| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Recent Research and Trends in the Removal of Various Toxic and Persistent Organic and Inorganic Pollutants by Utilizing Activated Carbons as Adsorbent Materials Prepared from Waste Vehicular Tires: A Review
Vagi C Maria1*, Petsas S Andreas2
1Laboratory of Environmental Quality & Geo-spatial Applications, Department of Marine Sciences, School of the Environment, University of the Aegean, Mytilene, Lesvos, Greece
2Department of Food Sciences & Nutrition, School of the Environment, University of the Aegean, Myrina, Lemnos, Greece
Received Date: April 02, 2020; Accepted Date: April 09, 2020; Published Date: April 22, 2020
*Corresponding author: Vagi C Maria, Department of Marine Sciences, School of the Environment, University of the Aegean, University Hill, GR-81100, Mytilene, Lesvos, Greece. Email: mbag@env.aegean.gr
Citation: Vagi MC, Petsas AS (2020) Recent Research and Trends in the Removal of Various Toxic and Persistent Organic and Inorganic Pollutants by Utilizing Activated Carbons as Adsorbent Materials Prepared from Waste Vehicular Tires: A Review. Envir Scie Pollu Res Mang: ESPRM-101.
Abstract
Α wide variety of toxic contaminants when is accidentally or illegally present in natural terrestrial or aquatic environmental compartments poses a threat to the inhabitants of those ecosystems owing to the non-biodegradable characteristics and the persistent nature of specific organic and inorganic components. In addition, the potential increase in the toxicity of parent compounds towards non-target organisms that live in these environmental compartments has also been observed and revealed through the qualitative and quantitative determination of transformation products with higher toxicity than the original chemicals. Simultaneously, the enormous volumes of waste tires which are produced around the world are considered as a serious source of environmental pollution and their recycling process is faced as a challenging task for the universal scientific community. According to the published data, various techniques and practices have been developed and optimized that transform car tire rubber wastes into more important, desirable, valuable, and efficient materials. Among others, activated adsorbent-materials are included in such products. Therefore, the main objective of the present survey is to review all the available published data regarding the recent research and future trends on using granulated adsorbent materials and activated carbons obtained and produced from waste tires and afterward applied for the removal of persistent and residual quantities of pollutants.
Keywords: Adsorption Isotherm; Persistent Contaminants; Recycled Discarded Tires; Remediation; Waste Rubber Tire
Introduction
The enormous dependence of human societies on the use of several different types of auto transport by land, sea or air has driven to the numeral increase of vehicles globally. Based on data provided by the biggest companies that produce, manufacture and sell tire and rubber products in the international market the quantities of the relevant products are estimated to reach 17 million tones yearly, whereas a dynamic increasing tendency in the annual numbers is also observed [1]. Almost 1.0-1.4 billion units of waste tires are produced yearly worldwide and constitute 90% from passenger car type and the rest 10% from other types of vehicles. According to published bibliographic sources, the larger amounts of solid wastes that are generated after the utilization of automobile tires by a variety of several different means of transportation are produced mainly by China, European Union countries, Japan and India
Which produce almost 90% of the total volume of waste tires produced around the world [1]. More specific, published literature refers that the number of withdrawn tires generated each year in the European Community, North America, and Japan are 1.5, 2.5 and 0.5 million tones, respectively [2]. Consequently, more than 330 million waste tires are discarded per year and accumulated over the years throughout the world [3].
Due to their unique characteristics in which non-biodegradability, large volume, and their currently inappropriate and illegal disposal into the environment are included, waste vehicular tires are considered as a major environmental issue and a serious source of environmental threat worldwide [4]. Among the several and severe negative impacts of dumped or stored non-pretreated tire wastes on the environmental sustainability, the promoted growth of unwanted organisms (such as pests and insects), the increase in the risk of fire ignitions, and finally the uncontrolled liberation and emission of harmful gas pollutants such as SO2, NOx and numerous volatile organic compounds (VOCs) which can penetrate into soil, atmosphere and water systems are included.
For all the above-mentioned reason and according to the relevant legislations, their disposal in landfills has been banned in EC country-members, whereas related waste management problems must be overcome and demanding solution. Subsequently, the management of end- of-life tires wastes has received an enormous global scientific attention to find economic and environmentally friendly treatments that simultaneously reduce solid wastes and allow energy recovery.
Consequently, as shown in figure 1, several methods for the reuse, recycling, and conversion of discarded tires have been proposed and applied in order not only to reduce relevant environmental pollution but also to produce valuable products such as energy (e.g. fuels), additives (e.g. road pavements, playground surfaces, rubber roofs, drainage systems, etc.) or carbonaceous adsorbent materials [5].
