| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Study of the Main Properties and Leach Behavior of Simulated MCCI Products
A S Aloy1, V I Almjashev2, V B Khabensky2, N F Karpovich1, T I Koltsova1, Y L Kretser1, P V Slastikhina1*, O V Bagryanov 3, S K Savin3
1RPA “V. G. Khlopin Radium Institute”, 194021, Saint Petersburg, Russia
2“Alexandrov Research Institute of Technology” (NITI), 188540, Sosnovy Bor, Leningrad Oblast, Russia
3JSC “Techsnabexport” (TENEX), 115184, Moscow, Russia
Received Date: March 15, 2022; Accepted Date: March 30, 2022; Published Date: April 05, 2022;
*Corresponding author: P V Slastikhina, RPA “V. G. Khlopin Radium Institute”, 194021, Saint Petersburg, Russia. Email : slastikhinapv@khlopin.ru
Citation: P V Slastikhina, A S Aloy, V I Almjashev, V B Khabensky, N F Karpovich, et al. (2022) Study of the Main Properties and Leach Behavior of Simulated MCCI Products. Enviro Sci Poll Res and Mang: ESPRM-120.
DOI: 10.37722/ESPRAM.202204
Abstract
The solidified product of melted core and concrete interaction (MCCI) was formed at the Fukushima NPP (1F), where the severe accident took place in March, 2011. In this study the structure, properties and leaching behavior of simulated MCCI samples has been performed. The solid model samples containing 40-70 wt. % of concrete components, (U,Zr)xOy and metallic (Cr, Ni, Fe) additives were obtained using induction melting in cold crucible (IMCC) at 2200-2500 ⁰C. The ratio of amorphous and crystalline phases as well as their composition has been evaluated. It was shown that initial structure of samples represented mainly amorphous silicate based matrix, in which crystalline solid solutions (U,Zr)xOy, Fe-Cr-Ni spinel and cristobalite (SiO2) were dispersed. Leach tests of previously characterized samples has been performed using deionized and nitride water with different pH = 4.01, 6.50, and 9.18 at temperature 25, 50, 90 and 120 ⁰C with exposure time till 84 days. Leaching kinetics was studied and composition of the secondary phases were detected. It was set that dissolution of silicate based phase was started at 25 ⁰C in deionized and nitride water forming secondary phases on the surface. Contrarily, such elements as U and Zr were detected in the solution only after 54 days leaching at 120 ⁰C. As a result of such severe condition the continuous surface layers from sedimental secondary phases were obtained and analyzed.
Keywords: Aging in Aqueous Solutions; Corium; Fukushima-Daiichi NPP; Leaching Rates; Molten Core Concrete Interaction Products (MCCI); Secondary Phases; Solid Solutions of Uranium and Zirconium Oxides; Structure
Introduction
After 10 years beyond design basis accident at the Fukushima Daiichi NPP (1F) the chemical and mechanical properties of solidified ex-vessel corium debris, representative product MCCI, are not well known [1, 2].
During tests in the Colima experimental facility ex-vessel corium, containing refractory oxides (UO2, ZrO2), steel and components of concrete (SiO2, Al2O3, CaO, Fe2O3), has been obtained. Experimental study of the different samples set on heterogeneous nature of them. Phase composition, microstructure of product, leaching rates also were investigated [3-6].
Nevertheless, from the point of debris defueling besides the physical and chemical properties of MCCI products, the amount of concrete erosion and formation of altered surface layers is important information.
The main goal of our study was to gain leaching data and morphology of altered layers as well as chemical composition of minerals deposited on their surface.
Experimental Part
Preparation of simulated MCCI products
For this study three simulated MCCI products representative different core-concrete ratio were selected in accordance with thermodynamic evaluation in [7].
Chemicals for batch preparation were taken in form pure oxides NiO, Cr2O3, FeO, SiO2, Al2O3 and ZrO2. Calcium was added in form preliminary synthesized compound CaZrO3. Nuclear depleted uranium dioxide (UO2) was used after grinding of the tablets till fraction less than 2 mm.
