Publication Information
ISSN 2692-1529
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Assessment of Plastic Degrading Genes from Sediments of River Brahmaputra, India through metagenomics
Niti Sharma1, Basanta Kumar Das2, Birendra Kumar Bhattacharjya1, Aparna Chaudhari3, Bijay Kumar Behera4 and A. Pavan-Kumar3
1ICAR-Central Inland Fisheries Research Institute, Regional Centre, Guwahati, Assam, India
2ICAR-Central Inland Fisheries Research Institute, Barrackpore, Kolkata, India
3ICAR-Central Institute of Fisheries Education, Mumbai, Maharashtra, India
4College of Fisheries, Rani Laxmi bai, Central Agricultural University, Jhansi, India
Received Date: April 04, 2023; Accepted Date: April 25, 2023; Published Date: May 07, 2024;
*Corresponding author: Niti Sharma, ICAR- Central Inland Fisheries Research Institute, Regional Centre, Guwahati, Assam, India; Email: sharma.niti352@gmail.com; Niti.Sharma@icar.gov.in
Basanta Kumar Das, ICAR-Central Inland Fisheries Research Institute, Barrackpore, Kolkata,–700120, West Bengal, India; Email: basantakumard@gmail.com; Basanta.das@icar.gov.in
Citation: Sharma N, Das BK, Bhattacharjya BK, Chaudhari A, Behera BK, Kumar AP (2024) Assessment of Plastic Degrading Genes from Sediments of River Brahmaputra, India through metagenomics. Jr Aqua Mar Bio Eco: JAMBE-131
DOI: 10.37722/JAMBE.2024202
Abstract
Extensive and uncontrolled use of plastics have caused accumulation of considerable plastic waste in aquatic environments, which is adding a new dimension to environmental pollution, since synthetic plastics, which are very difficult to degrade. Degradation of plastics by microbial species has gained attention as a potential eco-friendly countermeasures due to their special metabolic capabilities. In the present study we employed shotgun metagenomic sequencing for comprehensive profiling of plastic degrading genes identified in sediments of Brahmaputra River, India. Forty numbers of unique elements of plastic degrading genes were observed in the collected sediment samples. The results showed the presence of potential genes that is associated with biodegradation of different types of plastics such as polyethylene terephthalate (PET), polyethylene (PE), polyvinyl alcohol (PVA) and polystyrene (PS). Among the microbes, Pseudomonas pseudoalcaligenes bacteria dominated at all sampling sites. Further mapping predicted enrichment of plastic degrading enzymes such as polyesterase, esterase, depolymerase and dehydrogenase. The plastic degrading enzymes suggested variations between the sampling sites indicating impact of anthropogenic activities in the stretches of River Brahmaputra. The study provided baseline information for future considerations for detailed characterization of novel genes/enzymes and discovery of metabolic pathways in degrading plastic waste, which can help in cleaning of freshwater ecosystems.
Keywords: River Brahmaputra, metagenomics, sediments, plastic degrading genes, microbial community
Introduction
Plastic are inexpensive, highly durable and lightweight synthetic or semi-synthetic polymers that are used to create various products of numerous societal and economic benefits (Andrade et al. 2016; Purohit et al. 2020; Napper and Thompson 2023). It is one of the greatest finding in the millennium, which is now omnipresent part of our environment covering households, packaging, health care, construction, etc. Plastic are mainly non-biodegradable, petroleum derived materials with unique polymeric structure that provides low specific weight, low thermal and electrical conductivity, excellent mechanical properties and high durability (fishing line last for 600 years, plastic bottles 450 years and plastic bags 20 years), etc. (Eagle et al. 2016; Mazhandu et al. 2020). Plastic are of many variants and most common plastics are the high molecular weight polymers such as polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), polyvinylchloride (PVC), polyamine (PA), polystyrene (PS), and polyurethane (PU) contributing 80% of the annual plastic generation (PlasticsEurope 2019; Purohit et al. 2020).
The predominant plastic are the single use plastic having short life-cycle which is less than a month, leading to increase in accumulation in the environment (Panda et al. 2010). The biodegradation of plastic is extremely slow ranging from 100 to 1000 years, often burns in the open air leading to release of CO2 and poisonous chemical polluting the air (Welden 2020; Purohit et al. 2020; Pilapitiya and Ratnayake 2024). The annual global plastic production in 2016 was estimated around 322 million tonnes, increasing by 10% each year with half of all plastic produced are designed for single-use purpose (Crew et al. 2020; UNEP 2024). Popularity of plastic increased drastically with changing lifestyle and increasing population. Thus, massive plastic production covers the major share in global industry, due to low cost and multiple applications (Emmerik and Schwarz 2019; Sofi et al. 2020). Consequently, these materials generates about 350 to 400 million tonnes of plastic waste annually on global scale and 19-20 million tonnes of the plastic waste leaks into the aquatic ecosystem polluting rivers, lakes and seas (Ritchie and Roser 2020; UNEP 2024).