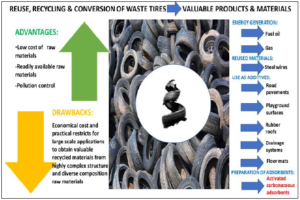
Figure 1. Some examples of reuses, and recycling or conversion strategies of waste vehicular tires.
Therefore, the scientific interest has been focused on the development of several recycling strategies and practices of converting them into more valuable products such as adsorbent materials which are widely utilized for the removal of several different inorganic and organic compounds from environmental samples. According to the relevant literature, the potential of using activated carbon adsorption materials from waste vehicular tires has been evaluated by numerous researchers [6-11].
Specifically, pyrolysis that is also described with the terms thermolysis and carbonization is a thermochemical process that has been used for many years in order to deal with various non-biodegradable solid wastes which are anthropogenically produced and afterwards disposed. This treatment is recognized as a polymer cracking/decomposition method (or thermal depolymerization) that transforms compounds of high molecular weights (polymers) into low-molecular-weight substances (monomers) and parallel produces reusable products.
Generally, pyrolysis involves complex reactions that take place intramolecular through the mechanism of free radicals and which according to the Arrhenius equation are depended on the two following parameters/pyrolysis conditions: the temperature (usually above 400oC and in the absence of O2 of inert atmosphere or under vacuum) and pyrolysis duration (or reaction time).
There are many studies in the literature which have demonstrated that large amounts of waste tires can be employed as feedstock for pyrolysis process. Usually, the pyrolysis of waste tires yields in a 40% wt high energy-density carbonaceous solid fraction of non-volatile matter that is also known as char and a 60% wt volatile fraction (gas and liquid volatile matter). Since pyrolysis allows the separation of produced chars (or carbon black materials) from the parallel produced volatile products it is reasonable to be considered as a significant waste-to-energy method that can be widely applied to produce renewable energy materials such as adsorbents.
The physicochemical properties of the formed tire pyrolysis products are depended on: (i) the employed pyrolysis conditions which determine the type of the applied pyrolysis method (oxidative-, hydro-, steam-, catalytic-, vacuum-pyrolysis), such as the temperature and heating rate of the heater system, type, pressure and flow rate of used carrier gas, pyrolysis duration, volatile residence time, etc., and (ii) the properties of the waste tire feedstock, such as composition, particle size, etc.
The adsorption characteristics of the produced adsorbent materials that are obtained after the applied pyrolysis and activation methods, including adsorption capacities, mesopore volumes, and BET (Brunauer–Emmett–Teller) surfaces have been investigated, compared and improved by several researchers throughout the years [2, 12]. A large number of excellent studies containing original results of scientific research studies or reviews or critical overviews have been published during the last years regarding the occurrence, effects of tire wear particles in the environment and the potential application of recycled rubber from scrap tire in the removal of toxic pollutants from liquid and gas media [13-27].
Hence, the main purpose of the present work is to review all the available data concerning the recent research and trends in the removal of various toxic organic and inorganic contaminants contained in water and wastewater matrices by utilizing activated carbons prepared from waste tires in order to provide essential information regarding the efficiency of such technologies in the remediation of aquatic polluted matrices. All reported results have been categorized regarding the type of pollutant that has been adsorbed onto tire derived adsorbent materials. The potential application of tire pyrolysis chars as adsorbent substrates is evaluated according to the yields and adsorption efficiencies reported for each category of target pollutant.
General composition of waste tires
The production of tires employs a variety of complex and diverse composition chemical mixtures that are used as tire materials and the composition differs and is always depending on the exact applications [13]. Poly-butadiene, styrene-butadiene, neoprene isoprene, and polysulphide, are included among other natural and synthetic petroleum-based rubbers that are contained as elastomers in a general percentage ratio of 40-60%. Apart from synthetic or/and natural rubber various chemicals are added such as reinforcement agents and fillers like carbon black, silica, and silanes (20-35% content), process mineral oils (12-15% content), textile and metal net (5-10% content), vulcanization agents and several additives including preservatives (halogenated cycloalkanes), anti-oxidants (amines, phenols), desiccants (calcium oxides), plasticizers (aromatic and aliphatic esters), and processing aids (mineral oils) (5-10% content) [13]. In figure 2 the general composition of waste tires is depicted.
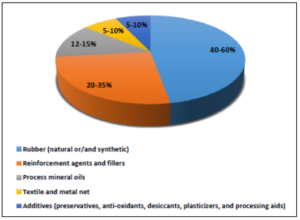
Figure 2. General composition of waste tires (Data taken from references [13-16]).
According to the relevant literature, the carbon content in waste tires is approximately as high as 70-75% [17], whereas almost 32% by weight of the solid vehicular discarded tires is carbon black [18]. Apart from the obvious difference in their physical appearance, the carbonaceous adsorbents that are produced from waste tire rubber and the activated carbons are quite similar if the distinction between their internal surface areas is ignored [28].
