Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
The Role of the Microbial Enhanced Protein ME-PRO® in Recirculation Aquaculture Systems (RAS) Using Precision Feeding
Brandon White1, Luke Fredrickson1, Oliver Araujo2, Napoleon Araujo2, Sergio F Nates1*
1Prairie AquaTech, Brookings, SD, USA
2Sumacua, Choluteca, Honduras
Received Date: May 01, 2020; Accepted Date: May 06, 2020; Published Date: May 15, 2020
*Corresponding author: Sergio F Nates, Prairie AquaTech, 705 32nd Ave S, Brookings, SD 57006, USA. Tel: +16056921266; Email: sergio@prairieaquatech.com
Citation: White B, Fredrickson L, Araujo O, Araujo N, Nates SF (2020) The role of the Microbial Enhanced Protein ME-PRO® in Recirculation Aquaculture Systems (RAS) Using Precision Feeding. Jr Aqua Mar Bio Eco: JAMBE-105.
Abstract
Direct effects of special ingredients such as fermented co-products can provide significant amounts of biologically active factors that can increase gut micro-biota, reduce intestinal-inflammation and boost metabolic processes for improved animal health. Changes in production technology and marketing and changes in feed ingredients are key structural transformations necessary for the aquaculture sector to grow. Supplementing aquaculture feeds with “functional” ingredients represents the largest opportunity towards improvement and optimization of Recirculation Aquaculture Systems (RAS). The experimental diets formulated with the microbial enhanced protein ME-PRO® showed no decrease in digestibility as compared to any reference diet or commercial feeds tested, indicating that ME-PRO® features replacing fish meal did not affect the physiological status of particular fish and shrimp species.
Introduction
The rapid growth of aquaculture worldwide has become increasingly dependent upon the use of external feed inputs, and in particular upon the use of compound aquafeeds. Pressures to reduce fishmeal consumption for sustainability reasons, combined with economic reasons, require intensive research efforts to find candidates for fish meal replacement [1]. A Microbial Enhanced Protein ME-PRO® is one of the leading alternative protein sources to replace fishmeal in aquaculture feeds.
Feed cost is by far the greatest input cost in aquaculture production and improving nutrient efficiency has great impact on profitability [2]. In aquaculture operations, feeding programs are designed to optimize population responses while minimizing feed costs. However, nutrient requirements vary greatly between aquaculture species and for each species, these requirements change over time depending on growth patterns.
Precision feeding is offered as a critical method to improve the utilization of dietary protein and other nutrients, therefore decreasing feed costs and nutrient excretion. Precision feeding requires true knowledge of the nutritional value of feedstuffs as well as animal nutrient requirements. Furthermore, the formulation of diets must be in accordance with environmental constraints and account for the steady changes of the dietary nutrient supply to match the requirements of the animals [3]. This technique is based on the fact that animals within a tank, a grow-out pond or RAS differ from each other in terms of “production potential” and therefore, each has different nutrient requirements. Precision feeding incorporates the use of feeding techniques that allow the right amount of feed with the right composition to be provided at the right time to each animal [4].
After the nutritional potential of feed ingredients has been precisely determined, the addition of environmental objectives to the traditional feed formulation algorithms can promote sustainability by reducing nutrient excretion with only small increases in feeding costs. Increasing the number of feeding phases can contribute to significant reductions in nutrient excretion and feeding costs. These techniques have been used in other terrestrial animal production systems for years with much success. However, the use of precision feeding techniques in which the aquatic species are fed with tailored diets can further improve the efficiency with which fish or shrimp utilize dietary nutrients. Using a daily formulation approach, it has been estimated that feeding costs can be reduced by more than 5% and nitrogen and phosphorus excretion can both be reduced by more than 40% [2].
Supplementing aquaculture feeds with “functional” ingredients represents the largest opportunity towards improvement and optimization of RAS. Physical characteristics of such ingredients include high viscosity, which can improve fecal material stability, low dust and small particle size, both of which can improve water quality. Some “functional” ingredients, such as fermented co-products, also offer significant amounts of short-chain peptides and free amino acids that confer excellent attractability and palatability properties [5]. The presence of biologically-active factors can increase gut micro-biota, reduce intestinal-inflammation and boost metabolic processes for which lead to improved animal health [6].