Plastic waste is ubiquitous present in air, soil and water. Plastic waste ranging from large debris to microplastics, continues to accumulate in aquatic environments, posing a severe threat to the ecosystems. The amount of plastic waste entering the aquatic ecosystems could nearly triple from 9-14 million tonnes per year in 2016 to a projection of nearly triple by 2040. The freshwater lakes and rivers are often the first receiver of plastic waste from urban and industrial pollution (Alimi et al. 2018; UNEP, 2023). It is estimated that 1000 rivers are accountable for nearly 80% of global annual riverine plastic emissions into the ocean with small urban rivers amongst the most polluting (Meijer et al. 2021; UNEP, 2024). Several studies showed that plastic pollution in river water and sediments have numerous consequences for freshwater biodiversity, environments and ecosystem services (Ballent et al. 2016; Fischer et al. 2016; Ebere et al. 2019; Gonçalves et al. 2020; Azevedo-Santos et al. 2021). Variety of plastic from macro- to microplastic are unknowingly consumed by fishes, aquatic animals, birds, turtles, etc causing blockage of intestine resulting in death due to starvation. They may also act as vectors in transferring plastic materials across food web. Another concerns are entangling of animals in plastic residues, fishing nets, effects on aquatic algae and plants, production of toxic chemicals and gases, causing several ecological damages (Reddy, 2018; Ryan 2018; Andrade et al. 2019; Blettler and Wantzen 2019; Parker 2019; Wu et al. 2019; Urbanski et al. 2020).
Several methods have been adopted for plastic waste management such as recycling, incineration and biodegradation. The focus of technological innovation in the management of plastic waste is currently on economical and environmentally friendly techniques. Consequently, with the use of biodegradable plastic, there has been a surge in the use of microbial resources for biodegradation of synthetic plastic wastes (Kumar et al. 2021). Thus, researchers have been actively exploring and identifying potential microbes having the ability to degrade the plastics and hunt the novel genes and enzymes from the microbes for the purpose. But due to difficulty in isolation and culturing of the microbes, very few microbial agents have been characterized for plastic degradation (Purohit et al. 2020; Shilpa et al. 2022). Metagenomics approach serves as a great tool by mining non-cultivable microbial communities from environment samples by exploring the potential plastic degrading microbes, novel genes and enzymes involved in the pathways (Ranjan et al. 2021; Hussein 2021). Therefore, the present study focused on exploring plastic degrading genes from Brahmaputra River sediments, one of the largest trans-boundary river of international importance, using metagenomics approach.
Materials and methods
Study area and sample collection
Sediment samples were collected from eight locations along the Brahmaputra River in Assam, India, covering the major landing centres of its upper, middle and lower stretches of the River during March-April 2022. The sampling locations were Sadiya (Code: S1; 27⁰ 49.14″ N 95⁰40.52″ E), Dibrugarh (Code:S2; 27⁰29.9″ N 94⁰ 54.13″ E), Tezpur (Code: S3; 26⁰36.58″ N 92⁰47.27″ E), Morigaon (Code: S4; 26°17.20″ N 92°06.40″ E), Guwahati (Code: S5; 26°11.43″ N 91°45.20″ E), Dhubri (Code: S6; 26⁰1.20″ N 89⁰59.41″ E), Tinsukia (Code: S7; 27⁰57.80″ N 95⁰32.54″ E) and Palasbari (Code: S8; 26⁰12.68″ N 91⁰54.11″ E). Sediment samples of 100 g (approx.) were collected from a depth of 15-20 cm at five locations within each sampling site. These samples were then combined to create a 500 g composite sample, increasing the probability of capturing microbial diversity. During transportation, the samples were stored in a sterile container in an icebox and preserved at -80°C for subsequent laboratory examination.
DNA extraction and high throughput sequencing
Total DNA was isolated using the Power soil DNA isolation kit TM (Qiagen, Germany) following the manufacturer’s instructions. The purity and quality of the extracted DNA were assessed by QubitTM (Thermofisher, USA) Fluorometer. DNA libraries were prepared using Truseq Nano library preparation kitTM (Illumina, USA). Subsequently, all libraries were quantified using a Qubit fluorometer (Thermofisher, USA) and a DNA HS assay kit (Thermofisher, USA). The insert size of the library was determined using high-sensitivity D1000 screentapes (Agilent) on Tapestation 4150 (Agilent). The libraries were index-coded and clustered using a cBot Cluster Generation System. Following cluster generation, paired-end reads were generated by sequencing the librarieson an Illumina NovaSeq 6000 platform.
Quality control, de novo assembly of reads and taxonomic assignment
Sequence reads were pre-processed to remove adaptors, poor-quality bases (QV<20 Phred Score) and short reads using the fastP tool (version 0.23.2). Therefore, high-quality reads were assembled de novo using Megahit version 1.1.3 to generate assembled metagenomes for downstream analysis. The resulting assembled contigs were subjected to taxonomic assignment using the Kraken2 tool (version 2.1.1) to identify operational taxonomic units. Kraken2 assigns taxonomic labels to contigs based on their k-mer content, comparing them to reference genome sequences. The Kraken genomic library, which includes the SILVA database, was used for microbial classification with a k-mer length of 35 and a minimizer length of 31. The Kraken-mpa-report tools were used to estimate microbial abundance across all taxonomic ranks for the classified reads.
