Publication Information
ISSN 2691-8803
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Gestational Diabetes Prevalence in North Italy.
Trend 2012-2019
Silvano Piffer1*, Riccardo Pertile1, Massimo Orrasch2, Francesca Zambotti2
1Servizio Epidemiologia Clinica e Valutativa, Azienda Provinciale per i Servizi Sanitari – Trento –Italy
2Centro Assistenza Diabetologica, Azienda Provinciale per i Servizi Sanitari, Trento – Italy
Received Date: August 21, 2021; Accepted Date: August 30, 2021; Published Date: September 07, 2021;
*Corresponding author: Silvano Piffer, Servizio Epidemiologia Clinica e Valutativa, Azienda Provinciale per i Servizi Sanitari – Trento –Italy, Centro per i Servizi Sanitari, Viale Verona, 38123 Trento (I). Email: piff.silv.tab@gmail.com
Citation: Piffer S, Pertile R, Orrasch M, Zambotti F (2021) Gestational Diabetes Prevalence in North Italy. Trend 2012-2019. Adv Pub Health Com Trop Med: APCTM-129.
DOI: 10.37722/APHCTM.2021301
Abstract
Purpose: The study aims to describe the temporal trends of prevalence of Gestational Diabetes Mellitus (GDM) in assisted pregnant women at hospitals in the province of Trento (North-East Italy) from 2012 to 2019, according to citizenship, age and educational level.
Methods: The diagnostic criteria of GDM used in the province of Trento are those of the International Association of Diabetes and Pregnancy Study Group (IADPSG). The pregnancy monitoring data are recorded in the Childbirth Assistance Certificate (CedAP), a mandatory document in Italy for monitoring pregnancy, childbirth and the health of the new-born and to be completed by the midwife who assisted the birth. Based on the data recorded in the CedAP, the trend in the prevalence of GDM over time was retrospectively analyzed, on all pregnant women, on pregnant women of Italian and foreign citizenship. The prevalence of GDM was also analyzed in relation to the age group and educational level of pregnant women.
Results: During the period 2012-2019, 35,577 pregnant women were assisted in the hospitals of the province of Trento. 1,950 cases of GDM have been recorded (244 cases on average per year), 1,143 in Italian women and 807 in foreign women. The average period prevalence of GDM for all pregnant women is 5.5% (95% CI 5.27-7.73); the average prevalence in Italian women is 4.30% (95% CI 4.06-4, 54), while in foreign women it is 8.80% (95% CI 8.23-9.37). The prevalence increases over time, both in Italians and in foreigners. Among the latter, Asian and African women have the highest values. The prevalence of GDM increases with the age of the mother and is also higher in pregnant women with low educational level.
Conclusion: A routine information flow, such as Cedap, if well managed, can provide useful data for a research activity on the GDM. The prevalence data of GDM reported in our study are consistent with European studies and inferior to previous Italian studies. The lower prevalence of GDM among pregnant women in the province of Trento, compared at least to national data, can be explained by a lower prevalence in the general population of subjects with known risk conditions, for example overweight and obesity. Foreign women and in particular those of Asian and African origin have the highest values and, at the same time, women of older age and with low educational level should be particularly considered in an overall control framework of the condition.
