Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
In Vitro Propagation of Acacia Hybrid from Axillary Buds
Pattama Tongkok1*, Jittraporn Chusrisom1, Pussadee Sukpiboon1, Eakpong Tanavat1, Pavina Badan1, Kasem Haruthaithanasan1, Tepa Phudphong1, Weerasin Sonjaroon2, Yuttana Banchong1 and Chatcharee Kaewsuralikhit3
1Kasetsart Agricultural and Agro-industrial Product Improvement Institute (KAPI), Kasetsart University, Bangkok, Thailand
2School of Integrated Science, Kasetsart University, Bangkok, Thailand
3Kasetsart University Research and Development Institute, Kasetsart University, Bangkok, Thailand
Received Date: April 10, 2021; Accepted Date: April 15, 2021; Published Date: April 22, 2021;
*Corresponding author: Pattama Tongkok, Kasetsart Agricultural and Agro-industrial Product Improvement Institute (KAPI), Kasetsart University, Bangkok, Thailand. Email: aappat@ku.ac.th
Citation: Tongkok P, Chusrisom J, Sookpiboon P, Tanavat E, Badan P, et al. (2021) In Vitro Propagation of Acacia Hybrid from Axillary Buds. Adv Agri Horti and Ento: AAHE-150.
DOI: 10.37722/AAHAE.2021401
Abstract
Acacia hybrid is a fast-growing tree species which plants in the forest plantation for energy in Southeast Asia, including Thailand. The efficient method for rapid propagation for forest plantation is tissue culture technology. A sterilization is one of the most important steps in tissue culture. The effects of type, concentration, and duration of disinfectants were examined. Axillary bud was used as explant for in vitro propagation. The procedures for sterilization were conducted six treatments and cultured in ½MS for 4 weeks. There was significantly different for all treatments in the percentage of survival, contamination, dead explants, and growth performance (P<0.05). The optimal sterilization procedure was rinsed in running water for 5 minutes +20% dishwashing liquids+70% (v/v) Ethanol for 30 seconds + 0.1% HgCl2 for 10 minutes and rinsing with sterile water for 4 times. The results showed 13.50±9.26% contaminated explants and 86.50±9.26% survived explants. The shoot length, leaf length and leaf number were 3.40±0.20 cm., 5.04±0.70 cm. and 3.3±0.48 no./explant, respectively. Axillary bud from sterilized explant was transferred to MS medium supplemented with 30 g/l sucrose+0.6 mg/l BAP+0.6 mg/l NAA+0.3 mg/l PVP for shoot multiplication. The maximum number of shoots after 8 weeks was 10.5±1.06 shoots/explant. Each microshoot was elongated and rooted on MS basal medium without plant growth regulators.
Keywords: Acacia, Axillary Bud; Disinfectants; Fast-Growing Tree; Tissue Culture
Introduction
Industrial forest plantations are playing an important role in the economic, social, environmental, and cultural lives (Thomson et al, 2018). There are many fast-growing tree species in Thailand which are endemic and exotic species, and have been using for energy purpose (Corvanich, 1982), for example Acacia hybrid. This species is a fast-growing tree species which grows in Indonesia, Malaysia, Thailand, Vietnam, and China (Rufelds, 1987, 1988; Doughty, 2000; FAO, 1982; Potts and Dungey 2004). The Acacia hybrid is a cross between Acacia mangium and A. auriculiformis which is a medium-sized tree that looks similar to A. mangium. The A. hybrid has been introduced to many countries and it is nowadays grown in Oceania and South-East Asia due to its rapid growth and high production. Moreover, this species is among the most useful of tropical trees with its good coppicing ability, and adaptability to a range of tropical environments. This tree can reach 8 to 10 m and 7.5 to 9.0 cm DBH. in 2 years (Kijkar, 2003; Kha and Ha, 2017; Maliwan et al, 2010). It has a slightly higher wood density (0.455 g per cm3), and is good for production paper production, medium density fiber board, oriented-strand board, furniture, and nitrogen-fixing (Kha, 2001; Nor Asmah, 2012). Therefore, it is the main species was recommended to grow in Thailand for industrial forest plantations (Kha et al. 1993; Maliwan, 2010).