Methods for preparation and development of activated carbonaceous adsorbent materials from waste rubber tire
Overall, the general steps and procedures that are commonly applied for the production of adsorbents by waste rubber are given in the scheme of figure 3. The preparation of tire rubber-derived activated carbons usually requires the initial step of the pyrolysis of the rubber which is followed by a controlled oxidation (activation) step of the previously obtained carbonized chars [29].
A variety of highly mesoporous activated carbons have been prepared via numerous and alternative methods that have been applied to discarded automobile tires, whereas several researchers have reported that the adsorption capacity of produced adsorption materials is enhanced by the co-presence of both mesopores and micropores especially in the case of large adsorbates like dyes, pesticides and many other macro-molecular chemical species [30].
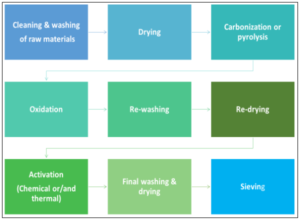
Figure 3. General steps and procedures applied for the preparation of adsorbents by waste rubber.
Despite other reported differences in the details of the used methods by the several scientists, there is a general agreement in the literature and many authors agree that after the pyrolysis or carbonization technique which is applied to untreated rubber tire the carbon black materials that are produced must undergo to further gas activation through steam, or air or carbon dioxide atmosphere heat process so as to obtain better adsorption behavior regarded their surface area and porosity values [12, 29, 31].
Additionally, the pyrolyzed product chars can be chemically activated with alkali chemical agents, such as KOH, K2CO3, NaOH and Na2CO3 [32-35], or acidic solutions among which HCl, HNO3, H2SO4, mixtures of H2SO4 and HNO3, HCl and BaCl2, or ZnCl2 are included [36-38]. Most comparative studies that were found in the present literature review have highlighted the fact that thermal, chemical and combined (thermal and chemical or vice versa) treatments enhanced the adsorption capacity of adsorbent materials produced from rubber of tires wastes [38].
The acquired variability in the adsorption characteristics of activated carbons produced such as surface area, pore structure affecting porosity and adsorption capacity has been investigated by several authors and has been attributed either to the differences in pyrolysis and activation conditions employed or to the dissimilar properties of the raw tire materials feed of the procedure [29].
In most cases of published literature, the textural and surface chemistry characteristics, the morphology, the point of zero charge (pHpzc), temperature programmed desorption (TPD) and several other adsorption features and characteristics of the prepared tire activated carbons have been measured and analyzed by using several technologies and techniques among which the scanning electron microscope, the energy dispersive X-ray analyzer and the Brunauer–Emmett–Teller surface area analyzer are included.
Efficiency in the removal of various toxic and persistent organic and inorganic pollutants
The efficient removal of a wide variety of toxic and persistent pollutants belonging to different chemical groups and exhibiting various physicochemical properties has been reported by a number of researchers worldwide.
According to the findings of the present review which are summarized in figure 4, the majority of the published scientific data are relevant to the study of the enhanced removal of heavy metals by adsorbents prepared from waste rubber tire (≈33% of the total found reports). Studies regarding the surveys on the application of activated carbons derived from scrap tires for the adsorption of dyes like methylene blue, methyl orange, rhodamine B, and many other substances of that class takes the second place (≈21% of the total found reports), followed by the studies on the sorption of organic solvents including toluene and xylene in aqueous solutions by recycled tires crumb rubber (≈10% of the total found reports).
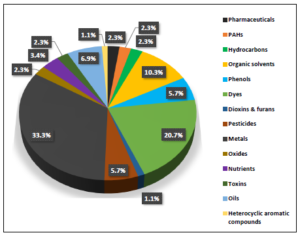
Figure 4. Trends in the scientific research regarding the removal of various toxic and persistent organic and inorganic pollutants by utilizing activated carbons as adsorbent materials prepared from waste vehicular tires.
The two most frequently used models that are employed to describe the sorption removal are the Freundlich and Langmuir isotherm models that both result in the best fit for the acquired experimental results and are described by the following equations, respectively:
qe = KF Ce1/n and qe = (qi b Ce) / (1 + b Ce)
where, (qe) is the solid-phase equilibrium concentration (in mg g-1); (Ce) is the aqueous phase concentration of equilibrium (in mg L-1); (KF) is the Freundlich equilibrium parameter (in mg g-1)(L mg-1) 1/n n); n represents the exponential parameter it ranges between zero and one; qe and qi are the solid-phase equilibrium concentration and uptakes at saturation (in mg g-1); Ce is the aqueous phase equilibrium concentration (in mg L-1).
