Publication Information
ISSN 2691-8803
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Antibacterial Activity of Different Compositions of Ethanol and Isopropanol in Hand Sanitizers
RM Kamal1, H Mudalige1*, U Bandaranayake1
1BMS School of Science, 591, Galle Road, Colombo 6, Sri Lanka
Received Date: November 27, 2021; Accepted Date: December 08, 2021; Published Date: December 14, 2021;
*Corresponding author: H. Mudalige, BMS School of Science, 591, Galle Road, Colombo 6, Sri Lanka, 9477 397 9016. Email: heshani.m@bms.ac.lk
Citation: Kamal RM, Mudalige H, Bandaranayake U (2021) Antibacterial Activity of Different Compositions of Ethanol and Isopropanol in Hand Sanitizers. Adv Pub Health Com Trop Med: APCTM-135.
DOI: 10.37722/APHCTM.2021402
Abstract
Hand hygiene is vital in the control of nosocomial and community spread infections. Alcohol-based sanitizers commonly contain ethanol, isopropanol and n-propanol at concentrations between 60-95%. This study explored the effect of altering ethanol and isopropanol compositions on the antibacterial activity of hand sanitizers. Hand sanitizer 1 (HS1) contained 40% each of ethanol and isopropanol giving a total alcohol concentration of 80% (v/v), while the Hand Sanitizer 2 (HS2) contained 42.5% of both alcohols, giving a total alcohol concentration of 85% (v/v). To analyse antibacterial susceptibility, well diffusion assay was performed using Mueller Hinton media. The zone of inhibition (ZOI) of HS1 was 23.5±1.41 mm against Staphylococcus aureus. No ZOI were observed against Escherichia coli. The ZOI of HS2 were 19.5±0.7 mm and 31.0±1.4 mm against S. aureus and E. coli, respectively. There was a significant statistical difference between the ZOI in relation to HS1 and HS2 for both E. coli (p=0.001; p< 0.05) and S. aureus (p=0.022; p<0.05). The HS2 was identified as more effective due to its broader antibacterial spectrum, and HS2 was subjected to a downstream hand swab analysis. The colony count method proved that HS2 reduced bacterial counts on hand surfaces from 1.093×109 cfu/mL to 2.2×105 cfu/mL. Additionally, biochemical tests confirmed the wide spectrum of HS2 activity against multiple bacterial species including Gram-positive and negative bacilli and cocci, lactose fermenters, oxidase and catalase producing bacteria. Broth macro-dilution was used to determine minimum inhibitory concentration (MIC) and concentration percentages of 20, 15, 10, 9, 8, 7, 6, 5,4,3,2 and 1of HS2 was analysed. The (MIC) of HS2 was 5% (2.5% ethanol and 2.5% isopropanol) against E. coli. It was lower at 4% (2% ethanol and 2% isopropanol) against S. aureus. The combination of ethanol and isopropanol produced higher ZOI and lower MIC values, implying the efficacy of antibacterial action.
Keywords: Hand Swab Analysis; Minimum Inhibitory Concentration; Well Diffusion
Abbreviation List
HS1
Hand Sanitizer 1
HS2
Hand Sanitizer 2
HSB
Hand Sanitizer 2 Before
HSA
Hand Sanitizer 2 After
MIC
Minimum Inhibitory Concentration
N/C
Negative Control
P/C
Positive Control
Introduction
Hands have been established as a primary route for the transmission of infectious pathogens. Hand hygiene is a vital aspect in the control of nosocomial and community spread infections. It is commonly achieved by either hand washing with antimicrobial soaps or using hand sanitizers. The use of hand sanitizers has drastically increased over the years, both due to their effectiveness and convenience.
The hand microbiome
Bacteria make up over 80% of all microorganisms present on hand surfaces (Park et al., 2017). The bacterial microbiome inhabiting hand surfaces are classified as transient or resident bacteria. Transient bacteria are the more pathogenic bacteria that colonize the superficial layers of the skin, making them prone to removal during hand hygiene. Resident bacteria are commensals, not typically connected with diseases that reside in the deeper skin layers.
The most abundant genera on hand surfaces are Propionibacterium, Staphylococcus and Corynebacterium (Kong and Segre, 2017; Fierer, 2008). It is therefore essential to use both Gram-positive and Gram-negative bacteria to represent this diverse range in hand sanitizer testing.