Functional annotation
The bioinformatics database KEGG (Kyoto Encyclopedia of Genes and Genomes) was used to annotate and interpret functional genes and metabolic pathways in metagenomic data using best hit with a known reference sequence (Kanehisa et al. 2017). With over 13,000 nodes, KEGG classification is represented as rooted tree, the leaves of which stand for several routes. The analysis has been carried out using Megan 6 (Huson et al., 2007).
Detection of plastic degrading genes (PDGs) from the metagenome
PlasticDB database was used to predict the plastic degrading enzymes (Gambarini et al. 2022). PlasticDB contains information on proteins and microorganism linked to plastic biodegradation, including data reported in scientific literatures. The metagenome assembled sequences were aligned using BLAST 2.10.1 against the plastic degrading enzyme database with a percent identity cutoff of 50. The hits with percentage identity ≥ 50% identity itself were designated as the plastic degrading enzyme.
Heatmap for PDGs was generated using average clustering method in R. It displays correlationsin both row and column clustes, with colors representing the correlation distances. The heatmap visualization allows for identification of patterns and relationships among PDGs. It represents presence or absence of genes in various samples. Each row corresponds to specific gene and each column represents a different sample site.
Results and Discussion
Data generation and quality control of sediment metagenome
To determine the microbial communities from the sediment metagenomic data of River Brahmaputra, Illumina NovaSeq 6000 platform was used. Total number of reads from 35.15 million to 49.67 million were generated from all the sampling sites with an average reads of 40.82 million per sample. Average contig length of about 275,712 bp to 200 bp with almost 99.13% of high quality reads Q20 and Q30 were generated. Shotgun metagenomics is increasingly employed to identify microbial communities associated with plastic degrading genes in various environmental samples (Pinnell and Turner 2019; Kumar et al. 2021) reported an average of 40 million sequence reads per sample using shotgun metagenomic sequencing. Similarly, using the Illumina HiSeq platform, Gaytan et al. 2020; Kumar et al. 2021 and Saleem et al. 2023 observed more than 35 million reads from landfill waste, respectively.
Microbial taxonomic and functional annotation
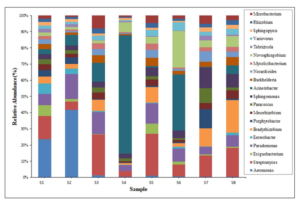
Metagenomic data comprises of bacterial (98.58%), archaeal (1.24%), eukaryotes (0.15%) and viral (0.03%) microbial diversity. The bacterial diversity comprises of 37 phyla with 1558 genera and 6200 species. Among the bacterial phyla, Proteobacteria (67.27%) dominates in all the sampling sites followed by Actinobacteria (18.30%), Firmicutes (4.79%), Bacteroidetes (4.65%), Planctomycetes (1.62%) and others (0.7-0.001%), respectively. Among the bacterial genera, Acinetobacter (8.0%) dominates followed by Aeromonas (6.26%), Streptomyces (3.8%), Pseudomonas (3.63%), Novosphingobium (2.30%) and others (2.02- 0.001%), respectively (Figure 1). Among the bacterial species, Aeromonas veronii dominates followed by Acinetobacter johnsonii in the population, mostly observed in upper stretches of the River near the city area sampling sites. The present investigations showed presence of mostly gram negative bacteria in metagenome sediments of River Brahamputra corroborates with River Ganga and Yamuna River metagenome data (Samson et al. 2019; Reddy et al. 2019; Behera et al. 2020; Das et al. 2020). The phylum level profiling shares similarity to other river sediments, indicating the presence of Proteobacteria. The proteobacteria is a diverse phylum, playing key role in several biogeochemical cycles (carbon, nitrogen and sulphur), reservoir of CO2 fixation, photo- and chemo- autotrophy enzymes (Badger and Bek 2008; Hicks et al. 2018; Liu et al. 2021). The complex organic materials in the river sediments support other microbes, collectively forming a complex biological niche in the sediment ecosystem under conditions of variable oxygen availability (Thoetkiattilkul et al. 2016; Srivastava and Verma 2022). The abundance of Acinetobacter genera in the samples showed anthropogenic pressure in the area of the River. Acinetobacter forms biofilm on abiotic and biotic surfaces as an effective strategy to increases their probability of survival under exposure to environmental stress, harbour pathways for degradation of various long-chain aromatic and dicarboxylic acids compounds (Jung and Park 2015; Bardbari et al., 2017; Hubeny et al. 2022). Aeromonas veronii was reported as fish pathogen and an opportunistic pathogen to humans (Li et al. 2020; Brar et al. 2023) and also been isolated from sediments of River and wetland (Behera et al. 2020; Kumar et al. 2023). In the present study, the abundance of bacterial diversity showed that the River may be under environmental stress due to increase in anthropogenic activities.