Almost all of the Acacia hybrid in Thailand were collected from natural stands in public land and farmland. Their productivity is not enough to supply all factory and power plant in the country. Recently, plantation forestry of Acacia hybrid in Thailand supplies high-quality raw material for bioenergy, it can reduce the pressure on native forests. Forest plantation of Acacia hybrids is not only for reforestation and short – term harvest but also for increasing local people’s income, it has also contributed significantly to environmental improvement (Kha and Ha, 2017). In Thailand, increasing the forest productivity and the quality of wood products by A. hybrid has become increasingly important to the forest industry. In addition, this species is capable of increasing soil fertility by undergoing in a symbiotic association with Rhizobium. Therefore, it was planted among crops for alternative agriculture of agriculturist (Kha, 2001; Nor Asmah, 2012; Skolmen, 1986).
Propagation of this species has been carried out mainly from seed. However, seed collected from Acacia hybrid trees is highly variable. Therefore, plant tissue culture technology has a potential to overcome this problem where it allows efficient and rapid clonal propagation of many economically important species, especially Acacia hybrid. A sterilization is one of the most important steps in tissue culture. The aim of this work was to examine the optimal kind and concentration of surface sterilization method and establish an efficient propagation system for Acacia hybrid using tissue culture technique. In this work, we studied a surface sterilization methodology and in vitro propagation of A. hybrid. In vitro propagation of the Acacia hybrid using tissue culture technique have an importance for genetic engineering work, bioenergy research and forestry industry in Thailand.
Materials and Methods
Collection of Plant Materials
Branch from mother plant of Acacia hybrid was used as explant source. The branch was collected from the hedge orchard of Kasetsart Agricultural and Agro-industrial Product Improvement Institute (KAPI), Thailand. A branch selection method, the branch of A. hybrid for tissue culture must obtain 15-20 cm in height, straight form, and healthy leaves. Then using sharp knife to divide each branch (Figure 1).
The branch was washed under running tap water by a soft brush. The branch was cut into nodal segments (5 cm in length), and washed under running tap water for 5 minutes, and rinsed with distilled water. The nodal segments were soaked with 20% dishwashing liquids for 1 minute and washed 4-5 times in sterile distilled water. 
Figure 1: Branch selection from mother tree.
Specimen Sterilization
Nodal segments were sterilized with 70% (v/v) ethanol for 30 seconds. The alcohol was decanted by washing the explants with sterile distilled water and followed by surface sterilization with different concentrations of disinfectant.
The nodal segment was sterilized with different concentrations of Clorox® (5, 10, and 15%) (v/v) for 10 minutes and with different concentrations of mercuric chloride (HgCl2) (0.05 and 0.1% (w/v) for 5 and 10 minutes, separately (Table 2). Clorox® and HgCl2 solution prepared in autoclaved distilled water. Clorox® was used for first and second treatments. Mercuric chloride was used for surface sterilization in third treatment – sixth treatment. The solutions were removed after surface sterilization. The sterilized explants were thoroughly washed with sterile distilled water for four times under aseptic conditions in a laminar airflow cabinet.
Data on contamination, dead explant and survival percentages were recorded. The following formula has been applied for calculation of percentage.

The sterilized explants were placed on a sterilized petridish covered with autoclaved tissue paper to remove excess moisture present on the surface of explants before cultivation. A total of 480 explants were used in all treatments with ten replicates of one explant each for surface sterilization experiment.
- Treatment 1: 10% Clorox® (0.5 sodium hypochlorite) for 10 minutes + 5% Clorox® (0.25 sodium hypochlorite) for 10 minutes
- Treatment 2: 15% Clorox® (0.75 sodium hypochlorite) for 10 minutes + 10% Clorox® (0.5 sodium hypochlorite) for 10 minutes
- Treatment 3: 0.05% HgCl2 for 5 minutes
- Treatment 4: 0.05% HgCl2 for 10 minutes
- Treatment 5: 0.1% HgCl2 for 5 minutes
- Treatment 6: 0.1% HgCl2 for 10 minutes
Media Preparation
Modified ½MS (Murashige and Skoog, 1962) medium supplemented with 30 g/l sucrose +15 mg/l Riboflavin +0.7% (w/v) Agar was used for tissue culture after surface sterilization. The pH of medium was adjusted to 5.8 with 1 N HCl and 1 N NaOH. The culture medium was poured into the bottom of the glass culture bottles (6 cm in diameter and 11 cm height). All media were sterilized by autoclaving at 15 lbs pressure, 121°C for 20 minutes.