Instead of the conventional method of growing fish and shrimp in open-water cages, ponds or raceways, recirculation aquaculture systems (RAS) can be used to rear any species of aquatic animals, freshwater or marine, in a closed system with a “controlled” environment [7]. RAS provides optimal environmental conditions, contributes to animal welfare and minimizes the feed conversion rate; hence improving feed efficiency. These changes in production technology, along with changes in feed ingredients, are key structural transformations necessary for the aquaculture sector to grow.
The increased interest in land-based culture methods and reduced use of fishmeal in aquaculture diets has generated the need to evaluate new and more environmentally friendly closed containment systems that utilize RAS technology. There are several factors that are relevant when developing RAS-specific diets. Key factors include: the digestibility and utilization of feed ingredients, consistency of the pellets, optimizing water stability and the composition of the feeds which can affect the removal efficiency of particles in the RAS. Additionally, the leaching of oils from the pellets can reduce feed attractability& palatability [8], which leads to uneaten pellets and interferes with protein skimmers, which play an essential role in the mechanical filtration process.
Material and Methods
Rainbow Trout Feeding Trials
Long and short-term feeding trials were completed using multiple lots of Rainbow trout (Oncorhynchus mykiss). Fish in all trials were fed either a commercial control feed, or feeds utilizing high inclusion (25-35%) of ME-PRO®. All feeds were formulated using commercial feed formulation software and manufactured using commercial extrusion methods. All chemical analysis (proximate analysis and mineral composition) of feeds were analyzed using 3rd party laboratories (Midwest Laboratories, Omaha, NE).
All chemical analysis (proximate analysis and mineral composition) of feeds were analyzed using 3rd party laboratories (Midwest Laboratories, Omaha, NE).
Grow out trials (~average individual initial weight 185 g – 1000g harvest weight) were conducted using a RAS composed of 3.41 m3 (900 gal) tanks. The RAS consisted of Cornell-style dual drain tanks, solids settling tanks, mechanical drum filtration, moving bed bioreactor (MBBR), UV sterilization, chilling, and oxygen injection. Water quality was monitored on a daily basis to ensure all parameters were within acceptable ranges.
At the day-0 starting sample, all fish were group-weighed by tank to determine biomass, and a sample of all the fish in 1 tank per treatment were anesthetized using 80 mg/L MS-222 and measured for fork length and weight. Group weights and identical individual fish sampling was completed at 3-week intervals throughout the 27-week trial. At week 16, 1 fish per tank (six fish per treatment) were sampled for general health evaluation including spleen, liver, visceral fat, and hematocrit.
Shrimp Feeding Trials
Larvae Development Trials
Approximately ninety million nauplii (Stage 5) of whiteleg shrimp Litopenaeus vannamei (Boone) were produced at Sumacua’s hatchery Choluteca, Honduras, and transferred to tanks containing 20 metric tons of salt water (28 ppt) in which all the experimental trials were conducted. Shrimp larvae were fed one of four diets containing ME-PRO® (inclusion levels up to 70%) from Zoea 3 to Postlarva13 (PL13). Survival (%) was determined from extrapolation of aliquots in which all surviving larvae were counted at the end of the trial.
Growth out Trials
Pacific white leg shrimp (L. vannamei) (mean, 1.6 g) were randomly stocked (n=700) at a density of 20 animals per tank. Each treatment was randomly assigned to 5 replicate tanks. The experimental diets were offered 3 times per day (0800, 1200, and 1600 hr) for 42 days. All shrimp were fed the same ration of a control diet prior to the start of the trial. Upon the start of the trial (Day 1) shrimp were offered experimental diets and fed to apparent satiation. Total feed consumption was used to estimate feed conversion ratio. Tank biomass was recorded when stocking of shrimp occurred (Day 0) and again at 3-week intervals until completion of the trial. Total tank biomass measurements were used to calculate relative growth (RG), specific growth rate (SGR), and biomass gain over the course of the trial.