Keywords: Birth Attendance Certificate; Gestational Diabetes; Socio-demographic variables
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy [1]. The GDM must be distinguished from manifest diabetes in pregnancy, i.e. pre-existing pregnancy itself, also taking into account that specific diagnostic criteria must be applied to the two conditions [2]. Pregnancy involves profound and progressive alterations in maternal metabolism. GDM occurs when the activity of β cells is insufficient to overcome the insulin resistance induced by a series of hormones of placental origin and which is especially typical of the late stages of pregnancy [3]. For this reason, GDM generally arises in the second part of pregnancy and the optimal time for screening is the 24-28th week of gestation. Risk factors for the development of GDM are maternal age> 35 years, family history of diabetes, the occurrence of GDM in previous pregnancies, overweight / obesity, belonging to ethnic groups at greater risk. Additional risk factors are represented by cigarettes smoking and polycystic ovary syndrome [4]. The prevalence of GDM reported in various studies is around 7%, however with a wide range (1-25%) depending on the ethnic group analyzed and the diagnostic criteria used [5]. Recent studies indicate an increase in this prevalence over time, both in high and low-middle-income countries, possibly linked to the increase in obesity in women of childbearing age [6, 7, 8]. The occurrence of GDM involves greater obstetric and neonatal risks and affected pregnant women have a 50-65% risk of GDM recurrence in the subsequent pregnancy compared to those unaffected. A woman who has had gestational diabetes has also a 7 times higher risk of developing type 2 diabetes mellitus within 5-10 years of delivery than a woman with a normoglycemic pregnancy [9, 10]. An accurate and timely identification of the GDM, through a screening in pregnancy, would be useful for the management of the pregnancy itself, but also for the planning of the birth, the containment of adverse effects for the new-born and the scheduling of postpartum checks. Furthermore, screening and diagnosis of GDM have represented a historically controversial topic [11, 12] The HAPO (Hyperglycemia and Adverse Pregnancy Outcome) study [13, 14], as well as other studies [15, 16] have demonstrated the relevant effects of even mild maternal hyperglycemia on the incidence of maternal and fetal complications and such as screening, diagnosis and treatment of gestational diabetes can be cost-effective. Starting from these bases, the International Association of Diabetes and Pregnancy Study Group (IADPSG) in 2010 established in a consensus [17] the indications for the diagnosis of GDM that have been received by several countries including Italy. These new recommendations have been implemented in the province of Trento since 2011. This study reports the trend in the prevalence of gestational diabetes in assisted pregnant women at the hospitals in the province of Trento (North East Italy, 540,000 inhabitants) from 2012 to 2019, using the Childbirth Assistance Certificate (CedAP) as an information source.
Materials and Methods
The Italian Physiological Pregnancy guideline recommends a fasting plasma glucose check for all pregnant women at the first pregnancy visit (within 12 weeks of gestation), in order to identify women with pre-pregnancy diabetes. Later in pregnancy, gestational diabetes screening is performed if certain defined risk factors are present and using the 75g Glucose Oral Load Test (OGTT). In detail, this test is prescribed at 16-18 weeks of gestation in the case of a diagnosis of gestational diabetes in a previous pregnancy, or a pregravidic body mass index ≥30 kg/m2, or plasma glucose values between 100 and 125 mg/dL before pregnancy or early pregnancy. It is performed at 24-28 weeks if maternal age is 35 years or older, or if pregravid body mass index is ≥25 kg/m2, or if fetal macrosomia was diagnosed in a previous pregnancy, or if there is first-degree family history of diabetes, or if the pregnant mother’s origin comes from areas with a high prevalence of diabetes, such as South Asia, the Caribbean and the Middle East. The diagnosis is also made for a single value equal to or above the predetermined thresholds which are 92 mg/dL fasting, 180 mg/dL at 60 minutes and 153 mg/dL at 120 minutes [18]. In the province of Trento the glycemic monitoring data are recorded on the personal obstetric guide of each pregnant woman, which is updated on the occasion of periodic checks. These data are also registered in the CedAP informative flow which represents the national information document of reference for the registration of parental characteristics, monitoring of assistance in pregnancy and childbirth and the registration of the characteristics of the new-born [19]. Its completion is mandatory by health professionals (usually midwives assisting the birth). The provincial CedAP archive is annually made available to the Clinical and Evaluation Epidemiology Service of the Provincial Health Services Agency of the Province of Trento. On the basis of the data recorded in the CedAP, the birth cohorts 2012-2019 were retrospectively analyzed. The temporal trend of the prevalence of gestational diabetes was calculated, using the Cochrane-Armitage test for the evaluation of statistical significant trends. The trend was also analyzed according to citizenship and if foreign, in relation to the geographical area of origin. The prevalence of gestational diabetes was also analyzed in relation to the age group and educational level of pregnant women. The significance of the differences between the categories under comparison was tested with the Chi-square test. Prevalence estimates are accompanied by 95% confidence intervals (CI).