Each explant from all treatments was placed on the culture medium. The cultures were kept at 25±2°C in darkness and maintained in a clean culture room for 4 weeks. Shoot length, leaf length, and number of leaves in all treatments were measured and morphological changes were recorded at 7, 14, 21, and 28 days of cultures.
Shoot Multiplication From Axillary Buds
The axillary bud from microshoot was cultured on modified MS medium (Murashige and Skoog, 1962) supplemented with 0.5 mg/l BAP+0.6mg/l NAA+0.3 mg/l PVP to induce multiple shoots for 8 weeks. The medium was adjusted to pH 5.7 before autoclaving at 120 oC for 20 minutes. The medium was supplied with 0.7% (w/v) Agar. Twenty five milliliters of the medium was poured into a culture bottles. All cultures were maintained under a 16 h of photoperiod with luminous intensity of approximately 50 µmol m-2s-1 emitted from cool fluorescent tubes (standard culture conditions) at 25 ± 2 oC. Young plantlet regenerated from a microshoot after 4 weeks on MS medium free of PGRs and induce to elongation.
Statistical Analysis
Data were statistically analyzed using SPSS. One-way analysis of variance (ANOVA) was used. When significant differences in ANOVA were found, means were compared using the Tukey’s post-hoc test at the 5% probability level.
Results and Discussion
Surface Sterilization Technique
Results revealed that the different type, concentration, and duration of disinfectants for surface sterilization of Acacia hybrid had effect on contamination, dead explant, and survival percentages of explants. There was significantly different all treatments in the percentage of contamination, death, survival, and growth performance on ½ MS media after 4 weeks (P<0.05). The present finding revealed that the optimal surface sterilization procedure of Acacia hybrid was sixth treatment, 70% (v/v) Ethanol for 30 seconds + 0.1% HgCl2 for 10 minutes and rinsed in 4 changes of sterile distilled water.
The result showed that using 0.1% HgCl2 for 10 minutes was the best result (T6). After 4 weeks of cultures, it provided the highest of survival percentage (86.50±9.26). The percentage of contamination in this treatment was 13.50±9.26 (Table 1). The shoot length, leaf length, and number of leaves after sterilization with this method was 3.40±0.20 cm, 5.04±0.70 cm. and 3.3±0.48 no./explant, respectively (Table 2). Microshoot was observed after 3 days and the leaf length of microshoot tends to increase since then (Figures 2, 3). Fourth treatment showed that the shoot length, leaf length, and number of leaves of explant was 3.40±0.21 cm, 5.03±0.69 cm. and 3.1±0.31 no./explant, respectively. The results showed that using HgCl2 for 10 minutes was the best result. However, the result showed that the contamination of explant was no different when used 0.05% and 0.1% HgCl2 for 10 minutes. While explant from third treatment to fifth treatment which use HgCl2 for 5 minutes for surface sterilization was more infectious than 10 minutes (Table 2). However, the growth of explant after 4 weeks from use of HgCl2 to sterilize (treatments 3-6) were not significantly different. First and second treatments showed that the shoot length, leaf length, and number of leaves were less developed than others (Table 2). The percentage of contaminated explants from use of Clorox® was extremely high (12.50±8.33) (Table 1). for 10 minutes. for 10 minutes. for 10 minutes. for 5 minutes. for 10 minutes. for 5 minutes. for 10 minutes. + 5% Clorox® + 10%Clorox®
Treatments
First sterilization
Second sterilization
%Survived explants
%Contaminated explants
%Dead explants
T1
10% Clorox®
5% Clorox®
12.50±8.33d
87.50±8.33d
0
T2
15% Clorox®
10% Clorox® for 10 minutes.