The experiment was conducted in an 8,246 L recirculating aquaculture system (RAS). The system was equipped with 35, 190 L semi-square tanks each equipped with a recirculating drain which withdrew water from the subsurface and a sludge drain which was affixed to the lowest point in the bottom at the center of the tank. Each tank had forced air diffusers fed by a blower, a water inlet flow bar that controlled the direction of current, and covers, which provided darkness to half of the tank and netting, which allowed light to penetrate the other half of the tank. The RAS was also equipped with a centrifugal water pump, bead filter, UV filter, biofilter, 3 solids settling sumps, clarifying sump, water inlet float valve, and a heater/chiller unit. The RAS replacement water was sourced with well water. Water flow to each tank was maintained at 6 to 7 L min-1 and water temperature was maintained between 28 and 30°C. Dissolved oxygen was maintained above 5.0 mg L-1, pH was held between 7 and 8, and salinity was maintained at 22 ppt throughout the experiment. Temperature, dissolved oxygen, and pH were monitored daily (0800 hr) while ammonia (NH3) and nitrite (NO2) were monitored weekly.
Challenge Trial
A trial was conducted to determine the effectiveness of diets containing ME-PRO® in mitigating the severity and impact of the Early Mortality Syndrome / Acute Hepatopancreatic Necrosis Disease (EMS/AHPND) in shrimp. The trial was conducted at the ShrimpVet Laboratory in Ho Chi Minh City, Vietnam and lasted 33 days including an adaption period for one day, twenty-one days of feeding period, one day of challenge, and following ten days of post-challenge.
Trials were carried out in 120L plastic tanks. All tanks were outfitted with an activated coral biological filter, aeration and covered with plastic cap to reduce the risk of cross contamination. Brackish water of 20 parts per thousand (ppt) of salinity was utilized in each trial. Shrimp were fed ad libitum with their respective diets with four meals per day during the trial. Feed consumption was recorded during the trial. Test diets included one basal diet and six treatment diets that contained ME-PRO® (inclusion levels 10-30%). Feeding amount was adjusted depending on the biomass and actual feed consumption. Water quality parameters such as dissolved oxygen (DO), pH, and temperature were measured daily. Total ammonia nitrogen, nitrite, and alkalinity were measured twice a week.
Specific pathogen free (SPF) shrimp (Litopenaeus vannamei) were utilized in this trial, with the original genetics obtained from Hawaii broodstock that were checked for important pathogenic agents including Enterocytozoon hepatopenaeid (EHP), Whitespot syndrome virus (WSSV), Taura syndrome virus (TSV), Infectious myonecrosis virus (IMNV), and EMS/AHPND disease using PCR technique. Nauplii were reared in a strict biosecurity facility. Post-larvae were also checked again for important pathogenic agents including EHP, WSSV, TSV, IMNV, and EMS/AHPND disease using PCR technique. Post-larvae were grown in biosecurity conditions. One day prior to start the study, shrimp were weighed in-group to determine initial weight. The initial average shrimp weight was 0.56 ± 0.04 gram.
An immersion challenge method was also used in this trial. Treatment tanks and positive controls, with total of 28 tanks were subjected to an immersion challenge. Tryptic Soy Broth +2% sodium chloride (TSB+) inoculated with a consistently virulent strain of Vibrio parahaemolyticus, incubated for 24 hours. The bacterial suspension was added into tanks to achieve the bacterial density measured by optical density absorbance (OD600 nm) at the density is expected to kill 90% in the positive control “LD90” within 10 days. Negative control (4 tanks total) was treated with sterile TSB+ added directly to the tanks. The challenge dosage was 3.25×105 CFU/mL which was lethal dose 90% (LD90). Standard histopathology, H&F stain was conducted in tissues of shrimp.
Results & Discussion
Recent studies developing practical diets for RAS operations using a microbial enhanced protein, ME-PRO®, have shown to be a promising solution for the production of eco-friendly aquaculture feeds. Besides having over 70 percent crude protein content and highly available phosphorus content (Table 1), ME-PRO® is manufactured from non-GM soybeans, which makes it a perfect match for the European aqua industry. The protein is processed at a state-of-the-art plant using non-GMO soybean meal and a natural occurring, non-toxigenic, fungi, Aureobasidium pullulans.
Results from numerous feeding trials has demonstrated that ME-PRO® can sustain fish and shrimp health, high-performance growth and feed efficiency with inclusion levels at super-dose levels as high as 70% of the total amount of ingredients in the diet. Feeding trials using Rainbow trout, Barramundi and Coho salmon have shown that fish fed ME-PRO® based feeds consistently utilized feed more efficiently than the fish fed the control feed. Studies conducted using these species reared in a RAS system displayed good growth rates when fed diets containing ME-PRO® at levels up to 25% and reduced feed conversion rates.