Figure 1: Abundance of top twenty genera of bacterial community in sediments of River Brahmaputra
The functional annotation of metagenomic sequences using KEGG database revealed 36 different pathways. A total of 1,70,88,172 ORFs were assigned to KEGG pathway genes. Analysis of KEGG predicted genes in sediment samples showed distinct pattern of functional gene abundance in each metagenome sample. Majority of the pathways were associated with carbohydrate metabolism, lipid metabolism, energy metabolism, amino acids metabolism, nucleic acid metabolism, glycan biosynthesis and metabolism, xenobiotic biodegradation and metabolism, translation, folding sorting and degradation and some unclassified functions (Figure 2). As the present study focused on degrading pathways, the major pathways associated with xenobiotics biodegradation and metabolism, xylene degradation, caprolactam degradation, polycyclic aromatic hydrocarbon degradation, chlorocyclohexane and chlorobenzene degradation. Similar observation was reported by (Qiu et al., 2020; Kumar et al. 2021; Yadav et al. 2021; Brar et al. 2023). Qiu et al. 2020 identified 46 small metabolic pathways from sediment of River, where carbohydrate, amino acid metabolism and energy were the major pathways. Yadav et al. 2021, determined xenobiotic bidegradation and metabolism using KEEG database, where the result prediction more than 350 pollutant degrading enzymes involved in plastic, hydrocarbon and dye degradation.
Figure 2: KEGG functional annotation showing major pathways from the sediment metagenome

Identification of PDGs from the sediment metagenome of R. Brahmaputra
From the metagenome data, plastic degrading genes (PDGs) were identified using PlasticDB database. A total of 100 elements with 40 unique elements were identified for PDGs (Figure 3). Highest number of PDGs elements were determined from S6 (37) followed by S2 (17) and S4 (17), respectively. These are the sampling sites of river passing through city areas where anthropogenic activities are increasing day-by-day. Plastic degrading microbes are mostly found in landfills, debris sites, marine and freshwater ecosystem. They can survive in harsh environments, besides having the capacity to synthesize secondary metabolites of industrial and clinical importance (Saleem et al. 2023). Zrimec et al. 2021 identified 11,906 enzyme homologues on soil and ocean data sets and 17 unique plastic types were recovered from the data sets. Plastic degrading genes for polymer such as polyethylene terephthalate (PET), polyethylene (PE), polyvinyl alcohol (PVA), polystyrene (PS), Polyhydroxyalkanoates (PHA) were mined from the datasets. A heatmap was developed showing abundance of PDGs in relation to sampling sites (Figure 4). The colour of each group scaled from red (2) to grey (-1) based on the relative abundance within the samples. Maximum number of PDGs were present in S6 followed by S2, S4, S1, S5 and S3, respectively. Similar observation was reported by Kumar et al., 2021 from landfills of Gujarat, where landfill microbes are linked to degradation of plastic such as PE, PET and PS. Putman et al. 2023 also identified plastic degrading genes from metagenome dataset for degradation of HDPE, PET and PC plastics containing abundant of aliphatic hydrocarbon and aromatic degradation genes.
Figure 3: Presence of total and unique elements of PDGs in sampling sites
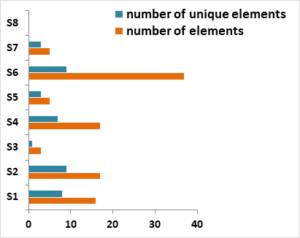
Figure 4: Heatmap showing the abundance of PDGs within the sampling sites
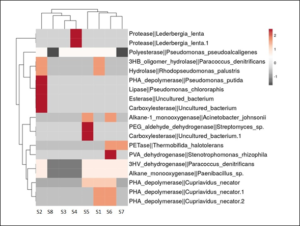
Figure 5: Abundance of plastic degrading microbial species from R. Brahmaputra
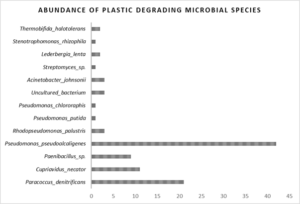
Table 1: List of major plastic degrading enzymes associated with microbial species
| Polymer name | Plastic degrading microbial species | Degrading enzymes |
| P3HV_PHBV_PHA | Paracoccus_denitrificans | 3HV_dehydrogenase |
| PHA_PHB | Cupriavidus_necator | PHA_depolymerase |
| LDPE | Paenibacillus_sp. | Alkane_monooxygenase |
| PBAT | Pseudomonas_pseudoalcaligenes | Polyesterase |
| LDPE | Rhodopseudomonas_palustris | Hydrolase |
| PHA | Pseudomonas_putida | PHA_depolymerase |
| PLA_PHA_PES_PCL | Pseudomonas_chlororaphis | Lipase |
| PLA | Uncultured_bacterium | Esterase |
| PBAT | Carboxylesterase | |
| PS | Acinetobacter_johnsonii | Alkane-1_monooxygenase |
| PEG | Streptomyces_sp. | PEG_aldehyde_dehydrogenase |
| PLA | Lederbergia_lenta | Protease |
| PVA | Stenotrophomonas_rhizophila | PVA_dehydrogenase |
| PET | Thermobifida_halotolerans | PETase |
Microbial Community Structure and novel enzymes associated with Plastic Degradation