The Freundlich model is an empirical equation, and it is widely used to describe much adsorption data for nonlinear sorption model with heterogeneous adsorbent surfaces. The Langmuir model has a theoretical basis, and it is generally the most straightforward non-linear isotherm model on monolayer sorption. Other alternative models such as Weber and Morris intraparticle diffusion model, the Boyd model, Redlich-Peterson model have been also used to analyze data and to distinguish between the pore and film diffusion steps.
According to the results of a recent paper published by Hüffer et al., (2020) concerning the investigation of the molecular interactions of organic compounds with tire crumb materials it was found that the phenomena and processes involved in sorption to tire materials were significantly different from that governing sorption to other microplastics [39]. Furthermore, in the same published work, it was suggested that beyond the hydrophobicity of sorbates that is a major factor in the sorption process onto tire materials adsorbents, additional interactions must be considered [39].
In (Table 1) are contained some selected examples of studies found during the current review evaluating the potential application of adsorbents obtained from waste vehicle tires in the removal of organic and inorganic pollutants from liquid or air samples. References are reported in chronological order. (Table 2) contains representative studies regarding more detailed information. Further discussion in details about the specific categories of the most studied target group of pollutants is presented in the following paragraphs. -Langmuir and Freundlich models • Equilibrium time: 60min for initial concentration of 700mg L-1→ Fast kinetics adsorption process • Decrease in granules particle size from 2.36 mm to 0.30 mm increased adsorption capacity from 5 mg g-1 to • Increase in temperature from 5oC to 45oC decreased the adsorption capacity from 13.4 mg g-1 to 9.9 mg g-1 • Conditions for maximum adsorption: pH=8.5; Adsorbent dosage:4g • Freundlich isotherm constants: KF=2.710mg g-1 (constant related to adsorption capacity), n=6.369 (constant related to adsorption intensity) (R2=0.721) •2.36mm - Column studies -Langmuir model • Conditions for maximum adsorption: pH=2; Pesticides initial concentration: 12 mg L-1; contact time: 60min; particle adsorbent size of 200–250 μm • Batch adsorption studies revealed Maximum adsorption of methoxychlor, atrazine and methyl parathion were 112.0 mg g−1, 104.9 mg g−1 and 88.9 mg g−1, respectively • Removal of methoxychlor, atrazine and methyl parathion from an initial concentration of 12mg L-1 signifying 93.3%, 87.4% and 74.1% respectively • Removal of 91%, 82.1% and 71.78% methoxychlor, atrazine and methyl parathion respectively by column experiments • Indications that diffusion of pesticide molecules into pores of the adsorbent mainly controls the adsorption process • Spontaneous, exothermic and random characteristics of the process are confirmed by thermodynamic studies -Fixed-bed columns -Langmuir and • Higher adsorption capacity was observed for larger mesopore volume of the prepared activated carbon than its commercial counterpart, even though the commercially activated carbon possessed a higher micropore volume and a higher surface area • Bulky adsorbate like Acid Blue 113, mesopore volume of the activated carbon played an important role in the adsorption phenomenon • Kinetic studies indicated that the adsorption process followed first order kinetics • Overall, the investigated adsorbent “waste rubber tire”– adsorbate system was evaluated as cost effective, efficient and fast for the removal of dyes from contaminated wastewater
Group of pollutant
Chemical compound (s)
Reference(s)
PHARMACEUTICALS:
Tetracycline
Acosta et al., 2016; Lian et al., 2013 [32, 40]