Fish Feeding Trials
ANCOVA results for multiple trials indicate that the control fish were significantly smaller and affected by both treatment (P=0.002) and week (P<0.001). In addition, there was no significant difference in FCR between the treatments or weeks (Figure 1). An analysis of covariance (treatment x week) revealed the vast majority of differences coming from the week rather than treatment feed. However, there were significant differences in average weight per fish for both treatment (P=0.046) and week (P<0.001) indicating fish fed the ME-PRO® based feed were consistently larger than the fish fed the control feed. There were also significant differences in FCR for both treatment (P=0.001) and week (P<0.001) indicating fish fed the ME-PRO® based feed consistently utilized feed more efficiently than the fish fed the control feed. The overall results of this study are similar to other experiments feeding fermented soybean meals to rainbow trout. Similarly, enteritis was not observed in these studies and no negative effects were found in the distal intestine of the rainbow trout fed ME-PRO® based feeds [9-12].
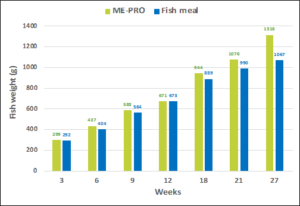
Figure 1: Final weight (g) of Rainbow trout fed diets containing ME-PRO® (25%) vs. fish meal diets.
In a second and similar trial, fish fed the ME-PRO® based feeds had significantly and consistently lower FCR (~1.0) than the control feed (~1.25) (Figures 2a, 2b). ANCOVA revealed a significant influence of the treatment (P<0.001) as well as week (P<0.001). Differences in average weight per fish were attributed to week (P<0.001) and not different between treatments (P=0.305). There were no significant differences in K-value (P=0.758), splenosomatic index (P=0.998), hepatosomatic index (P=0.475) or viscerosomatic index (P=0.411). Fish fed ME-PRO® based feeds had significantly larger viscerosomatic index (P=0.040) and lower Hematocrit (P=0.005) [13].
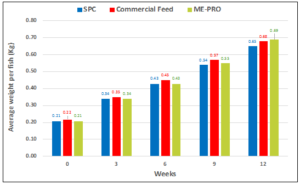
Figure 2a: Average weight per fish (Kg) performance of Rainbow trout fed ME-PRO®.
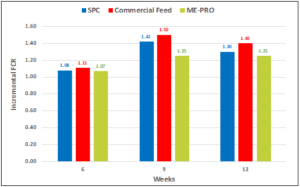
Figure 2b: Incremental feed conversion rate (FCR) of Rainbow trout fed ME-PRO®.
Shrimp Feeding Trials
Larvae Development Trials
Similar to other studies [14, 15] the inclusion of different percentages of ME-PRO® in hatchery diets showed promising results, improving several productivity parameters, including survival of the larval stages (Figure 3).
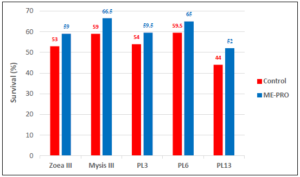
Figure 3: Survival of first feeding L. vannamei larvae (Z3 -PL13) fed artificial feeds containing ME-PRO®.
Growth out Trials
Weekly growth rates of shrimp fed on ME-PRO® at levels up to 50% was not significantly different from those fed commercial feeds (Figure 4). While shrimp growth can be affected by numerous other factors such as water quality conditions and genetics, based on the observed response of the shrimp from this experiment, there were no differences across the ME-PRO® treatments in the experiments, confirming the use of ME-PRO® in commercial feed formulations. The results of the growth trial revealed that the higher apparent digestibility coefficient of ME-PRO® along with higher amino acid digestibility resulted in better growth of the animals. These results are similar to the findings of other studies in which fermented soybean meal increased the growth performance of whiteleg shrimp [16, 17].
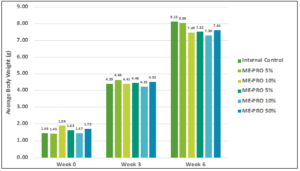
Figure 4: Average Body Weight (g) of L. vannamei juveniles fed artificial feeds containing ME-PRO®.