All three batches after mixing of the chemicals in needed proportion were thoroughly blended and melted in the IMCC [8].
Metallic zirconium was used as a starting material for initial inductive heating of the batches.
After heating up to 2200-2500 ⁰C under nitrogen atmosphere and aging ~20 minutes the melt was cooled with middle rate 3-4 ⁰C⁄sec. As a result, the solidified ingots weighting in 1.5-2 kg each, were produced. One of them before and after fragmentation is shown in Figure 1.

Figure 1: View of ingot before and after fragmentation.
Initial characterizations
Initial characterizations has been done using several centimeters size samples in order to collect preliminary information on: density, porosity, micro Vickers hardness, microstructure, the main chemical phases and the distribution of elements between the different phases. This initial information are needed for interpreting the leaching experiments, including secondary compounds formation on the sample surfaces.
The equipment and methods used for these initial characterization are named below.
Phase analysis was performed using X-ray diffractometer (XRD) D2 PHASER (Bruker, Germany) with CuKα radiation.
Scanning Electron Microscopy (SEM) observation coupled with Energy-Dispersive X-ray Spectroscopy (EDS) using a MIRA3 scanning electron microscope TESCAN was performed to study morphology, microstructure as well as elemental analysis.
The density and porosity measurements were made by hydrostatic weighting using analytical scales OHAUS Explorer E12140.
Micro Vickers hardness was determined using a Tinius Olsen microhardness tester at a load of 9.8 N, the loading time 10 s on the basis of 10-15 measurements of indentation prints.
Analysis of leaching solutions were performed by ICP-EOS (Perkin Elmer) PlasmaQwont mod. PQ 9000. Minimal detectable concentration of elements (U, Zr, Si, Al, Fe, Ca, Ni, Cr) was 10 µg⁄dm3.
Leaching protocol
The samples were weighted and surface area were measured by applying a film to the surface of the sample [9].
Leach tests were carried out using monolithical samples in accordance with MCC-1 method at 25, 50, 90 and 120 ⁰C in aerated deionized (pH≈6.50, Eh≈50 mV) and nitrided (pH≈6.50 Eh≈ - 20 mV) water with solution renewal after 1, 3, 7, 10, 14, 21 and 28 days [10]. Solution with pH=4.01 and 9.18 were prepared using standard buffers.
Nitrided water was obtained from deionized boiled and cooled water by bubbling with rate 50 l/h highly pure nitrogen at 4-5°C through it for 4 hours. The concentration in solutions as function of time, temperature and pH for all higher listing elements were measured by ICP-EOS.
Normalized mass loss-NL(i) g⁄cm2 and leach rates-RL(i) g⁄cm2‧day were calculated according to the following formulas:

where NL(i) – normalized loss of element i, g/cm2; RL(i) – leaching rate of element i, g/cm2‧day; mi – mass of element i in aqueous solution, g; S – surface area of sample, cm2; fi – fraction of element i in the sample.
Experimental Results and Discussion
Phase composition and microstructure
The three types of simulated MCCI products are shown in Figure 2 together with data of their density and micro Vickers hardness.

Figure 2: SEM observation for three simulated MCCI products 1 (a), 2 (b), 3 (c).
All samples represented in Figure 2 are very dense non-porous ceramics, which bulk density and micro Vickers hardness became bigger due to higher concentration of UO2. There are no visible pores, which were observed in for Colima 4 test [2].
Table 1 summarize the composition of their in terms of mass percentage (wt. %), according to SEM/EDS data.
Oxide
Number MCCI product
1
2
3
UO2
12.78
26.21
36.78
ZrO2
5.56
8.84
11.78
FeO
3.01
4.31
5.45
Cr2O3
0.75
1.04
1.40
NiO
0.30
0.56
0.60
SiO2
63.03
47.32
33.00
CaO
5.94
4.72
4.38
Al2O3
8.62
6.91
6.61
Corium : Concrete mass ratio
22:78
41:59
56:44
XRD analysis data pointed to formation of a number of crystalline phases and amorphous one. Solid solutions with a stoichiometry close to U0,95Zr0,05O2 and Zr0,88U0,12O2 as well as spinel like Fe(Fe,Cr,Ni)O2 and SiO2 in form of cristobalite were identified. Interaction of UO2 and ZrO2 with concrete’s additives was not detected because formation of uranium or zirconium silicates was not identified nowhere.
