child. Apley, J. (Ed.). Clinics in Developmental Medicine No. 67. Philadelphia. 99-110.
- Thelen E (1987) The role of motor development in developmental psychology: a view of the past and an agenda for the future. In: Eisenberg, N. (ed.): Contemporary Topics in Developmental Psychology. New York, Wiley, 3-33.
- Deisseroth K, Singla S, Toda H et al (2004) Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42:535-552.
- Schalow G (2009) Building of New Motoneurons in the Human Spinal Cord upon Coordination Dynamics Therapy to Improve Finger Functions in Motoric Complete Cervical Spinal Cord Injury. In: Berkovsky, T.C. (Ed.), Handbook of Spinal Cord Injuries, Chapter 4. 231-264, Nova Science Publishers.
- Schalow, G (2005) Tremor in Parkinson’s disease patients can be induced by uncontrolled activation and uninhibited synchronization of α2-motoneuron firing to which α1-motoneuron firing synchronizes. Electromyogr. Clin. Neurophysiol., 45:393-406.
- Schalow, G., Pääsuke, M., Jaigma, P. Integrative re-organization mechanism for reducing tremor in Parkinson’s disease patients. Electromyogr. Clin. Neurophysiol, 45:407-415, 2005.
- Sperry RW (1945) The problem of central nervous reorganization after nerve regeneration and muscle transposition. Rev. Biol., 20:311-369.
- Sperry RW (1947) Effect of crossing nerves to antagonistic limb muscles in the monkey. Neurol. Psychiat. Chicago, 58:452-473.
- Tsukahara, N. (1978) Synaptic plasticity in the red nucleus. In: Cotman, C.W. (ed.), Neuronal plasticity. Raven Press, New York. 113-130.
- Weiss P and Brown PF (1941) Electromyographic study on recoordination of leg movements in poliomyolitis patients with transposed tendons. Proceedings of the Society for Exper. Biology and Medicine, 48:384-387.
- Thelen E and Smith LB (1994) A dynamic approach to the development of cognition and action. MIT Press, Cambridge.
- Schalow G (2005) Tapering of human nerve fibres. Gen. Physiol. Biophys. 24:427-448.
- Haken H, Kelso JA & Bunz H (1985) A theoretical model of phase transitions in human hand movements. Biological Cybernetics 39:139-156.
- Carlsson CA and Sundin T (1980) Reconstruction of afferent and efferent nervous pathways to the urinary bladder in two paraplegic patients. Spine 5:37-41.
- Schalow G, Aho A and Lang G (1992) Microanatomy and number of nerve fibers of the lower intercostal nerves with respect to a nerve anastomosis. Donor nerve analysis. I(IV). Electromyogr Clin Neurophysiol 32:171-185.
- Schalow G (1992) Number of fibres and fibre distributions of nerves innervating the urinary bladder in humans. Acceptor nerve analysis. II (IV). Electromyogr Clin Neurophysiol 32:187-196.
- Schalow G and Barth H (1992) Single fibre action potential recording from the nerve to the musculus obliquus externus abdomonis following pin-prick in humans. III (IV). Electromyogr Clin Neurophysiol 32:197-205.
- Schalow G (1992) Impulse pattern, innervation density and two point discrimination of skin and mucosal afferents in humans. Consideration for a sensory innervation of urinary bladder and anal canal in spinal cord lesions. IV (IV). Electromyogr Clin Neurophysiol 32: 259-285.
- Schalow G and Schmidt H (1975) Action potentials induced in slow muscle fibres by partial denervation. Nature (London) 253:122-123.
- Miledi R, Parker I and Schalow G (1977) Calcium transients in frog slow muscle fibres, in frog skeletal muscle fibres using arsenazo III. Nature 268 No. 5622:750-752.
- Miledi R, Parker I and Schalow G (1981) Calcium transients in normal and denervated slow muscle fibres of the frog. J. Physiol. 318:191-206.
- Schalow G & Zäch GA (1993) Control of oscillatory firing sacral sphincteric motoneurons (FR) by secondary muscle spindle afferents in humans. Poster 322.29/P at the XXXIInd International Congress of Physiological Sciences in Glasgow.
- Schalow G, Zäch GA and Warzock R (1995) Classification of human peripheral nerve fibre groups by conduction velocity and nerve fibre diameter is preserved following spinal cord lesion. J. Auton. Nerv. Syst. 52:125-150.
- Schalow G, Bersch U, Göcking K and Zäch GA (1995) Detrusor-sphincteric dyssynergia in paraplegia compared with the synergia in a brain-dead human by using the single-fibre action potential recording method. J. Auton. Nerv. Syst. 52:151-180.
- Schalow G, Bersch U, Michel D and Koch HG (1995) Detrusor-sphincteric dyssynergia in humans with spinal cord lesions may be caused by a loss of stable phase relations between and within oscillatory firing neuronal networks of the sacral micturition centre. J. Auton. Nerv. Syst. 52:181-202.
- Kandel ER, Schwartz JH and Jessell TM (1991) Principles of neural sciences. Prentice-Hall International Inc.
- Schalow G and Yamamura M (1971) Investigation of ground state correlation in an extended Lipkin-Meshkov-Glick-Modell. Nuclear Physics A161:93-104.
Contents
Movement-based learning strategies to repair the human CNS.
Basal ganglia and cortex injury due to hypoxia.
Functional anatomy of the cerebral cortex and basal ganglia for understanding the repair
Repair of phase and frequency coordination through exercising on special CDT devices.
Measuring CNS functioning by the arrhythmicity of exercising (coordination dynamics value)
Unique properties of special CDT devices.
Motor learning and problem-solving therapy.
Interpersonal coordination and co-movement
Repair strategies at the neuron membrane and genetic levels.
CNS functioning at the beginning of coordination dynamics therapy.
Brain repair through 6 weeks of Coordination Dynamics Therapy.
Training of other arm, leg and trunk movements to improve symmetrical grows of the body.
Neuron as a coincidence detector with respect to coordinated afferent input
CNS repair through 3 months of CDT.
Improvement of hand and finger functions on the paretic (left) side.
Training of left leg functions
Further improvement of hand functions
Pattern variability and forefoot training by running backwards.
CNS repair through 4 months of CDT.
Correction of leg length discrepancy.
CNS repair through 6 months of CDT.
CNS repair through 2 years of CDT.
Improvement of the paretic leg.
Improvement of the coordination dynamics support the motor improvements on the paretic side.
General state of the treatment and the educational systems.
Comparison of basal ganglia repair with cerebellum repair
Comparison of basal ganglia repair with spinal cord and cerebellum injury repair
Basal ganglia and cerebellum repair with respect to the ontogenetic landscape for locomotion.
Jendrassik maneuver, co-movement, jumping maneuver and interpersonal co-ordination.
Jumping/bumping maneuver explained on the basis of human neurophysiology.
Tapering of afferents, g-motoneurons and preganglionic parasympathetic and sympathetic fibers.
Organization time for pattern change.
Analyzation of the jumping/bumping maneuver and its organization, based on human neurophysiology