In the present study, river sediment metagenome showed abundance of several enzymes and microbes involved in plastic degradation (Figure 5). The abundance analysis showed dominance of Pseudomonas pseudoalcaligenes in all the sampling sites having the capacity of PET degradation followed by Paracoccus denitrificans and Cupriavidus nectar for PHA degradation. For PE biodegradation, microbes uses dioxygenase and monooxygenase enzymes to add one or two oxygen atoms to form aldehydes ketones, alcohols and carboxylic groups through a free radical reaction. Through oxidation process, the extracellular enzymes contribute to mineralization of PE, making the polymers more hydrophilic and open up the surface for microbial adhesion (Jacquin et al. 2019; Kumar et al. 2021; Raoufi et al. 2023). In this study, sediment metagenme dataset showed abundance of degrading enzymes like alkane-monooxygenase, alkane-1-monooxygenase, hydrolase for PE degradation associated with microbial species Paenibacillus sp., Acinetobacter johnsonii, Rhodopseudomonas palustris. Similarly enzymes for PET degradation identified were polyethylene terephthalate hydrolase (PET hydrolase) and PETase, Lipase, Carboxylesterase, etc. linked with Thermobifida halotolerans, Pseudomonas chlororaphis, other uncultured bacterial species. A list of all the degrading enzymes with the associated microbes are mentioned in Table 1. Hydrolytic enzymes act on PET ester bonds and break them into simple monomers (Soong et al. 2022; Temporiti et al. 2022). Similarly, Kumar et al. 2021 observed enzymes such as PET hydrolase that hydrolyzes PET to mono-(2-hydroxyethyl) terephthalate, bis (2-hydroxyethyl) terephthalate and terephthalic acid, involved in biodegradation of PET. Soong et al. 2022 reported polyester hydrolases linked to bacterial species such as Thermobifida fusca, Thermomonospora curvata, and Ideonella sakaiensis (Soong et al., 2022). It has been reported that thermophilic Pseudomonas sp. are capable of biosynthesis and biodegradation of PHA and other synthetic plastic (Mozejko-Ciesielska et al., 2019; Saleem et al. 2023) which corroborates with our study. Since many Pseudomonas species such as Pseudomonas pseudoalcaligenes, Pseudomonas putida, and Pseudomonas chlororaphis were observed in the river sediment metagenome, capable of plastic degrading.
Conclusions
The present study employed high-throughput metagenomic approach to deliver insight into the abundance of plastic degrading genes and microbial community structures present in sediments of River Brahamputra. The microbial diversity revealed high microbial richness comprising of highest bacterial species in S2 (polluted area) and lowest in S8 (pristine area) sampling sites. Bacterial isolates Pseudomonas pseudoalcaligenes, Paracoccus denitrificans, Cupriavidus nectar, Thermobifida halotolerans, Streptomyces sp., Acinetobacter johnsonii were present in the metagenomics data which is being explored for plastic degradation. Functional analysis showed the abundance of potential enzymes involved in metabolism, xenobiotic and plastic degradation. Potential degrading enzymes alkane monooxygenase, hydrolase, PETase, carboxylesterase, lipase, esterase, etc were mostly present in the sampling areas. These plastic degrading enzymes were associated with the bacterial species for degradation of plastic such as PET, PE PS, PBA and PHA. Thus, these findings provide a baseline information and technique for development of metagenomics approach for the degradation of plastic waste from freshwater ecosystem. Further studies are required for detailed characterization of enzymes, determining common gene cluster at higher taxonomic level. These efforts may result in identification of novel metabolic pathways for degradation of plastics waste.
Acknowledgment
The authors are highly thankful to Indian Council of Agricultural Research, New Delhi and ICAR-Central Inland Fisheries Research Institute, Barrackpore, West Bengal for supporting this research work.
Funding: The work did not receive external funding
Data availability: The datasets presented in this study can be found in online repositories of SRA at NCBI with BioProject id- PRJNA944918 and submission id- SUB14320240.
Statements and Declarations
Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate: Not Applicable
Consent for publication: Not applicable
References
- Alimi OS, Farner, BJ, Hernandez LM, Tufenkji N (2018) Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ Sci Technol 52(4):1704–1724.
- Andrade MC, Winemiller KO, Barbosa PS, Fortunati A, Chelazzi D, Cincinelli A, Giarrizzo T (2019) First account of plastic pollution impacting freshwater fishes in the Amazon: Ingestion of plastic debris by piranhas and other serrasalmids with diverse feeding habits. Environ Pollut 244:766–773.