PAHS:
Naphthalene
Gupta et al., 2016; Gunasekara et al., 2000 [41, 42]
Fluorene
Gupta et al., 2016 [41]
Phenanthrene
Gupta et al., 2016 [41]
HETEROTRICYCLIC AROMATIC COMPOUNDS:
Dibenzothiophene
Danmaliki et al., 2016 [43]
HYDROCARBONS:
Cyclooctane
Prpich et al., 2008 [44]
2,2,4,4,6,8,8-heptamethylnonane
Prpich et al., 2008 [44]
1‐octadecene
Prpich et al., 2008 [44]
Methane
Lehmann et al., 1998 [27]
ORGANIC SOLVENTS:
Benzene
Shahrokhi-Shahraki et al., 2020; Lu et al., 2015 [45, 46]
Ethylbenzene
Shahrokhi-Shahraki et al., 2020; Lu et al., 2017; Lu et al., 2015 [45-47]
Toluene
Shahrokhi-Shahraki et al., 2020; Lu et al., 2017; Lu et al., 2015; Alamo-Nole et al., 2011; Oh et al., 2009; Gunasekara et al., 2000 [45-49, 42]
Xylenes (ortho-, meta-, and para- isomers)
Shahrokhi-Shahraki et al., 2020; Lu et al., 2017; Lu et al., 2015; Alamo-Nole et al., 2011 [45-48]
cis-1,2-dichloroethylene
Lu et al., 2017; Lu et al., 2015 [46, 47]
Trichloroethylene
Lu et al., 2017; Lu et al., 2015; Lian et al., 2012a [46, 47, 50]
Methyl tert-butyl ether
Lu et al., 2017 [47]
Dichloromethane
Saleh et al., 2015 [51]
Chloroform
Saleh et al., 2015 [51]
Carbon tetrachloride
Saleh et al., 2015 [51]
Acetone
Lehmann et al., 1998 [27]
PHENOLS (not including the ones with pesticide action):
Phenol
Trubetskaya et al., 2019; Tanthapanichakoon et al., 2005; Nakagawa et al., 2004; San Miguel et al., 2003; Helleur et al., 2001 [52, 53, 30, 29, 26]
DYES:
Methylene blue
Mukherjee et al., 2019b; Daraei et al., 2017; Lian et al., 2012b; Quek et al., 2011; Mui et al., 2010a; Mui et al., 2010b; San Miguel et al., 2003; Lin et al., 2002; Sainz-Diaz et al., 2000 [54, 55, 35, 56-58, 29, 31, 59]
Procion Red H-E2B
San Miguel et al., 2003 [29]
Black 5
Tanthapanichakoon et al., 2005; Nakagawa et al., 2004 [53, 30]
Red 31
Tanthapanichakoon et al., 2005 [53]
Methyl orange
Chennouf-Abdellatif et al., 2017; Lian et al., 2012b [60, 35]
Acid blue 25
Mui et al., 2010a; Mui et al., 2010b [57, 58]
Acid yellow 117
Mui et al., 2010a; Mui et al., 2010b [57, 58]
Rhodamine B
Tuzen et al., 2018; Chennouf-Abdellatif et al., 2017; Li et al., 2010 [61, 60, 62]
Direct Scarlet 4BS
Han et al., 2016 [34]
Cresol red
Khudhair et al., 2015 [63]
DIOXINS (Polychlorinated Dibenzo-p-Dioxins (PCDD)) & FURANS:
2378-substituted PCDD/F
Hajizadeh et al., 2011 [64]
PESTICIDES:
Methoxychlor
Gupta et al., 2011a [2]
Methyl parathion
Gupta et al., 2011a [2]
Atrazine
Gupta et al., 2011a [2]
Paraquat dichloride
Hamadi et al., 2004 [65]
2,4-dichlorophenol
Joseph et al., 2013 [37]
1,3-dichlorobenzene
Lian et al., 2012a; Lian et al., 2011 [50, 66]
1,3-dinitrobenzene
Lian et al., 2012a; Lian et al., 2011 [50, 66]
2,4-dichlorophenol
Lian et al., 2011 [66]
γ-hexachlorocyclohexane
Lian et al., 2012a [50]
METALS:
Copper (II) (Cu2+)
Shahrokhi-Shahraki et al., 2020; Chang et al., 2016a; Chang et al., 2016b; Deng et al., 2016; Song et al., 2016; Ramola et al., 2014; Feroze et al., 2013;Shahtalebi et al., 2013; Quek et al., 2009; Oladoja et al., 2006 [45,67-75]
Chromium (III and/or IV) (Cr3+ and/or Cr6+)
Benjamin et al., 2017; Song et al., 2016; Gupta et al., 2013; Hamadi et al., 2001 [36, 70, 76, 77]
Cadmium (II) (Cd2+)
Dimpe et al., 2017; Alexandre-Franco et al., 2011; Entezari et al., 2006 [78-80]
Zinc (II) (Zn2+)
Liu et al., 2018; Deng et al., 2016; Song et al., 2016; Solano et al., 2012 [81, 69,70,82]
Lead (II) (Pb2+)
Shahrokhi-Shahraki et al., 2020; Dimpe et al., 2017; Deng et al., 2016; Song et al., 2016; Ramola et al., 2014; Saleh et al., 2013; Mousavi et al., 2010 [45, 78, 69-71, 83, 84]
Mercury (II) (Hg2+)
Ramola et al., 2014; Lin et al., 2006a; Lin et al., 2006b; Manchón-Vizuete et al.,2005; Gunasekara et al., 2000; Lehmann et al., 1998; Knocke et al., 1981 [71, 85, 86, 38, 42, 27, 28]
Arsenite, As (III) and Arsenate, As(V) (As3+ and As5+)
Imyim et al., 2016 [87]
Nickel (II) (Ni2+)