The Gram-positive Staphylococcus aureus, a transient and resident bacterium, was chosen as it is a significant cause of nosocomial and community infections (Kampf and Kramer, 2004). Moreover, the Staphylococcus species is the most abundant on hands.
The Gram-negative Escherichia coli was chosen as it is a transient bacterium that causes a broad spectrum of diseases ranging from meningitis to urinary tract infections. It is also the principle cause of infectious enteric diseases with a very high mortality rate. Both S. aureus and E. coli are opportunistic pathogens that mainly express virulence in the presence of a primary infection or during immune compromised states.
Hand sanitizers
Alcohol-based hand sanitizers
Alcohol-based sanitizers commonly contain ethanol, isopropanol and n-propanol either as the sole active ingredient or in combined formulations. Proper hand sanitization can be achieved by using alcohol-based sanitizers which, unless hands are visibly soiled, outperform hand washing with regular antimicrobial soaps (Forer, Block and Frenkel, 2017; CDC, 2002).
The World Health Organization recommends the use of ethanol and isopropanol in hand sanitizers (WHO, 2009). These short-chained amphipathic alcohols are highly formidable against a broad spectrum of pathogens. They rapidly inactivate vegetative bacteria, enveloped viruses and fungi. However, they exert a more subdued attack against non-enveloped viruses, protozoa and oocytes.
Ethanol and isopropanol stage a powerful multipronged assault on bacteria leading to a bactericidal impact. By the lowering of surface tension, they break open bacterial cell walls (Restrepo, 2015). The alcohol duo elutes lipopolysaccharides on the outer cell membrane, and peptidoglycans on the inner cell wall of Gram-negative bacteria like E. coli (Hu et al., 2018). Although the thick peptidoglycan cell wall on Gram-positive bacteria like S. aureus confers an increased resistance against membrane lipid leakage, ethanol, and isopropanol displace their phosphate groups (Abualizadeh et al., 2017). Both Gram-positive and Gram-negative bacteria, therefore, show higher membrane fluidity on exposure to these alcohols as shown in Figure 1 (Huffer et al., 2011).
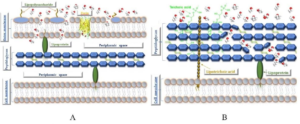
Figure 1: A– Disruption of Gram negative bacterial outer membranes by alcohols. The alcohols cross the membrane and displace lipopolysaccharides. B– Alteration of protein function resulting in peptidoglycan disarrangement in Gram-positive bacteria (Man et al., 2017).
The attack on bacterial cell walls and the loss of membrane integrity leads to an envelope stress response that produces reactive oxygen species (Horinouchi, Maeda, Furusawa, 2018). These reactive oxygen species induce genome damage and decrease oxygen aeration (Cao et al., 2017). The membrane damage also induces a reduced proton flux which leads to a detrimental drop in aerobic respiration (Goodarzi et al., 2010). The lowered ATP (adenosine triphosphate) production has fatal downstream implications contributing towards the bactericidal outcome. Although for the most part, ethanol and isopropanol show similar antimicrobial mechanisms, there are a few notable differences. Isopropanol has higher hydrophobicity due to the presence of two methyl groups. It can, therefore, dislodge lipopolysaccharides more vehemently than ethanol and is more damaging towards Gram-negative bacteria. On the other hand, the liposoluble hydrocarbon tail in ethanol penetrates membranes more voraciously (Morente et al., 2013). The resulting membrane disarrangement makes Gram-positive bacteria more susceptible to osmotic shock and subsequently, leakage and cell lysis in the presence of ethanol (Dyrda et al., 2019).
In addition to being membrane-active agents, electron microscopy studies have also detailed the denaturation of cytoplasmic and membrane proteins by ethanol and isopropanol through desiccation. The denaturation process requires water, and this explains the reduced antibacterial potency of absolute alcohol.
Alcohol-based sanitizers are not known to induce resistance in bacteria allowing for their widespread general application (Kampf, 2018). Alcohols, due to their volatility, have a questionable sustained action. Their lipophilic nature may also cause dermal irritation with frequent use (Cartner et al., 2017). The skin barrier, however, can be protected by coalescing alcohol-based sanitizers with emollients or humectants (Draelos, 2012). Apart from these minor drawbacks, alcohols are well tolerated in antibacterial hand sanitizers.