- Andrade, AJM, Grande, SW, Talsness, CE, Grote K, Chahoud I (2016) Dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP), non-monotonic dose–response and low dose effects on rat brain aromatase activity. Toxicol A 227: 185-192. doi: 10.1016/2006.07.022.
- Azevedo-Santos VM, Brito MFG, Manoel PS, Perroca JF, Rodrigues-Filho JL, Paschoal LRP, Gonçalves GRL, Wolf MR, Blettler MCM, Andrade MC, Nobile AB, Lima FP, Ruocco AMC, Silva CV, Perbiche-Neves G, Portinho JL, Giarrizzo T, Arcifa MS, Pelicice FM (2021) Plastic pollution: A focus on freshwater biodiversity. Ambio 50(7):1313-1324. doi: 10.1007/s13280-020-01496-5.
- Badger MR, Bek EJ (2008) Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle. J Exp Bot59(7):1525–1541. https://doi.org/10.1093/jxb/erm297
- Ballent A, Corcoran PL, Madden O, Helm PA, Longstaffe F (2016) Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar Pollut Bull 110:383–395
- Bardbari AM, Arabestani MR, Karami M, Keramat F, Alikhani MY, Bagheri KP (2017) Correlation between ability of biofilm formation with their responsible genes and MDR patterns in clinical and environmental Acinetobacter baumanniiMicrob Pathog 108:122–128. https://doi.org/10.1016/j.micpath.2017.04.039
- Behera BK, Patra B, Chakraborty HJ, Sahu P, Rout AK, Sarkar DJ, Parida PK, Raman RK, Rao AR, Rai A, Das BK, Jena J, Mohapatra T (2020) Metagenome analysis from the sediment of river Ganga and Yamuna: In search of beneficial microbiome. PLoS ONE 15(10): e0239594
- Blettler MCM, Wantzen KM (2019) Threats underestimated in freshwater plastic pollution: Mini-Review. Water, Air, & Soil Pollut230:174
- Brar B, Kumar R, Sharma D et al.(2023)Metagenomic analysis reveals diverse microbial community and potential functional roles in Baner rivulet, India. J Genet Eng Biotechnol 21: 147. https://doi.org/10.1186/s43141-023-00601-x
- Crew A, Gregory-Eaves I, Ricciardi A (2020) Distribution, abundance, and diversity of microplastics in the upper St. Lawrence River. Environ Pollut 260: 113994
- Das BK, Behera BK, Chakraborty HJ, Paria P, Gangopadhyay A, Rout AK, Nayak KK, Parida PK, Rai A (2020) Metagenomic study focusing on antibiotic resistance genes from the sediments of River Yamuna. Gene 758: 144951
- Eagle L, Hamann M, Low DR (2016) The role of social marketing, marine turtles and sustainable tourism in reducing plastic pollution. Mar Pollut Bull 107(1):324–332
- Ebere EC, Wirnkor VA, Ngozi VE, Chukwuemeka IS (2019) Macrodebris and microplastics pollution in Nigeria: first report on abundance, distribution and composition. Environ Anal Health Toxicol 34:e2019012
- Emmerik TV, Schwarz A (2019) Plastic debris in rivers. Wires water, 7:e1398.https://doi.org/10.1002/wat2.1398
- Fischer EK, Paglialonga L, Czech E, Tamminga M (2016) Microplastic pollution in lakes and lake shoreline sediments – A case study on Lake Bolsena and Lake Chiusi (central Italy) Environ Pollut 213:648–657
- Gambarini V, Pantos O, Kingsbury JM, Weaver L, Handley KM, Lear G (2022) PlasticDB: a database of microorganisms and proteins linked to plastic biodegradation, Database, 2022: baac008, DOI: org/10.1093/database/baac008.
- Gaytán I, Sánchez-Reyes A, Burelo M, Vargas-Suárez M, Liachko I, Press M, Shawn, S, Cruz-Gómez, MJ Loza-Tavera H (2020) Degradation of Recalcitrant Polyurethane and Xenobiotic Additives by a Selected Landfill Microbial Community and Its Biodegradative Potential Revealed by Proximity Ligation-Based Metagenomic Analysis. Front Microbiol 10,https://doi.org/10.3389/fmicb.2019.02986
- Gonçalves M, Schmid K, Andrade MC, Andrades R, Pegado T, Giarrizzo T (2020) Are the tidal flooded forests sinks for litter in the Amazonian estuary? Mar Pollut Bull 161:111732.