Microstructure, shown in Figure 3, illustrate the heterogeneous nature of the products under study. It can be seen from Figure 3, that phases, containing heavy elements (white) uniformly enough spread throughout amorphous matrix (grey).

Figure 3: SEM-BSE observation for three simulated MCCI products 1 (a), 2 (b), 3 (c).
For each three MCCI products the quantitative SEM/EDS analysis was carried out on several distinct spots. Ratio of different phases, based on the obtained results, are shown in Figure 4.
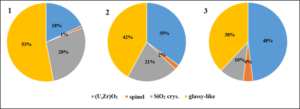
Figure 4: Ratio of phases which were formed in simulated MCCI products.
More detailed composition of the amorphous glassy-like phase in all three products are shown in Table 2, which demonstrates very close content of each chemical form everywhere.
Oxide
Number MCCI product
1
2
3
SiO2
71
70
68
CaO
12
12
13
Al2O3
15
15
15
Spinel
2
3
4
Total
100
100
100
As opposed to earlier data [11] silicate glassy-like phase containing dissolved uranium was not found.
Comparison of leach behavior in different conditions
Only elements from amorphous glassy-like phases (Si, Al, Ca) were detected in the leachates during tests at 25, 50 and 90 оС in deionized and nitrided water. No any amount of uranium, zirconium, nickel and chromium were found in the leachates.
Normalized weight losses of elements in total (Si, Al, Ca) and the only for calcium are shown in Figure 5.

Figure 5: Total normalized losses of Al, Si, Ca and only Ca in deionized (а) and nitrided (b) water (28 days, Т=90оС, pH=6.50).
As follows from Figure 5 it could be concluded that total mass losses has very close tendency and mainly characterized by calcium leaching in the same conditions.
Altered surface layer after leach test at 90 ⁰C for products 1, 2 and 3 in deionized water has differences in total amount. Figure 6 demonstrates that such layer became less when amount of amorphous glassy-like phase (grey) reduces from product 1 to 3.

Figure 6: SEM observations of surface products 1(a), 2(b), 3(c) after leaching in deionized water (pH=6.50, 28 days, 90 ⁰C).
Influence of pH solution on altered surface layer after 28 days at 90 ⁰C leaching in deionized and nitride water are shown in Figure 7. These SEM observations clear demonstrate the differences in morphology of altered layers, which depend on water pH and Eh.
Chemical compositions of all altered layers, according to SEM/EDS analysis are shown in Table 3.
рН
Oxide
Total
Al2O3
SiO2
CaO
Cr2O3
FeO
NiO
Deionized water (Eh≈50 mV), Product 2
Initial
11
78
7
1
4
1
100
4.01
6
85
4
0
5
1
100
6.50
7
70
2
2
20
0
100
9.18
6
64
2
0
28
0
100
Nitrided water (Eh≈ - 20 mV), Product 1
Initial
11
77
7
1
3
1
100
4.01
8
83
5
0
3
0
100
6.50
9
79
7
0
5
0
100
9.18
4
76
3
0
14
4
100
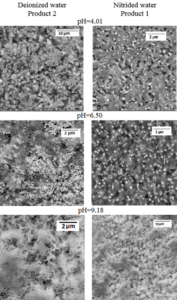
Figure 7: SEM observations of altered surface layers (pH=4.01, 6.50, 9.18; 28 days; 90 ⁰C).
Comparison the data in Table 3 indicates that pH value of water has more significant influence on alteration process than Eh.
The concentration of SiO2 in layers became higher in acidic condition, while at alkaline condition its amount became less. Amount of FeO was increased, probably, due to hydrolysis.