Alcohol-free hand sanitizers
Alcohol-free sanitizers contain antimicrobials like quaternary ammonium compounds, triclosan, iodine compounds and chlorhexidine. These are often formulated as water-based rubs. Chlorhexidine and triclosan, similarly to ethanol and isopropanol, impair membrane function and coagulate proteins (Cheung et al., 2012). Quaternary ammonium compounds like benzalkonium chloride also disrupt membranes, but they work by adsorbing to membranes and causing cell content leakage (Pereira and Tagkopoulos, 2019). Iodine infiltrates cells and complexes with molecules interrupting synthetic metabolic pathways (Jing et al., 2020). These chemicals are predominantly bacteriostatic but are bactericidal at higher concentrations. Alcohol-free sanitizers show persistent antimicrobial activity unlike that usually seen with alcohol- based hand sanitizers (Bondurant et al., 2020; Bondurant, Duley and Harbell, 2019). Because of this, they are added to alcohol-based sanitizers to increase their potency.
Significance of the study
The presence of an ongoing pandemic has made us susceptible to secondary infections by opportunistic pathogens. It is of vital importance to take necessary preventive measures to avoid the emergence of multiple pandemics. The global hand sanitizer shortage has underlined the requirement for more effective formulations (Berardi et al., 2020). The preparation of an efficacious and potent hand sanitizer is, therefore, a timely need to ensure personal and community health.
In this study, we hope to elucidate the optimum composition of ethanol and isopropanol and the presence of a synergistic action, if any.
Methodology
Subculturing
Streak Plates
Nutrient agar (Himedia, Mumbai, India) was prepared according to the manufacturer’s instructions. The agar was then dispensed into petri plates labelled for each of the bacterial strains: Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922). Isolated colonies from the primary culture were used to prepare streak plates. They were incubated at 37ºC for 24 hours. After overnight incubation, the plates were stored at 4ºC.
Liquid culture
Nutrient broth (Himedia, Mumbai, India) was prepared according to the manufacturer’s instructions. The broth was dispensed up to a volume of 7mL in each tube. The tubes were labelled for E. coli and S. aureus. The broth was inoculated with a well isolated colony from the respective primary culture. The tube was thoroughly mixed to emulsify the culture. Once even turbidity was achieved, the tubes were incubated overnight at 37ºC and stored at 4°C.
Preparation of Hand Sanitizer Samples
Two falcon tubes were labelled HS1 and HS2. Distilled water, ethanol and isopropanol were pipetted into the tubes according to the volumes given in Table 1 to prepare a total volume of 10.0mL. 40% Isopropanol 42.5% Isopropanol
Sample
Composition of active ingredients
Volume of Ethanol (100%) /mL
Volume of Isopropanol (100%) /mL
Volume of distilled water /mL
HS1
40% Ethanol
4
4
2
HS2
42.5% Ethanol
4.25
4.25
1.5
Full hand sanitizer samples were prepared by compounding 5.0mL of the hand sanitizer with 72.5μL of glycerin to achieve the WHO (2009) suggested glycerin concentration of 1.45%.
Well Diffusion
Mueller Hinton agar (Himedia, Mumbai, India) was prepared as per manufacturer’s instructions for 4 petri plates. After the plates solidified, two of the plates were streaked, using a sterile cotton swab, with overnight S. aureus liquid culture that had been adjusted in distilled water to the turbidity of a 0.5 McFarland solution. The other two were streaked similarly using E. coli instead. The plates were divided into 4 quadrants as shown in Figure 2.
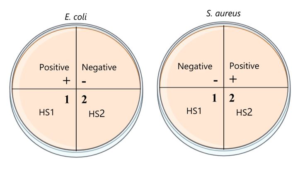
Figure 2: The division and labelling of the plates for the well diffusion assay.
Wells were bored into three of the quadrants using the end of a sterile 1000 µL pipette tip. Into the respective wells 50 µL of DMSO (negative control), full HS1 and full HS2 were pipetted in. Gentamicin disks (10µg) were used as a positive control. The petri plates were incubated for 24 hours at 37ºC. After incubation, the diameter of the zones of inhibition were measured and the results were statistically analysed using independent t tests.
The HS2 was shown to have superior performance and was selected to be used in further downstream analysis.
Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration of HS2 was determined to further assure its quality. Based on a literature review, final alcohol concentrations of 20, 15, 10, 9, 8, 7, 6, 5, 4, 3, 2, 1 and 0 percent were chosen (Man et al., 2017; Mazzola et al., 2009). Mueller Hinton broth (Himedia, Mumbai, India) was prepared according to manufacturer guidelines. Overnight cultures were adjusted in distilled water to the turbidity of a 0.5 McFarland solution to prepare the inoculum. Into each test tube, except the positive control, 100 µL of culture was added along with 900 µL of Mueller Hinton broth separately for E. coli and S. aureus. The alcohol dilutions were created according to Table 2. Tube 13 served as a negative control while tube 14, which was devoid of bacterial inoculation, served as the positive control. Two sets of test tubes were prepared, one each for E. coli and S. aureus. Each tube contained a total volume of 2mL reaction mixture. The tubes were sealed with foil and incubated overnight at 37 ºC. After incubation they were examined for turbidity. The lowest concentration of the tube showing no visible turbidity was selected as the minimum inhibitory concentration.
Tube
100% Eth- anol / μL
100% Iso- propanol / μL
Autoclaved d.H2O / μL
Final Composition (Total Alcohol in 2mL) (v/v)
1
200
200
600
20%
2
150
150
700
15%
3
100
100
800
10%
4
90
90
820
9%
5
80
80
840
8%
6
70
70
860
7%
7
60
60
880
6%
8
50
50
900
5%
9
40
40
920
4%
10
30
30
940
3%
11
20
20
960
2%
12
10
10
980
1%
13 (N/C)
0
0
1000
0%
14 (P/C)
200
200
1600
20%
Hand swab analysis
Peptone water was prepared as per the manufacturer’s instructions. Then, 5.0 mL each of peptone water was aliquoted into 2 falcon tubes labelled HSB and HSA. Distilled water of volume 1 mL was added to a 4 x 4 cm area on the right palm using a Pasteur pipette. The water was rubbed in by gently massaging with the fingertips of the right hand for 20 seconds. The sterile cotton swab was then rubbed over the area and then used to inoculate the peptone water in the tube labelled HSB. Afterwards, 1 mL of the full HS2 was dispensed on the right palm and massaged in for 20 seconds before swabbing, as before. The swab was used to inoculate the peptone water in the tube labelled HSA. This process is represented in Figure 3. The tubes were sealed and incubated at 37ºC for 24 hours.
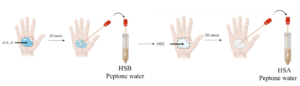
Figure 3: Schematic diagram of the hand swab analysis.
Preparation of Spread Plates
Nutrient agar was prepared according to the manufacturer’s guidelines. The agar was dispensed into 5 petri plates labelled B-10-3, B-10-4, B-10-5, A-10-1 and A-10-2 for the dilutions.
The overnight peptone water cultures were serially diluted ten-fold. The HSB overnight peptone water was diluted up to 10-5 by the serial transfer of 100 μL of culture into 900 μL of autoclaved distilled water. Similarly, the HSA overnight culture was diluted up to 10-2 as decided from observation of bacterial growth.
The final three dilutions of the HSB and the final two HSA dilutions were inoculated on nutrient agar plates as shown in Figure 4. The spread plate technique was followed and 15μL of the dilutions were used. The plates were sealed and incubated at 37ºC for 24 hours. The plates showing between 30-300 colonies were selected and the number of colonies counted. The initial colony forming units per milliliter (cfu/mL) was calculated from the raw colony counts.
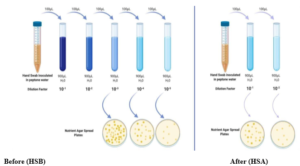
Figure 4: Schematic diagram of the serial dilution and spread plate assays
Before– the overnight HSB culture was diluted up to a factor of 10-5 and the last three dilutions plated.
After– the overnight HSA culture was diluted up to a factor 10-2 and the dilutions were plated.
Gram Staining
Gram staining was performed on the HSB and HSA overnight peptone cultures. A loop full of inoculum was transferred to glass slides and then heat-fixed by briefly passing over the top of a Bunsen flame. The slides were flooded with crystal violet for 1 minute and rinsed using distilled water. Then Gram’s iodine was introduced and left for a minute before rinsing. Gram’s decolouriser was added and left on the slides for 30s before washing. Finally, safranin was added and left for a minute before washing off. The slides were observed under the microscope at 40x and using the 100x oil immersion lens.