Results
During the period 2012-2019, 35,577 pregnant women were assisted at the hospitals in the province of Trento and of these only 4.4% are resident outside the province. The average proportion of pregnant women with foreign citizenship is 25.8% (26.2% in 2012, 24.9% in 2019). The average age of all pregnant women is 32, 33 in Italian pregnant women and 30 in pregnant women of foreign citizenship. 1,950 cases of GDM have been recorded (244 cases on average per year), 1143 in Italian women and 807 in foreign women. The average age of the overall cases is 34 years, 35 years in Italians and 32.5 years in foreigners. The average period prevalence of GDM for all pregnant women is 5.5% (95% CI 5.27-7.73), the average prevalence in Italian women is 4.30% (95% CI 4.06-4.54), and while in foreign women it is 8.80% (95% CI 8.23-9.37). The annual trend in the prevalence of GDM in all pregnant women and respectively in Italian and foreign women is shown in (Fig. 1). In both groups there is an increase over time, greater in foreigners than in Italians. The prevalence of GDM in foreign women is higher than in Italian women with a statistically significant difference (p <0.01). Among foreigners, the prevalence is higher in Asians, followed by Africans (Fig. 2). These two groups show a statistically significant difference compared to both Italians (p <0.0001) and all foreigners (p <0.001). The prevalence of GDM grows linearly with age, with a statistically significant trend (p <0.001) (Fig. 3). Prevalence increases over time in each age group, with a greater increase in the 35-39 and 40+ year classes and a smaller increase in the 25-29 year class (Fig. 4). The prevalence of GDM grows linearly with the reduction in the level of education with a statistically significant trend (p <0.001). It could be defined as a sort of watershed between those who studied up to 8 years and those who studied for more than 8 years (Fig. 5). The prevalence according to the qualification does not show significant changes from one year to the next.
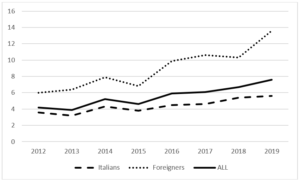
Figure 1: Province of Trento. Temporal trend of GDM prevalence. Italians, Foreigners and All pregnant women assisted at birth units. Period 2012-2019.
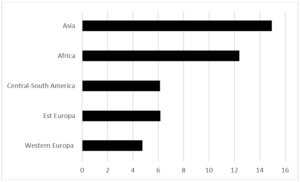
Figure 2: Province of Trento. GDM prevalence in Foreigners. According the geographical area of origin. Period 2012-2019.
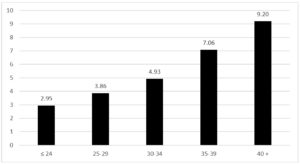
Figure 3: Province of Trento. GDM prevalence according the age class of women. Period 2012-2019.
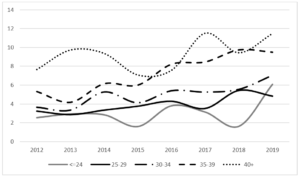
Figure 4: Province of Trento. Temporal trend of GDM prevalence for each age class. Period 2012-2019.
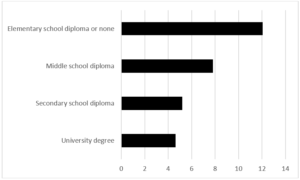
Figure 5: Province of Trento. GDM prevalence according the education level of women. Period 2012-2019.