30.00±6.45c
70.00±6.45c
0
T3
0.05% HgCl2
–
72.50±7.90b
27.50±7.90b
0
T4
0.05% HgCl2
–
80.00±8.74ab
20.00±8.74ab
0
T5
0.1% HgCl2
–
76.50±12.34b
23.50±12.34b
0
T6
0.1% HgCl2
–
86.50±9.26a
13.50±9.26a
0
*Means±SD values within a column followed by the different superscript letters are significantly different (p<0.05)
Treatments
Sterilizing agents
Shoot length (mm)
Leaf length (mm)
Leaves (no./plant)
T1
10% Clorox®
3.31±0.16b
4.68±0.48 b
3.0±0.47 a
T2
15% Clorox®
3.20±0.09c
3.74±0.38 c
2.6±0.69 b
T3
0.05% HgCl2
3.41±0.17a
5.04±0.67 a
3.2±0.42 a
T4
0.05% HgCl2
3.40±0.21a
5.03±0.69 a
3.1±0.31 a
T5
0.1% HgCl2
3.44±0.23a
5.10±0.71 a
3.1±0.56 a
T6
0.1% HgCl2
3.40±0.20a
5.04±0.70 a
3.3±0.48 a
*Means±SD values within a column followed by the different superscript letters are significantly different (p<0.05)
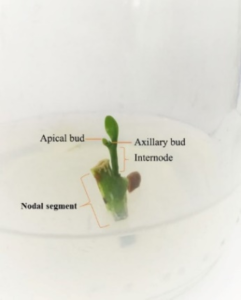
Figure 2: Acacia hybrid on ½ MS medium after surface sterilization using 0.1% HgCl2 (sixth treatment) for 1 week in darkness.
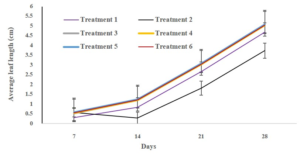
Figure 3: Leaf development of Acacia hybrid after surface sterilization for 28 days (4 weeks).
This sterilization procedure with method from sixth treatment could reduce sterilization process rang from 2-3 steps. The report showed that the surface sterilization of Acacia chundra with 0.1% HgCl2 for 20 minutes was highly antimicrobial (Rout et al., 2008). Ismail et al (2016) reported that surface sterilization of A. auriculiformis had to use both HgCl2 and Clorox®. Furthermore, the surface sterilization of A. auriculiformis had many procedures, the explant was soaked in 0.1% Benlate (fungicide) combination with 1% Boric acid for 24 hours before sterilized with 0.1% HgCl2 for 10 minutes (first sterilization) and 10% Clorox® for 5 minutes (second sterilization). Although, mercuric chloride is highly antimicrobial, with action against both bacteria and fungi, it should be used at 0.05 – 0.1% for 5-10 minutes (Rathore et al., 2012; Banerjee, 2013; Monteuuis et al., 2013; Nagashree et al., 2015). The sterilization studies of Acacia sp. in many reports showed use of HgCl2 for surface sterilization to reduce tissue loss and to achieve good sterilization results, although there are many toxic compounds. Therefore, the optimal surface sterilization technique of Acacia sp. does not depend only on the kind or concentration of the disinfectant, but also on the preparation method of explants to clean up before sterilized with disinfectants. Therefore, use of HgCl2 at low concentrations (0.05- 0.1%) is sufficient for the surface sterilization of Acacia hybrid. This may be the reason why some reports prefer to use HgCl2 at 0.05-0.1%. However, mercuric chloride should really be avoided, several research laboratories around the world have completely banned its use because it is highly toxic to nearly every other organism.
Shoot Multiplication From Axillary Buds
In vitro propagation of Acacia hybrid from axillary bud collected from explant which sterilized with surface sterilization technique from sixth treatment. New shoot was initiated on ½ Murashige and Skoog (MS) basal medium for 4 weeks in dark condition. The maintenance of shoot cultures in darkness increases the shooting rates. However, when the shoot was maintained in the medium more than 4 weeks, its axillary buds became brunette and later turned brown. Therefore, the Acacia hybrid tissue should be subcultured within 4 weeks.
Axillary bud was developed and maintained by regular subcultures every 4 weeks onto modified MS culture medium supplemented with 30 g/l sucrose+7 g/l agar+0.6 mg/l BAP+0.6 mg/l NAA+0.3 mg/l PVP (Figures 4, 5). The results of shoot multiplication from axillary bud were observed after cultivated for 8 weeks in light condition (Figure 5). Microshoot was produced by axillary buds. The culture showed 100% multiple shoot formation after culture, 10.5±1.06 shoots/explant. The average multiplication rates were 2–3 shoots every 2 weeks. This procedure enhances the capacity for shoot multiplication by axillary bud. The elongated and rooted microshoots from axillary bud were induced on MS medium free of PGRs for 4 weeks (Figure 5D).