Biochemical Tests
Lactose Fermentation
MacConkey agar (Himedia, Mumbai, India) plates were prepared according to the manufacturer’s guidelines. The plates were streaked with the HSB and HSA overnight cultures, as described earlier and incubated at 37ºC for 24 hours.
Catalase Test
A few drops of hydrogen peroxide were added on to glass slides. To it, isolated colonies from the nutrient agar overnight spread plates were added.
Indole Test
Isolated pink colonies from the MacConkey streak plates were used to inoculate 5mL of peptone water. This was incubated overnight at 37ºC. To this, 5 drops of Kovac’s reagent were added.
Oxidase test
On oxidase disks, 100 µL of the overnight HSB and HSA cultures were added. These were observed after 30 seconds.
Results
Well Diffusion
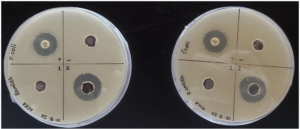
Figure 5: E. coli well diffusion plates. Sample 2 produced the larger inhibition zone in both duplicates.
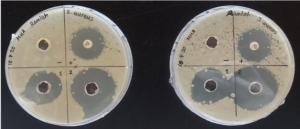
Figure 6: S.aureus well diffusion plates. Sample 2 produced the larger inhibition zone in both duplicates.
The HS2 produced greater zones of inhibition than HS1 in the case of both bacterial strains as shown in Table 3. The HS2 (19.5±0.7 mm) had a statistically significant difference against E. coli compared to the HS1 (0 mm), p=0.001; p< 0.05. Likewise, HS2 (31.0±1.4 mm) also had a statistically significant difference against S. aureus compared to the HS1 (23.5±1.41 mm), p=0.022; p< 0.05
Sample
Mean ± Standard Deviation /mm
E. coli
S. aureus
HS1
0±0
23.5±0.7
HS2
19.5±0.7
31.0±1.4
Minimum Inhibitory Concentration determination
For the E. coli dilution series, the final tube with no visible turbidity was tube 8 while it was tube 9 for S. aureus as seen in Figures 7 and 8. The minimum inhibitory concentration of the HS2 was 5% (2.5% ethanol and 2.5% isopropanol) for E. coli and 4% (2% ethanol and 2% isopropanol) for S. aureus.
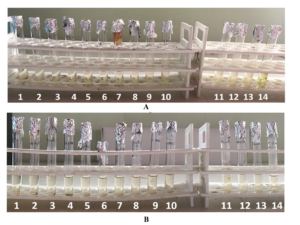
Figure 7: Dilution series of the Minimum Inhibitory Concentration test of E. coli and S. aureus.

Figure 8: A close up of the dilution series showing the consecutive tubes used to determine the MIC A– E.coli, tube 8 was the final tube with no visible turbidity B– S. aureus, tube 9 was the final tube with no visible turbidity.
Determination of Colony forming units reduction
After the use of the sanitizer, the bacterial count reduced from 1.093 x 109 cfu/mL to 2.2 x 105 cfu/mL as shown in Figure 9 and 10. This corresponds to a 4log10 reduction in bacteria.
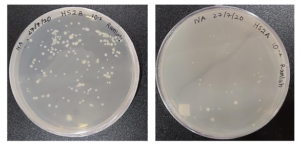
Figure 9: Nutrient agar spread plates of HS2 Before-10-5 dilution of HSB showing 164 colonies
After- 10-2 dilution of HSA showing 33 colonies.
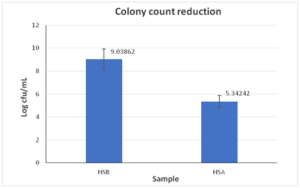
Figure 10: Bar graph of the colony count reduction before and after using HS2.
Gram Staining
The use of HS2 reduced the number of gram-positive rods and gram-positive cocci on hand surfaces.
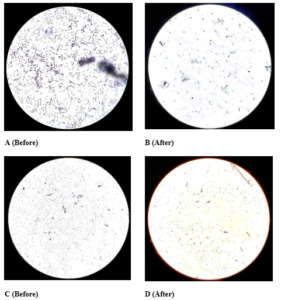
Figure 11: Gram Staining under 40x objective lens A. Before HS2-multiple blue rods B.