Leach test in deionized water (pH=6.50, 56 days, 90 ⁰C)
Total normalized mass loss for main elements (Si, Al, Ca) of amorphous glassy-like phase are shown in Figure 8.
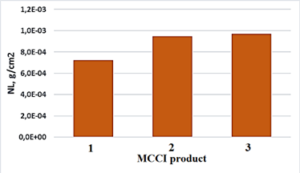
Figure 8: Summarized normalized mass loss of Si, Al, Si during 56 days in deionized water, pH=6.50, 90 ⁰C.
Results of SEM/EDS analysis of the altered surface layers for all three product are shown in Figure 9.
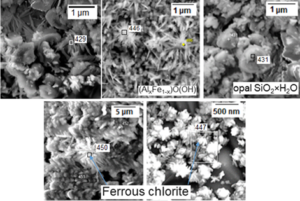
Figure 9: SEM observation of the surface after leach in deionized water (pH=6.50, 56 days, 90 °C).
Results of SEM/EDS analysis for different local spots, indicated on Figure 9, are presented in Table 4.
Spectrum number
O
Al
Si
Fe
Ni
Zr
U
Total
429
59.80
19.11
16.20
3.26
0.00
1.63
0.00
100
431
65.13
0.90
33,97
0.00
0.00
0.00
0.00
100
447
62.10
8.41
12.83
15.66
0.00
0.00
0.00
100
450
62.24
8.77
12,54
16.45
0.00
0.00
0.00
100
446
63.66
23.05
3.74
9.55
0.00
0.00
0.00
100
The secondary reprecipitated phases, as follows from Figure 9 and Table 4, could be identified as mineral formation kaolinite (spectrum 429), spherical formation of hydrolyzed silicon oxide SiO2·H2O (spectrum 431) and extended crystals of ferrous chlorites (spectrum 447 and 450).
The secondary reprecipitated phases containing any amount of uranium or zirconium were not observed at these conditions.
Leach behavior of the products under hydrothermal condition
Leach test under hydrothermal condition has duration of 84 days at 120 °C. Deionized water with pH=6.50 was used.
At these hard conditions even uranium and zirconium were detected in leachates, but in different time of renewal water. So, zirconium was founded after 49 days, while uranium only after 56 days.
Figure 10 demonstrates leach rates for concrete (Si, Ca, Al) and core (U, Zr) components.
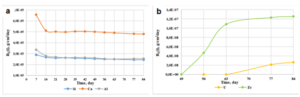
Figure 10: Dependence of the leaching rate of Si, Ca, Al (a) and U, Zr (b) on time for product 2.
The common tendency of leaching at the named hard condition to keep the same character as could be seen before. Maximum value belong to calcium, but silicon and aluminum have leach rates less by factor of two, approximately. Leach rates of zirconium and uranium after 84 days were equal 1.26·10-7 and 3.47·10-7 g/cm2·day, correspondingly. The time-table of leach rates dependence in Figure 10 indicates that they are close to residual rates, when solution becomes saturated and secondary minerals begin to form.
Such secondary minerals, which formed after 84 days, produced the continuous surface layer of secondary phases, which are shown in Figure 11.
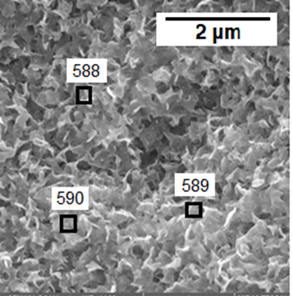 Figure 11: SEM observation of the surface after leach in deionized water for product 2 (pH=6.50, 84 days, 120 оС,).
Figure 11: SEM observation of the surface after leach in deionized water for product 2 (pH=6.50, 84 days, 120 оС,).
According to SED/EDS analysis (Table 5) the composition of this layer is practically the same and represents alumosilicates of iron and nickel. Uranium and zirconium were not found in the surface layer.