Siddiqui et al., 2016 [88]
Uranium (VI) (U6+)
Belgacem et al., 2014 [89]
OXIDES:
NOx : NO
Al-Rahbi et al., 2016 [33]
SOX : SO2
Nieto-Márquez et al., 2016 [90]
NUTRIENTS:
Ammonia (NH4+)
Hossain et al., 2010 [91]
Nitrite, nitrate (NO2-, NO3-)
Krayzelova et al., 2014; Hossain et al., 2010 [92, 91]
Orthophosphate, total dissolved phosphorus (PO43-, P)
Ramola et al., 2014; Hossain et al., 2010 [71, 91]
RESIDUAL CHLORINE:
Chlorine
Trubetskaya et al., 2019 [52]
OILS:
Spilled engine oil
Lin et al., 2010 [93]
Oil
Aisen et al., 2003; Aisen et al., 2002 [94, 95]
Crude oil
Aisen et al., 2006 [96]
Petroleum oil
Lin et al., 2008 [97]
TOXINS:
Cylindrospermopsin
Mashile et al., 2019 [98]
Microcystin-LR
Mashile et al., 2018 [99]
PARABENS
Methylparaben
Mashile et al., 2020 [100]
Propylparaben
Mashile et al., 2020 [100]
Type of adsorbent
Adsorbent preparation method & Particle size
Tested pollutant(s)
Adsorption method & Kinetic studies
Main findings-Conclusions
Reference
Rubber granules from scrap tire
Washed (with d. H2O), dried (air), cut (with knives and electric grinding machine), mechanically sieved, washed (with distilled H2O) by agitation (mechanical shaker at 150rpm for 3h), and dried (oven at 60oC for 5h)
Phenol
(UV–visible spectrophotometer at λ=248 nm)-Batch method (mechanically agitated 250mL Erlenmeyer flasks containing 100mL of phenol aqueous solution & adsorbent appropriate dose)
• Adsorption process was affected by operational parameters: contact time, initial concentration of phenol, adsorbent dosage and solution temperature
10.6 mg g-1 and percentage removal of phenol from 20.5% to 40%
• Langmuir isotherm constants: Qo=15.6mg g-1 (maximum sorption capacity), b=87.09L mg-1 (sorption constant) (R2=0.995)→Better fit of experimental data→ Mono-layer type of adsorptionAisien et al., 2013 [4]
•0.212mm
•0.425mm
•0.60mm
•1.18mm
Carbonaceous adsorbent of higher mesopore, macropore content and a favorable surface chemistry prepared from waste rubber tire
Initial cleaning, carbonization of the ground tire granules, mixing of 2g of dried material with 8g of KOH (for 10min) & thermal activation to 900oC (for 2h), treatment with HCl (1M) for ash removal, washing
with d. H2O)Methoxychlor, atrazine, methyl parathion (GC-ECD method analysis)
-Batch method (mechanically agitated 250mL Erlenmeyer flasks containing 100mL of pesticide aqueous solution & adsorbent appropriate dose)
(A glass column of length 30 cm and 1 cm internal diameter, filled with weighed amount of prepared adsorbent material having particle size 200–250 μm)• After the application of successive chemical and thermal treatment, a basically carbonaceous adsorbent is prepared which exhibited not only a higher mesopore, macropore content but also has a favorable surface chemistry
Gupta et al., 2011a [2]
• 100–150 μm
• 150–200 μm
• 200–250 μm
Mesoporous activated carbon material
Cleaned, washed (with deionized H2O), dried (oven at 100oC for 2h), heated for carbonization (500 oC for 5h), oxidized with H2O2 solution (for 24h at 60oC), washed with deionized H2O (x3 times) and dried (at 110 ◦C for 2h in vacuum oven), activated (to 900oC for 2h in a covered silica crucible by heating in a muffle furnace), cooled (in a desiccator). Treated with 1M HCl solution (to remove the ash content), washed (with deionized H2O), dried (at 100oC for 24h), and sieved.
Methoxychlor, atrazine, methyl parathion (GC-ECD method analysis)
-Batch method
(250mL Erlenmeyer
flasks containing
100mL of dye aquatic
solution &
adsorbents
appropriate dose
agitated in an orbital
shaker at 100 rpm)
method (glass
column with length
30 cm and 1 cm
internal diameter,
filled with weighed
amount of prepared
adsorbent material
having particle size
200–250 μm)
Freundlich models• The dye adsorption depended on both the surface properties as well as the porous properties.