Discussion
The data provided by a current information flow such as Cedap can be conveniently used for estimates of the prevalence of some conditions affecting the mother. This is the case, for example, with GDM.The CedAP information flow, as organized in the province of Trento, can be considered a reliable source in the registration of the frequency of the GDM, especially because the midwife, present at the birth, refers to the obstetric guide, a personal document that collects all the information relating to the pregnancy in progress and that the pregnant woman must carry with her during the periodic checks and at the time of delivery. The prevalence estimates of GDM reported in the present study increase over cohorts of subsequent births, in agreement with what is reported in the international literature. In part, this increase could be associated with an expansion in the prevalence of overweight and obesity in the population and in particular in women of childbearing age (5-7). A comparison between the data relating to the province of Trento and other areas can be problematic, also taking into account the persistence of a certain heterogeneity in diagnostic tests, in the reference information sources and the possible error generated by the presence of type 2 diabetes mellitus (T2DM) undiagnosed pre-pregnancy [20-23]. The chosen study period, however, protects us from possible problems of local variability in the diagnosis and classification of the condition considering that starting from 2011/2012 a criterion defined by an International Consensus Conference [17] and substantially consistent with the National Guidelines on Care for Physiological Pregnancy [18]. The prevalence of GDM reported in our study appears overall to be slightly lower than that reported by international studies [17, 24] and by previous national studies [25-30]. The lower prevalence of GDM among pregnant women in the province of Trento, compared at least to the national figure, can be explained in no small part by a lower prevalence in the general population of subjects with known risk conditions, for example overweight and obesity, as reported by the investigations of the National Institute of Statistics (ISTAT) and the PASSI (Progress of the Health Authorities for Health in Italy) surveillance system [31, 32]. Overall, the prevalence estimates reported by us are consistent with European data [33]. The prevalence of GDM is confirmed to be higher in older pregnant women and in those with low educational qualifications, aspects that can be correlated with the different socio-demographic stratification of known risk factors [4-7]. The data relating to age must be taken into particular consideration given that the maternal age at birth is gradually increasing and this leads to hypothesize a further increase in the prevalence of GDM in future birth cohorts. In particular, however, the prevalence of GDM is confirmed to be higher in women of foreign citizenship, especially in Asian and African ethnic groups [34]. The values found in these ethnic groups are consistent, albeit in the variability between individual surveys, with what is reported by studies carried out in Asian and African countries [35, 36]. In conclusion, foreign women, especially from Asia and Africa and women with childbirth age over 35, especially if overweight or obese, and women with low educational qualifications represent a significant share of GDM cases and therefore of subpopulations. at greater risk to be considered as a priority in public health action aimed at minimizing the risk of developing a GDM and its possible short and long-term consequences for the mother and child. Ad hoc programs for these higher risk subgroups should be developed and implemented before conception and during pregnancy. All health professionals active in the birth path should be involved.
Declarations
Funding: The authors did not receive support from any organization for the submitted work.
Financial interests: No financial interests were gained for this study.
Non-financial interests: No non-financial interests were gained for this study.
Conflicts of interest/Competing interests: All authors of this study have no conflicts of interest/competing interests.
Consent for publication: All authors agree to the publication of this study.
Bibliography
- Metzger BE, Coustan DR for the Organizing Committee. (1998) Summary and recommendations of the Fourth International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care. 21(Suppl 2):B161-B1671.
- Reece EA, Leguizamón G, Wiznitzer A (2009) Gestational diabetes: the need for a common ground. Lancet. 373:1789-97.
- Butte NF (2000) Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J ClinNutr. 71(5 Suppl):1256S-1261S.
- National Collaborating Centre for Women’s and Children’s Health. Diabetes in pregnancy. Management of diabetes and its complications from preconception to the postnatal period. RCOG Press, London, 2008.
- Lapolla A, Dalfrà MG, Ragazzi E et al. (2011) New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria: a retrospective study on pregnancy outcome. Diabet Med. 28:1074-1077.
- Feig Ds, Hwee J, Shah BR, Booth GL, Bierman AS et al. (2014) Trends in incidence of Diabetes in Pregnancy and serious perinatal outcomes. A large population based study in Ontario, Canada, 1986-2010. Diabetes Care. 37 1590-1596.
- Guariguata L, Linnenkamp U, Beagley J, Whiting D, Cho N (2014) Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 103:176-85.
- Goldenberg RL, McClure EM, Harrison MS, Miodovnik M (2016) Diabetes during Pregnancy in Low and Middle-Income Countries. Amer J Perinatol. 33:1227-1235.
- Group HSCR (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 358:1991-2002.
- Bellamy L, Casas JP et al. (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 373:1773-9.
- Waugh N, Royle P et al. (2010) Screening for hyperglycaemia in pregnancy: a rapid update for the National Screening Committee. Health Technol Assess. 14:1-183.