After HS2-few blue rods C. Before HS2-blue cocci D. After HS2-red rods and red cocci.
Lactose Fermentation
Lactose fermenting and non-lactose fermenting bacteria were present on the hand surface before the use of HS2 but were not present after its use.
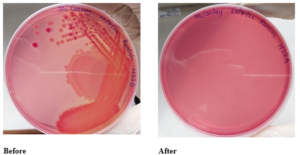
Figure 12: Mac Conkey streak plates of HS2
Before– The pink colonies of lactose fermenters and yellow colonies of non-lactose fermenters are visible
After– No colonies visible.
Catalase Test
There were catalase-positive bacteria before the use of HS2, but this reduced drastically after the usage of HS2.

Figure 13: Catalase test
Before- The characteristic beer froth of the positive catalase result
After- Almost no bubbles seen.
Indole Test
Some of the lactose fermenters present in hand surfaces before the use of HS2 metabolise tryptophan to indole while others do not. To confirm the presence of E. coli, a Gram stain was done on the completion of the indole test. The Gram stain showed Gram-negative rods which confirmed the presence of E. coli as per the Bergey’s manual.
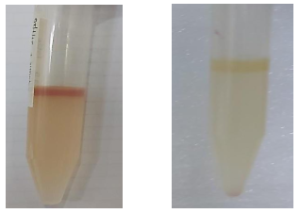
Figure 14: Indole Test
Presence of the cherry red ring indicating an indole positive test
Indole negative test.
Discussion
The activity of HS2 was higher than that of HS1. This was seen in the preliminary screening well diffusion test where HS2 had significantly higher zones of inhibition when compared with HS1 for both the bacterial strains. This was expected as the total alcohol concentration in HS2 was greater than HS1. The better performance of HS2 also suggests that water is not a limiting factor when the alcohol concentration is increased up to 85%. The usage of HS2 can be promoted as it demonstrated a good standard of antibacterial effects during the microbiological assays. The prepared hand sanitizers consistently produced higher zones of inhibition for S. aureus than E. coli. So, although the sanitizer is effective against both Gram-negative and Gram-positive species, it remains more virulent against the latter.
To elucidate the synergistic activity of the sanitizer, the results obtained were compared with similar research. The zone of inhibition of S. aureus, in a similar study, for 80% isopropanol was 10±1 mm (Thaddeus et al., 2018). The HS1 which had a total alcohol concentration of 80%, composed of 40% ethanol and 40%, produced a zone diameter of 23.5±0.7 mm. This shows that by combining ethanol and isopropanol, a higher zone of inhibition is achieved. From this it can be derived that although the total alcohol concentration was the same, the compounding of ethanol and isopropanol seems to have a synergistic effect. An analogous conclusion was also reached by Odebisi-Omokanye and his colleagues (2015).
The MIC for both bacterial strains was much less than the working concentration of the hand sanitizer, which is 85%, ensuring its high quality. The MIC of ethanol is 6.57% for E. coli and 8.75% for S. aureus (Mazzola et al., 2009; Penna, Mazzola and Martins, 2001). The MIC of HS2, a combination of ethanol and isopropanol, resulted in lower MIC’s for both bacteria suggesting a synergistic action. This validates the shift towards using both ethanol and isopropanol instead of only one in hand sanitizer products. This conclusion is noteworthy as we can use both alcohols, as opposed to one, to increase potency, instead of adding chemical antimicrobials like benzalkonium chloride. Alcohols do not induce resistance or cross resistance unlike chemicals which makes it more suitable for hand sanitizers that are used multiple times a day. Also isopropanol is less expensive when compared with ethanol so the combination we have developed and tested is affordable and can be produced in bulk without high production costs ensuring an affordable hand hygiene solution.
The HS2 was able to reduce the bacterial burden by achieving 4 log10 reduction (99.99% bacterial killing) which surpasses the FDA and European standards that require a minimum of a 3 log10 reduction (99.9% bacterial killing). In addition to this, it also proved to have a wide range of activity against lactose fermenters, oxidase and catalase producing bacteria as judged by the biochemical tests.
Glycerin was added as a humectant to minimize the drying effect alcohol has on our skin. The percentage of glycerin required, however is a heated debate as the increase in glycerin concentration impairs the antibacterial effects of the alcohol (Menegueti et al., 2019). Therefore, the WHO (2009) suggested concentration of 1.45% was utilized in the sanitizers.