• The rate determining stage of the adsorption phenomenon was particle diffusion and increased mobility of adsorbate was observed with increasing temperatureGupta et al., 2011b [12]
• 100–150 μm
• 150–200 μm
• 200–250 μm
Heavy metals
Several highly toxic metals or metalloids that are released into the environment and can cause a series of potential negative health effects and/or severe environmental impacts (among which cadmium, mercury, arsenite, and arsenate, lead, zinc, copper, chromium and others are included) must be removed from waste effluents and other environmental matrices. Toxicity of these elements is depended on essential parameters such as oxidation state, exposure level or concentration, target organisms, etc. Therefore, the enhanced removal of a wide variety of heavy metals by using mesoporous adsorbents prepared from waste rubber tire has been investigated by numerous researchers [27, 28, 36, 38, 42, 45, 67-89, 101, 102].
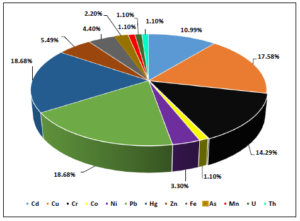
Figure 5. Trends in the scientific research regarding the removal of various metals by utilizing activated carbons as adsorbent materials prepared from waste vehicular tires.
As illustrated by the data contained in figure 5 the scientific interest has been focused mostly on the removal of lead and mercury since almost 19% percentage of the total number of found articles regarding the elimination of metals from environmental substrates through adsorption process onto tire produced adsorbents was relevant to Pb or Hg. In the second place was found copper (almost 18%), followed by chromium (almost 14%) and cadmium (almost 11%), while zinc, iron, nickel, arsenate, manganese, and cobalt were the metals that followed in aforementioned diminishing order and those heavy metal elements on which research has been focused the least.
The efficient recovery of copper (II) from liquid matrices by utilizing tire-derived adsorbents has been studied by numerous scientific teams around the world [45, 67-75, 103]. For instance, Al-Asheh and Banat (2000) have examined the adsorption ability of three types of adsorbents prepared by tire rubber that were untreated, chemically and physically activated rubber materials towards bivalent copper ions and reported that the decreasing range for the measured adsorption capacity of the tested adsorbents was the below: untreated materials> chemically activated rubber> physically activated rubber [103]. In a published study regarding the removal of copper via adsorption phenomenon by the use of a green sorption media composed of recycled tire rubber, expanded clay aggregate, and coconut coir revealed that the aforementioned adsorption media mixture could be effective and reliable for this purpose [67].
Dimpe et al. (2017) reported the successful adsorptive removal of cadmium (Cd(II) and lead (Pb(II)) from real environmental samples via the utilization of chemically activated carbonaceous materials obtained from waste tires (H2O2 was chosen as the more efficient activating agent compared to H3PO4), while under the optimum conditions that were: pH value 6.5; the mass of adsorbent 0.2 g; contact time 32.5 min and metals initial concentration 55 mg L−1 the achieved adsorption capacities of Cd(II) and Pb(II) were 201 and 196 mg g−1, respectively [78]. Adathodi et al. (2018) applied a novel adsorbent- aircraft tire rubber ash for the removal of the harmful and toxic heavy metal chromium from wastewater that originated from various manufacturing industries and simultaneously they evaluated the effect of several factors and parameters such as dosage, initial concentration, pH of the solution, contact time, and temperature on the efficiency of the process [104]. On the whole, 92.24 % percentage removal of chromium was accomplished, whereas the adsorption efficiency was observed to be decreased by the change in pH value of the solution (from pH 3 to pH 9), and decrease in the adsorbent dose. On the contrary, the effectiveness of chromium removal through the adsorption onto aircraft tire rubber waste carbon was increased by the decrease in the chromium initial concentration (from 49.02% to 59.79%), or/and in the temperature, or/and in the dosage of adsorbent [104]. The mechanism of the sulfur component in pyrolyzed char prepared from the waste tire was investigated by Li et al. (2015) in the removal of gas-phase elemental mercury (Hg0) in the presence of oxygen. Based on the published results of this study, the significant capture of the toxic metal that was achieved after its adsorption on the pyrolyzed (at 600°C) adsorbent materials was attributed mainly to the chemisorption phenomenon. The assumed main pathway of the performed reactions included the initial reaction of surface ZnS on the char with O2 that produced S which in turn reacted with metallic Hg0 to form HgS [105].
Dyes
Natural and synthetic dyes are usually water-soluble and colored chemicals that are used in several applications of plastic, textile, food, cosmetic, paper and printing industries. As a consequence, they find their way in several aquatic environmental matrices and wastewaters and hence they are considered as a potential source of pollution. Many of these compounds, especially the synthetic ones, are reported to cause a variety of indirect and direct undesirable harmful effects on both biotic and abiotic components of the ecosystems that they enter due to their toxic character.
Unquestionably, dyes are one of the most representative groups of organic chemicals that are characterized as persistent pollutants owing to the fact that their removal from wastewater requires more demanding strategies and techniques as conventional treatment methods are not efficient enough for that purpose [63].