- Hicks N, Liu X, Gregory R, Kenny J, Lucaci A, Lenzi L, Paterson DM, Duncan KR (2018) Temperature Driven Changes in Benthic Bacterial Diversity Influences Biogeochemical Cycling in Coastal Sediments. Front Microbiol 9, doi: 10.3389/fmicb.2018.01730
- Hubeny, J., Korzeniewska, E., Buta-Hubeny, M., Zieliński, W., Rolbiecki, D., & Harnisz, M. (2022). Characterization of carbapenem resistance in environmental samples and Acinetobacter spp. isolates from wastewater and river water in Poland. Sci Total Environ822: 153437. https://doi.org/10.1016/j.scitotenv.2022.153437
- Huson DH, Auch AF, Qi J, Schuster SC (2007) MEGAN analysis of metagenomic data. Genome Res 17:377–386
- Hussein A (2021) Molecular Techniques to Assess Microbial Community Structure, Function, and Dynamics in the Environment, Int J Emerg Trends, Sci Technol 4–14.
- Jacquin J, Cheng J, Odobel C, Pandin C, Conan P, Pujo-Pay M, Barbe V, Meistertzheim A-L, Ghiglione J-F (2019) Microbial ecotoxicology of marine plastic debris: a review on colonization and biodegradation by the “Plastisphere”. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.00865.
- Jung J, Park W (2015) Acinetobacterspecies as model microorganisms in environmental microbiology: current state and perspectives. Appl Microbiol Biotechnol 99: 2533–2548 https://doi.org/10.1007/s00253-015-6439-y
- Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K (2017) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45:D353–D361
- Kumar R, Pandit P, Kumar D, Patel Z, Pandya L, Kumar M, Joshi C, Joshi M (2021) Landfill microbiome harbour plastic degrading genes: A metagenomic study of solid waste dumping site of Gujarat, India, Sci Total Environ 779:146184.
- Kumar V, Bera T, Roy S, Vuong P, Jana C, Sarkar DJ, Devi MS, Jana AK, Rout AK, Kaur P, Das BK, Behera BK (2023) Investigating bio-remediation capabilities of a constructed wetland through spatial successional study of the sediment microbiome. npj Clean Water6: 8. https://doi.org/10.1038/s41545-023-00225-1
- Li T, Raza SHA, Yang B, Sun Y, Wang G, Sun W, Qian A, Wang C, Kang Y, Shan X (2020) Aeromonas veroniiInfection in Commercial Freshwater Fish: A Potential Threat to Public Health. Animals 10(4): 608. https://doi.org/10.3390/ani10040608
- Li J, Jia C, Lu Q, Hungate B A, Dijkstra P, Wang S, Wu C, Chen S, Li D, Shim H (2021) Mechanistic insights into the success of xenobiotic degraders resolved from metagenomes of microbial enrichment cultures. J Hazard Mater 418: 126384 https://doi.org/10.1016/j.jhazmat.2021.126384.
- Liu J, Zhou H, Yang Z, Wang X, Chen H, Zhong L, Zheng W, Niu W, Wang S, Ren X, Zhong G, Wang Y, Ding X, Müller R, Zhang Y, Bian X (2021) Rational construction of genome-reduced Burkholderiales chassis facilitates efficient heterologous production of natural products from Proteobacteria. Nat Commun12(1): 4347. https://doi.org/10.1038/s41467-021-24645-0
- Mazhandu ZS, Muzenda E, Mamvura TA, Belaid M (2020) Integrated and consolidated review of plastic waste management and bio-based biodegradable plastics: challenges and opportunities. Sustainability 12(20):8360
- Meijer LJJ, van Emmerik T, van der Ent R, Schmidt C, Lebreton L (2021) More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci Adv 7(18): eaaz5803
- Mozejko-Ciesielska J, Szacherska K, Marciniak P (2019) PseudomonasSpecies as Producers of Eco-friendly Polyhydroxyalkanoates. J Polym Environ 27: 1151–1166. https://doi.org/10.1007/s10924-019-01422-1
- Napper IE, Thompson RC, (2023) Plastics and the Environment. Annu Rev Environ Resour 48: 55-79
- Panda AK, Singh RK, Mishra DJR (2010) Thermolysis of waste plastics to liquid fuel: a suitable method for plastic waste management and manufacture of value added products—a world prospective. 14(1):233–248
- Pilapitiya PGCNT, Ratnayake AS (2024) The world of plastic waste: A review. Cleaner Mater 11:100220. https://doi.org/10.1016/j.clema.2024.100220.
- Pinnell LJ, Turner JW (2019) Shotgun metagenomics reveals the benthic microbial community response to plastic and bioplastic in a coastal marine environment, Front Microbiol 10 (2019) https://doi.org/10.3389/fmicb.2019.01252.
- PlasticsEurope(2019) Plastics—the facts 2019: an analysis of European plastics production, demand and waste data PlasticsEurope Messe Düsseldorf, Frankfurt.