Spectrum number
O
Al
Si
Fe
Ni
Total
588
66.13
15.17
12.01
3.22
3.48
100
589
66.26
15.19
11.74
3.56
3,24
100
590
64.07
16.26
12.21
3.80
3.66
100
Conclusion
The structure of completely melted at 2200-2500 °C simulated MCCI products represents silicate-based amorphous glassy-like phase where solid solutions of (UxZry)O2, iron-chromium spinel and crystobalite in form SiO2 are distributed.
Leach behavior of MCCI products are practically the same in deionized and nitrided water with the most mass loss of calcium in acidic condition while of silicon and aluminum in alkaline media.
Leachates at 25, 50 and 90 °C after 28 and 56 days contained only Si, Ca, Al as a concrete’s components. Uranium and zirconium in leachates were detected after 56 days at 120 °C. Normalized masses loss after 84 days were equal 10-5 and 10-6 g/cm2 for zirconium and uranium correspondingly.
The secondary surface layers after leaching at 90 °C for 56 days were formed by compounds like (AlxFe1-x)O(OH), ferrous chlorite and opal SiO2·2H2O.
Any secondary phases, containing uranium and zirconium, were not detected at all testing conditions.
Acknowledgements
The study was carried out at the expense of the subsidy of the Mitsubishi Research Institute (Japan) No. NSU 51-18 dated 27.11.2019 for the implementation of the project “Decommissioning and treatment of contaminated water at the Fukushima Daiichi NPP”.
The authors are grateful to the persons from analytic laboratory A. A. Lumpov, N.V. Sapozhnikova, Yu. A. Naumova for the solutions analysis.
References
- Kitagaki T, Ikeuchi H, Yano K, Brissonneau L, Tormos B, et al. (2019) Effect of quenching on molten core-concrete interaction product, Journal of Nuclear Science and Technology 56:902-914.
- Nakayoshi A, Jegou C, De Windt L, Perrin S, Washiya T (2020) Leaching behavior of prototypical corium samples: A step to understand the interactions between the fuel debris and water at the Fukushima Daiichi reactors, Nuclear Engineering and Design 360:110522.
- Kitagaki T, Ikeuchi H, Yano K, et al. (2018) Characterization of the VULCANO test products for fuel debris removal from the Fukushima Daiichi nuclear power plant. Progress in Nuclear Science and Technology 5:217-220.
- Journeau Ch, Boccaccio E, Brayer C, Cognet G, Haquet J-F, et al. (2003) Ex-vessel corium spreading: results from the VULCANO spreading tests. Nuclear Engineering and Design 223:75-102.
- Journeau Ch, Piluso P, Haquet J F, Boccaccio E, Saldo V, et al. (2009) Two-dimensional interaction of oxidic corium with concretes: The VULCANO VB test series/ Annals of Nuclear Energy 10:1597-1613.
- Brissonneau L, Ikeuchi H, Piluso P, Gousseau J, David C, et al. (2020) Material characterization of the VULCANO corium concrete interaction test with concrete representative of Fukushima Daiichi Nuclear Plants, Journal of Nuclear Materials 528:151860.
- Kitagaki T, Yano K, Ogino H, Washiya T (2017) Thermodynamic evaluation of the solidification phase of molten core–concrete under estimated Fukushima Daiichi nuclear power plant accident conditions. Journal of Nuclear Materials 486:206-215.
- Almjashev V, Khabensky V, Krushinov E, Vitol S, Sulatsky A, et al. (2020) Studies carried out at the “Rasplav” platform of severe accidents research department of FSUE Alexandrov NITI, Lifecycle technologies of nuclear power plants 1-19. P. 54-73 (In Russian).
- Patent: RU 2078301 C1 (1997).
- Standard Test Method for Static Leaching of Monolithic Waste Forms for Disposal of Radioactive Waste, ASTM C1220-92, American Society for Testing and Materials, 1916 Race St., Philadelphia, PA, 19103 (MCC-1).
- Washiya T, Ogino H, Takano M, Yano K, Kaji N (2016) Approach to estimating fuel debris properties generated in Fukushima Daiichi NPS, The Proceedings of HOTLAB S1.