Challenge Trial
The results obtained indicated that the application of different inclusion levels has positive effects on the improvement of survival rate of the EMS-infected shrimp. Based on the results, the improvement of survival was observed in all treatments with diets containing ME-PRO®. The trial indicated that the application of 30% of ME-PRO® has positive effects on the improvement of survival rate of the EMS-infected shrimp (Figure 5).
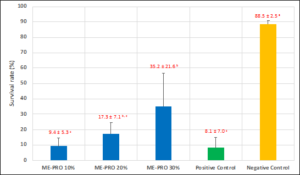
Figure 5: Survival rate (%) of L. vannamei juveniles fed artificial feeds containing ME-PRO®on day 10th of a post-challenge with EMS. Means sharing the same superscript are not significantly different from each other (Tukey’s HSD, P<0.05).
Water Quality
Formulated feeds are the major driver allowing intensification of production but are also the major source of N and P in RAS. In addition, phosphorous is one of the most critical minerals when formulating aquaculture feeds for RAS projects. On average, ME-PRO® contains 0.4% phosphorous (Table 1) and phytic acid concentration on the final product is on average less than 0.12 (g/100g). After 18 months of continuously using feeds containing ME-PRO® data taken at a commercial trout operation showed a reduction in phosphorus discharge of 69% with initial phosphorus discharge levels of 0.21 mg/liter to levels of 0.065 mg/liter.
Protein Source
Phosphorus (% dmb)
Phosphorus Availability (%)
Phosphorus Discharged (% dmb)
ME-PRO®
0.4
90
0.05
Fishmeal
4.0
50
1.50
Poultry meal
2.3
50
1.00
Soybean meal
0.7
12
0.60
During the shrimp growth and challenge trials, water quality parameters were recorded daily. Values of water quality parameters (temperature, DO, pH, TAN, nitrite, and alkalinity) are presented (Table 2).
Treatment
ME-PRO 10%
ME-PRO 20%
ME-PRO 30%
Positive Control
Negative Control
Temp (oC)
27.53 ± 0.54a
27.38 ± 0.49a
27.49 ± 0.50a
27.39 ± 0.51a
27.45 ± 0.46a
DO (mg/L)
6.19 ± 0.06a
6.19 ± 0.06a
6.19 ± 0.06a
6.20 ± 0.06a
6.18 ± 0.05a
pH
7.78 ± 0.05a
7.79 ± 0.05a
7.78 ± 0.05a
7.79 ± 0.05a
7.79 ± 0.06a
Alkalinity (ppm)
124.44 ± 5.27a
125.56 ± 5.27a
124.44 ± 5.27a
124.44 ± 7.26a
124.44 ± 5.27a
TAN (ppm)
0.28 ± 0.26a
0.22 ± 0.26a
0.11 ± 0.22a
0.22 ± 0.26a
0.22 ± 0.26a
Nitrite (ppm)
3.50 ± 2.26a
3.61 ± 2.15a
3.61 ± 2.15a
3.61 ± 2.15a
3.61 ± 2.15a
Sanility (ppt)
20.00 ± 0.00a
20.00 ± 0.00a
20.00 ± 0.00a
20.00 ± 0.00a
20.00 ± 0.00a
Comparing fish trials testing ME-PRO® and fishmeal water quality measurements have shown particular differences. Total dissolved solids in RAS water for ME-PRO® and fishmeal treatments were 774 and 822 ppm respectively. Turbidity (NTU) measured in system tanks showed that ME-PRO® (0.4) was slightly better than our fishmeal control (0.6).
A central problem that confronts sustainable RAS involves two interlocking limitations that their operators must balance to run successful aquaculture operations. The first limitation involves an understanding of present-day water re-use systems that limit fish or shrimp production. The second limitation centers on sustainable fish feeds where use of soy-based feeds in such present-day water re-uses systems make their management more difficult. The resulting situation requires present day aquaculture farmers to balance the risk-benefit of each limitation so as to achieve profitability as well as fulfil the demands of their customers [18].
Conclusion
Formulating low fish meal aquaculture feeds for RAS systems requires the use of combinations of several ingredients since most feedstuffs have been shown to have significant nutrient and functional limitations. Fermentation of plant ingredients can reduce the anti-nutritional factors and enhance the digestibility. The significant differences observed in the present study with growth performance of fish and shrimp fed ME-PRO® is probably due to the elimination of anti-nutritional factors and increased digestibility of the ingredient. The results also indicate that a Microbial Enhanced Proteins such as ME-PRO® can directly replace at least 80% of the dietary fishmeal in commercial feeds for shrimp larvae, and several juvenile and adult fish species.