- Purohit J, Chattopadhyay A, Teli B (2020) Metagenomic Exploration of Plastic Degrading Microbes for Biotechnological Application. Curr Genom 21: 253-270
- Putman LI, Schaerer LG, Wu R, Kulas DG, Zolghadr A, Ong RG, Shonnard DR, Techtmann SM (2023) Deconstructed Plastic Substrate Preferences of Microbial Populations from the Natural Environment. Microbiol Spectr 11(4). 10.1128/spectrum.00362-23
- Qiu H, Gu L, Sun B, Zhang J, Zhang M, He S, An S, Leng X (2020) Metagenomic Analysis Revealed that the Terrestrial Pollutants Override the Effects of Seasonal Variation on Microbiome in River Sediments. Bull Environ Contam Toxicol 105:892–898. https://doi.org/10.1007/s00128-020-03033-2
- Ranjan K, Bharti MK, Siddique RA, Singh J (2021) Metatranscriptomics in Microbiome Study: A Comprehensive Approach, in: Microb Metatranscriptomics Belowgr, Springer, pp. 1–36.
- Raoufi H, Taqwa S, Fagiryaar F (2023) Enzymatic Degradation of Polyethylene and Polyethylene Terephthalate: A Mini Review. American J Environ Climate 2: 3: 41-50.
- Reddy B, Pandey J, Dubey SK (2019) Assessment of environmental gene tags linked with carbohydrate metabolism and chemolithotrophy associated microbial community in River Ganga. Gene 704: 31-41
- Reddy, S (2018) Plastic pollution affects sea life throughout the ocean. https://www.pewtrusts.org/en/research-and analysis/articles/2018/09/24/plastic-pollution-affects-sea-life throughout-the-ocean
- Ritchie H, Samborska V, Roser M (2023) Plastic Pollution. Our World in Data. https://ourworldindata.org/plastic-pollution
- Ryan PG (2018) Entanglement of birds in plastics and other synthetic materials. Marine Pollut Bull 135:159–164.
- Saleem M, Ali A, Razzak SA, Khawaja S, Yahya S (2023) Shotgun Metagenomic insights into the Plastisphere microbiome: Unveiling potential for clinical and industrial enzymes production along with plastic degradation. https://doi.org/10.21203/rs.3.rs-3333696/v1
- Samson R, Shah M, Yadav R, Sarode P, Rajput V, Dastager SG, Dharne MS, Khairnar K (2019) Metagenomic insights to understand transient infuence of Yamuna River on taxonomic and functional aspects of bacterial and archaeal communities of River Ganges. Sci Total Environ 674: 288–299
- Shilpa, Basak N, Meena SS (2022) Exploring the plastic degrading ability of microbial communities through metagenomic approach, Materials Today: Proceedings, https://doi.org/10.1016/j.matpr.2022.02.308
- Sofi IR, Manzoor J, Bhat RA, Munvar R (2020) Plastic Pollution and the Ecological Impact on the Aquatic Ecosystem. 80-93
- Soong YV, Sobkowicz MJ, Xie D (2022) Recent Advances in Biological Recycling of Polyethylene Terephthalate (PET) Plastic Wastes. Bioengineering (Basel) 9(3). https://doi.org/10.3390/ bioengineering9030098
- Srivastava A, Verma D (2023) Ganga River sediments of India predominate with aerobic and chemo‑heterotrophic bacteria majorly engaged in the degradation of xenobiotic compounds. Environ Sci Pollut Res 30(1): 752–772
- Temporiti MEE, Nicola L, Nielsen E, Tosi S (2022) Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 10(6). https://doi. org/10.3390/microorganisms10061180
- Thoetkiattikul H, Mhuantong W, Pinyakong O, Wisawapipat W, Yamazoe A, Fujita N, Champreda V(2017) Culture-independent study of bacterial communities in tropical river sediment. Bioscience, Biotechnol Biochem 81 (1): 200-209.
- UNEP (2023). United Nations Environment Programme. Turning off the Tap. How the world can end plastic pollution and create a circular economy. Nairobi.
- UNEP (2024). United Nations Environment Programme. Beat Plastic Pollution. Visual Features.https://www.unep.org/interactives/beat-plastic-pollution
- Urbanski BQ, Denadai AC, Azevedo-Santos VM, Nogueira MG (2020) First record of plastic ingestion by an important commercial native fish (Prochilodus lineatus) in the middle Tietê River basin, Southeast Brazil. Biota Neotropica 20:e20201005.
- Welden NA (2020) The environmental impacts of plastic pollution. Plastic Waste and Recycling. 8:195-222 https://doi.org/10.1016/B978-0-12-817880-5.00008-6.
- Wu Y, Guo P, Zhang X, Zhang Y, Xie S, Deng J (2019) Effect of microplastics exposure on the photosynthesis system of freshwater algae. J Hazard Mater 374:219–227
- Yadav R, Rajput V, Dharne M (2021) Functional metagenomic landscape of polluted river reveals potential genes involved in degradation of xenobiotic pollutants. Environ Res 192: 110332
- Zrimec J,Kokina M,Jonasson S, Zorrilla F, Zelezniak A, Plastic-Degrading Potential across the Global Microbiome Correlates with Recent Pollution Trends. mBio12:10.1128/mbio.02155-21.https://doi.org/10.1128/mbio.02155-21