- Royal College of Obstetricians and Gynaecologists (RCOG). Scientific Advisory Committee. Opinion Paper 23. Diagnosis and treatment of gestational diabetes. RCOG Press, London, 2011.
- The HAPO Study Cooperative Research Group (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 358:1991-2002.
- The HAPO Study Cooperative Research Group (2010) Hyperglycaemia and adverse pregnancy outcome (HAPO) study: associations with maternal body mass index. BJOG. 117:575-84.
- Landon MB, Spong CY et al. (2009) A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 361:1339-48.
- Durnwald CP, Mele L et al. (2011) Glycemic characteristics and neonatal outcomes of women treated for mild gestational diabetes. ObstetGynecol. 117:819-27.
- Boyd E. Metzger (2010) International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 33:676-82.
- Sistema Nazionale Linee Guida. La gravidanza Fisiologica: Ministero della Salute, Istituto Superiore di Sanità e CeVEAS. Rome 2011.
- Ministry of Health. Birth attendance certificate. Ministerial Decree no. 349 of 16 July 2001.
- AMD e SID. Standard italiani per la cura del diabete mellito 2018.
- WHO (2013) Diagnostic criteria and classification of hyperglycemia first detected in pregnancy. WHO/NMH/MND/13.2 Geneva: WHO.
- Sovio U, Murphy HR, Smith GC (2016) Accelerated fetal growyhpior to diagnosis of gestational diabetes mellitus: A prospective cohort study of nulliparous women. Diabetes Care. 39:982-7.
- Siegel AM, Coxwell CA, Biggio JR, Tita A, Harper LM (2016) Impact of interval between screening and diagnosis of gestational diabetes on pregnancy outcomes. Am J Perinatol. 34:557-562.
- Seshiah V, Das A, Balaji V, Joshi SR, Parikh M et al. (2006) Gestational diabetes mellitus-guidelines. JAPI. 54:622
- Lapolla A, Dalfrà MG, Lencioni C, Di Cianni G (2004) Epidemiology of diabetes in pregnancy: a review of Italian data. Diabetes Nutr Metab. Dec, 17:358-67.
- Murgia C, Berria R, Minerba L et al. (2006) Gestational diabetes mellitus in Sardinia: results from an early universal screening procedures. Diabetes Care. Jul, 29:1713-4.
- Lapolla A, Dalfrà MG, Bonomo M et al. (2009) Gestational diabetes mellitus in Italy: a multicenter study; Scientific Committee of GISOGD Group. Eur J ObstetGynecolReprod Biol. Aug, 145:149-53.
- Corrado F, Pintaudi B, Di Vieste G, et al. (2014) Italian risk factor-based screening for gestational diabetes. J Matern Fetal Neonatal Med. Sep, 27:1445-8.
- Lacaria E, Lencioni C, Russo L et al. (2015) Selective screening for GDM in Italy: application and effectiveness of National Guidelines. J Matern Fetal Neonatal Med. 28:1842-4.
- Nicotra F, Molinari C, Dozio N et al. Screening for gestational diabetes in the Lombardy region: A population-based study. Diabetes Metab 2015 Sep, 41: 319-325.
- Indagini multiscopo sulle famiglie. Anno 2016. ast accessed December 2020.
- Sistema sorveglianza Passi. datiPassi 204-2016. Last accessed december 2020.
- Eades CE, Cameron DM, Evans JM (2017) Prevalence of gestational diabetes mellitus in Europe: a meta-analysis. Diabetes Res Clin Pract. 129:173-81.
- Hedderson MM, Darbinian JA, Ferrara A (2010) Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. PaediatrPerinatEpidemiol. Sep, 24:441-8.
- Mwanri AW, Kinabo J, Ramaiya K, Feskens EJ (2015) Gestational diabetes mellitus in sub-Saharan Africa: systematic review and metaregression on prevalence and risk factors. Tropical Med Int Health. 20:983-1002.
- Lee KW, Ching SM, Ramachandran V et al. (2018) Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy and Childbirth. 18:494-514.