In addition to being efficacious, hand sanitizers should also have appealing organoleptic properties to promote compliance with routine sanitization. There are many forms of sanitizers available; rubs, foams, gels and these variations can influence antimicrobial properties. A balance should be struck between efficacy of the sanitizer and user appeal in order to achieve optimum benefit.
Future Scope
There is a dearth of literature that analyses the antibacterial effect of alcohol combinations and further research can be based on more combinations with the inclusion of a varied range of alcohols. The study did not focus on other microbes like spores, viruses, fungi and the activity of the prepared hand sanitizers against these organisms remain inconclusive. This drawback can be addressed in extended studies. The study can be extrapolated to cover a wider range of alcohol combinations. The future experiments based on the premise of this study will incorporate the Centers for Disease Control and Prevention (CDC) recommended guidelines for consumers on alcohol-based hand sanitizer for a minimum of 60% ethanol, as a control to evaluate and compare the efficiency of the novel formulation.
Conclusion: The HS2 had better antibacterial activity in comparison to HS1. Ethanol and isopropanol show a synergistic antibacterial activity.
References
- Abbas SZ, Hussain K, Hussain Z, Ali R, Abbas T (2016) ‘Anti-bacterial activity of different soaps available in local market of Rawalpindi (Pakistan) against daily encoun- tered bacteria’ Pharmaceutica Analytica Acta, 7.
- Abualizadeh E, Bumah VV, Masson-Meyers DS, Eells JT, Hirschmugi CJ et al. (2017) ‘Understanding the antimicrobial activity of selected disinfectants against methicillin-resistant Staphylococcus aureus (MRSA)’ Public Library of Science One, 12.
- Berardi A, Perinelli DR, Merchant HA, Bisharat L, Basheti IA et al. (2020) ‘Hand sanitisers amid CoViD-19: a critical review of alcohol- based products on the market and formulation approaches to respond to increasing demand’ International Journal of Pharmaceutics, 584.
- Bondurant S, McKinney T, Bondurant L, Fitzpatrick L (2020) ‘Evaluation of a ben- zalkonium chloride hand sanitizer in reducing transient Staphylococcus aureus bacterial skin contamination in health care workers’ American Journal of Infection Control, 48:522-526.
- Bondurant SW, Duley CM, Harbell JW (2019) ‘Demonstrating the persistent anti- bacterial efficacy of a hand sanitizer containing benzalkonium chloride on human skin at 1, 2, and 4 hours after application’ American Journal of Infection Control, 47:928-932.
- Cao H, Wei D, Yang Y, Shang Y, Li G et al. (2017) ‘Systems- level understanding of ethanol-induced stresses and adaptation in E. coli’ Scientific Re- ports, 7.
- Cartner T, Brand N, Tian K, Saud A, Carr T et al. (2017) ‘Effect of different alcohols on stratum corneum kallikrein 5 and phospho- lipase A2 together with epidermal keratinocytes and skin irritation’ International Journal of Cosmetic Science, 39:188-196.
- Centers for Disease Prevention (2002) Guideline for hand hygiene in health-care settings: rec- ommendations of the healthcare infection control practices advisory committee, 51 (16) Atlanta: United States Epidemiology Program Office.
- Cheung H, Wong MMK, Cheung SH, Liang LY, Lam YW et al. (2012) ‘Differential actions of chlorhexidine on the cell wall of Bacillus subtilis and Escherichia coli’ Public Library of Science One, 7.
- Draelos DZ (2012) ‘Rethinking hand sanitizers’ Cosmetic Dermatology, 25:347-348.
- Dyrda G, Boniewska-Bernacka E, Man D, Barchiewicz K, Słota R (2019) ‘The effect of organic solvents on selected microorganisms and model liposome membrane’ Molec- ular Biology Reports, 46:3225-3232.
- Fierer N, Hamady M, Lauber CL, Knight R (2008) ‘The influence of sex, handedness and washing on the diversity of hand surface bacteria’ Proceedings of the National Academy of Sciences in the United States of America, 105:17994-17999.
- Food and Drug Administration (2020) Policy for the temporary compounding of certain alco- hol-based hand sanitizer products during the public health emergency immediately in effect guidance for the industry. Rockville: Centre for Drug Evaluation and Research.