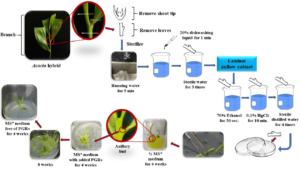
Figure 4: Procedure of the optimal method for in vitro propagation of Acacia hybrid through axillary buds.

Figure 5: In vitro propagation from axillary buds. (A) the culture after 2 weeks, (B) multiple shoot formation after 4 weeks, (C) shoot multiplication from axillary bud after 8 weeks, (D) the elongated and rooted microshoot on MS medium free of PGRs for 4 weeks.
Conclusion
The optimal surface sterilization procedure of Acacia hybrid was rinsed in running water for 5 minutes +20% dishwashing liquids+70% (v/v) Ethanol for 30 seconds + 0.1% HgCl2 for 10 minutes and rinsing with sterile water for 4 times. Multiple shoot induction after cultivating for 8 weeks on MS medium supplemented with 30 g/l sucrose+0.6 mg/l BAP+0.6 mg/l NAA+0.3 mg/l PVP.
Acknowledgement
We acknowledge that the research was funded by Kasetsart University Research and Development Institute (KURDI) and Kasetsart Agricultural and Agro-industrial Product Improvement Institute (KAPI).
References
- Banerjee P (2013). Rapid in vitro propagation of Acacia auriculiformis on solid and liquid media: role of organic additive, antioxidant and plant growth regulators. Cibtech J. Bio-Protocols. 2:39-49.
- Corvanich A (1982). Fast growing tree species and concept for forest plantation in Thailand. Forest of Thailand Association, Bangkok.
- FAO (1982). Seed sources establishment and tree improvement project, Sabah, Malaysia. In Variation in Acacia mangium (ed. L. Pedley) 41-42.
- Ismail HS, Kumar M, Aziah MY, Hasnida NH (2016) In Vitro micropropagation of Acacia auriculiformis from selected juvenile sources. Dendrobiology 75:157-165.
- Kha LD, Nguyen DH, Nguyen VT (1993). Natural hybrid between Acacia mangium and Acacia auriculiformis (in Vietnamese). Forest Journal 7:18-19.
- Kha LD (2001). Studies on the use of natural hybrids between Acacia mangium and Acacia auriculiformis in Vietnam. Agriculture Publishing, Hanoi.
- Kha LD, CE Harwood, ND Kien, BS Baltunis, ND Hai, et al. (2012). Growth and wood basic density of Acacia hybrid clones at three locations in Vietnam. New Forests. 43:13-29.
- Kha LD, HT Ha (2017). Research and development of Acacia hybrids for commercial planting in Vietnam. Vietnam Journal of Science, Technology and Engineering. 59:36-42.
- Kijkar S (2003). Part II-Species Descriptions Acacia hybrid ( mangium x A. auriculiformis).4 April 2021, 250-252.
- Maliwan H, K Haruthaithanasan, E Thanawat, S Phromlert, A Baysangchan (2010). The potential of Leucaena leucocephala, Eucalyptus camaldulensis, Acacia mangium and Acacia spp. (mangium x auriculaeformis) as plantation crops for energy. In Proceedings of 48th Kasetsart University Annual Conference: Plants, 3-5 February 2010, Kasetsart University, Bangkok.
- Marashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15:473-497.
- Monteuuis O, Galiana A, Goh D (2013). In Vitro propagation of Acacia mangium and mangium xA. auriculiformis. In: Maurizio L. (Eds.). Protocols for Micropropagation of Selected Economically-Important Horticultural Plants, Methods in Molecular Biology. Springer. NY, USA. 199-211.
- Nagashree BR, Santosh Kumar HS, Gurumurthy BR, Nataraja Karaba N, Shivannai MB (2015). PCR-based RAPD technique to determinutese induced salinity tolerance in vitro in Acacia auriculiformis. Plant Biosyst. 149:15-23.
- Rathore JS, Rai MK, Shekhawat NS (2012). Induction of somatic embryogenesis in gum arabic tree [Acacia senegal (L.) Willd.]. Physiol. Mol. Biol. Plants. 18:387-392.
- Rout GR, Senapati SK, Aparajeta S (2008). Micropropagation of Acacia chundra (Roxb.) DC. Hort. Sci. (prague) 35:22-26.
- Skomen RG (1986). Acacia (Acacia koa Gray). In: Biotechnology in Agriculture and Forestry, vol Tree 1. Springer, Berlin 375-383.