References
- Dani D (2018) A review of replacing fish meal in aqua feeds using plant protein sources. International Journal of Fisheries and Aquatic Studies 6: 164-179.
- Baki B, Yucel S (2017) Feed cost/production income analysis of Seabass (Dicentrarchus labrax) aquaculture. International Journal of Ecosystems and Ecology Sciences 7: 859-864.
- Teles AO, Couto A, Enes P, Peres H (2020) Dietary protein requirements of fish – a meta-analysis.
- Føre M, Frank K, Norton T, Svendsen E, Alfredsen JA, et al. (2018) Precision fish farming: A new frame work to improve production in aquaculture. Biosystems Engineering 173: 176-193.
- Suresh AV, Kumaraguru KP, Nates S (2011) Attractability and palatability of protein ingredients of aquatic and terrestrial animal origin, and their practical value for blue shrimp, Litopenaeus stylirostris fed diets formulated with high levels of poultry byproduct meal. Aquaculture 319:132-140.
- Brezas A, Hardy RA (2020) Improved performance of rainbow trout selected strain is associated with protein digestion rates and synchronization of aminoacid Scientific Reports 10: 4678.
- Espinal CA, Matulić D (2019) Recirculation Aquaculture Technologies. In: Goddek S., Joyce A., Kotzen B., Burnell G. (eds) Aquaponics Food Production Systems. Springer, Cham.
- Tantikitti C (2014) Feed palatability and the alternative protein sources in shrimp feed. Songklanakarin Journal Science Technology 36: 51-55.
- Barnes ME, Brown ML, Bruce TJ, Neiger R, Sindelar S (2015) Effects of fermented soybean meal diet on rainbow trout mortality and immune function during a disease outbreak. Journal of Aquaculture Feed Science and Nutrition7: 6-15.
- Barnes ME, Brown ML, Neiger R (2015) Comparative performance of two rainbow trout strains fed fermented soybean meal. Aquaculture Internationa l23: 1227-1238.
- Barnes ME, Brown ML, Rosentrater KA (2012) An initial investigation replacing fish meal with a commercial fermented soybean meal product in the diets of juvenile rainbow trout. Veterinary and Animal Science 2: 234-243.
- Barnes ME, Brown ML, RosentraterKA, Sewell JR (2013) Preliminary evaluation of rainbow trout diets containing PepSoyGen, a fermented soybean meal product, and additional amino acids. The Open Fish Science Journal 6: 19-27.
- Krogdahl A, Kortner TM, Jaramillo-Torres A, Gamil AAA, Chikwati E, et al. (2020). Removal of three proteinaceous antinutrients from soybean does not mitigate soybean-induced enteritis in Atlantic salmon (Salmo salar, L). Aquaculture 514: 774495
- Shao J, Zhao W, Liu X, Wang L (2018) Growth performance, digestive enzymes, and TOR signaling pathway of Litopenaeus vannameiare not significantly affected by dietary protein hydrolysates in practical conditions. Frontiers in Physiology 9: 998.
- Shao J, Zhao W, Han S, Chen Y, Wang B (2018) Partial replacement of fishmeal by fermented soybean meal in diets for juvenile white shrimp (Litopenaeus vannamei). Aquaculture Nutrition 25: 145-153.
- Bae J, Hamidoghli A, Djaballah MS, Maamri S, Hamdi A, et al. (2020) Effects of three different dietary plant protein sources as fishmeal replacers in juvenile whiteleg shrimp, Litopenaeus vannamei. Fisheries and Aquatic Sciences 23: 2-6.
- Moniruzzaman M, Damusaru JH, Won S, Cho S-J, Chnag KH, et al. (2019) Effects of partial replacement of dietary fish meal by bioprocessed plant protein concentrates on growth performance, hematology, nutrient digestibility and digestive enzyme activities in juvenile Pacific white shrimp, Litopenaeus Journal of the Science of Food and Agriculture 100: 1285-1293.
- Chen Z, Chang Z, Zhang L, Jiang Y, Ge H, et al. (2019) Effects of water recirculation rate on the microbial community and water quality in relation to the growth and survival of white shrimp (Litopenaeus vannamei). BMC Microbiology 19: 192.