- Forer Y, Block C, Frenkel S (2017) ‘Preoperative hand decontamination in ophthalmic surgery: a comparison of removal of bacteria from surgeons’ hands by routine antimi- crobial scrub versus an alcoholic hand rub’ Current Eye Research, 42:1333-1337.
- Goodarzi H, Bennett BD, Amini S, Reaves ML, Hottes AK et al. (2010) ‘Regulatory and metabolic rewiring during laboratory evolution of ethanol tolerance in E. coli’ Molecular Systems Biology, 6.
- Horinouchi T, Maeda T, Furusawa C (2018) ’Understanding and engineering alcohol- tolerant bacteria using OMICS technology’ World Journal of Microbiology and Biotech- nology, 34.
- Hu L, Zhou C, Li H, Zhang M, Xu W (2018) ‘Instantaneous response of bacteria to external stimuli monitored by syringe spray mass spectrometry’ Analytical Chemistry, 90:11417-11422.
- Huffer S, Clark ME, Ning JC, Blanch HW, Clark DS (2011) ‘Role of alcohols in growth, lipid composition, and membrane fluidity in yeasts, bacteria and archaea’ Applied and Environmental Microbiology, 77:6400-6408.
- Jing JLJ, Yi TP, Bose RJC, McCarthy JR, Tharmalingam N et al. (2020) ‘Hand sanitizers: a review on formulations aspects, adverse effects and regula- tions’ International Journal of Environmental Research and Public Health, 17.
- Kampf G (2018) ‘Biocidal agents used for disinfection can enhance antibiotic resistance in gram-negative species’ Antibiotics, 7.
- Kampf G, Kramer A (2004) ‘Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs’ Clinical Microbiology Reviews, 17:863-893.
- Kong HH, Segre JA (2017) ‘The molecular revolution in cutaneous biology: investigat- ing the skin microbiome’ Journal of Investigative Dermatology, 137:119-122.
- Man A, Gâz AS, Mare AD, Berta L (2017) ‘Effects of low-molecular weight alcohols on bacterial viability’ Revista Romana de Medicina de Laborator,
- Menegueti MG, Laus AM, Ciol MA, Basile-Filho MAA, Pires D et al. (2019) ‘Glycerol content within the WHO ethanol-based handrub formulation: balancing tolerability with antimicrobial efficacy’ Antimicrobial Resistance and Infection Control, 8.
- Morente EO, Fernández-Fuentes MA, Grande-Burgos MJ, Abriouel H, Pérez Pulido R et al. (2013) ‘Biocide tolerance in bacteria’ International Journal of Food Mi- crobiology, 162:13-25.
- Mazzola PG, Jozala AF, Novaes LCD, Moriel P, Penna TCV (2009) ‘Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents’ Brazilian Journal of Pharmaceutical Sciences, 45.
- Odebisi-Omokanye MB, Ahmed El-Imam AM, Arshad SO, Oke MA (2015) ‘Com- parative assessment of antibacterial efficacy of four popular hand sanitizers sold in Nigeria’ Fountain Journal of Natural and Applied Sciences, 4:1-9.
- Park J, Kim SJ, Lee JA, Kim SB (2017) ‘Microbial forensic analysis of human- associated bacteria inhabiting hand surface’ Forensic Science International, 6:510-520.
- Penna TC, Mazzola PG, Silva-Martins AM (2001) ‘The efficacy of chemical agents in cleaning and disinfection programs’ Journal of Infectious Diseases, 1.
- Pereira BMP, Tagkopoulos I (2019) ‘Benzalkonium chloride: uses, regulatory status and microbial resistance’ Applied and Environmental Microbiology, 85.
- Restrepo D, Laconi N, Alcantar NA, West LA, Buttice AL et al. (2015) ‘Inhibition of heparin precipitation, bacterial growth, and fungal growth with a combined isopropanol-ethanol locking solution for vascular access devices’ Journal of Pediatric Surgery, 50:472-477.
- Thaddeus NIN, Chukwemeka E, Jane OO, Obumneme AC, Okechukwu EC (2018) ‘Effects of some common additives on the antimicrobial activities of alcohol- based hand sanitizers’ Asian Pacific Journal of Tropical Medicine, 11:222-226.
- World Health Organization (2009) WHO guidelines on hand hygiene in health care. Geneva: World Health Organization.