A variety of adsorbent materials that are widely available and result in promising and efficient capacities have been tested to diminish the concentration of dyes in aquatic samples. Therefore, their removal through the adsorption process by the use of low-cost adsorbents prepared from tire rubber waste is a topic that has gained a stable and undiminished scientific interest [106, 107].
Based on the findings of the current survey concerning the review of the published literature dealing with the recent research and trends in the use of waste vehicular tires in the adsorption technology, it became obvious that numerous surveys have been performed on waste rubber tire activated carbon for several applications of dye removal from liquid phase media such as effluents [12, 29-31, 34-35, 54-63].
In figure 6 the trends in the relevant published articles that were reviewed during the current study and concerned the removal of various organic dyes by utilizing activated carbons as adsorbent materials prepared from waste vehicular tires are shown. From this figure, it can be observed that the majority of the conducted studies concerned the adsorption of Methylene Blue dye onto porous carbon obtained from waste tires yielding in almost 45% percent of the total number of reports found dealing with the removal of dyes. Methyl Orange and Acid Blue 25 are the two synthetic dyes that follow in the second place of the relative surveys with equal 8% percent, while the third-place belongs to the three separate dyes of Acid Yellow 117, Rhodamine B, and Black 5 giving almost 6.5% percentage of the relevant literature. According to the same data, the adsorption removal of a wide variety of other synthetic substances by vehicular adsorbents has also been studied.
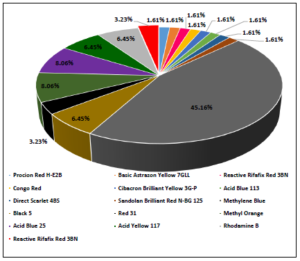
Figure 6. Trends in the scientific research regarding the removal of various organic dyes by utilizing activated carbons as adsorbent materials prepared from waste vehicular tires.
In a recently published study of Elmaslar Özbaş et al. (2019) who examined the production phases in the preparation of activated carbon-adsorbents obtained from end-of-life tires as raw materials the good efficiency in the removal of Methylene Blue dye was reported [108]. The production of the two different acquired types of activated carbon materials started with the wash-impregnation phase in two different aquatic solutions of KOH (1:1 and 1:2 rations), followed by thermal activation step, and finally the pyrolysis stage followed. After the Brunauer-Emmett-Teller analysis of the obtained adsorptive materials, the surface area was determined to be equal to 2.945 m2 g-1. Under the optimum conditions, 83% removal of the tested dye was accomplished (temperature 30°C; pH value 6.5; adsorbed dose 7.5 g L-1, and contact time 30 min) [108].
These results are in agreement with the ones that have been reported previously from Daraei et al. (2017) [55]. The Langmuir isotherm model has been used for the experimental values and good fitness in the kinetics of the adsorption process of Methylene Blue dye over tire activated carbon was observed. The maximum capacity of the prepared adsorbent was achieved at ambient temperature, media pH value 3, contact time 90 min and adsorbent dose 4 g [55].
Similar observations have been reported and for other organic molecules that belong to the group of synthetic dyes, such as in the case of dye Direct Scarlet 4BS [34]. At an ambient temperature of 35°C, pH 4.0, in the co-presence of 60 mg L-1 of dye (original fortification level) and 0.6 g of activated carbon that has been prepared from tire by NaOH activation, and after 60 min contact time the removal of Direct Scarlet 4BS dye in the aquatic solutions reached the percent value of 85.14% [34]. On the contrary, the results of Khudhair et al. (2015) indicated that a different dye substance, and more specifically Cresol Red dye, can be physically adsorbed onto the surfaces of waste tire rubber through a natural and significant slower process of a total duration of 21 days that yielded in more than 81% removal of the dye from its liquid solutions [63]. Therefore, it was concluded that despite the drawbacks of the lower rates achieved and the time-consuming demand, however the benefits of the low-cost and energy-saving (no agitation or activation stage) overscale and made the studied adsorption technique as an efficient method for the removal of the tested organic substance. The physical properties of the used adsorbents were particle diameter 2 mm and the used weight 12 g [63].
The removal of the cationic dye Rhodamine B from aquatic matrices via adsorption strategies has been also examined by numerous researchers that tried to optimize several operating parameters and critical factors affecting the capacity of the investigated adsorption systems [60-62]. According to the findings of the scientific group of Li et al. (2010) who conducted experimental batch equilibrium, kinetics and thermodynamic tests the activated pyrolytic adsorbent prepared from tire wastes exhibited greater adsorption efficiency than most adsorbents [62]. The parameter of ionic strength value had insignificantly influence on the process, while contradictory, both of the factors of pH and temperature values showed a significant effect on the removal phenomenon. Moreover, experimental data fitted the Langmuir isotherm model and pseudo-second-order kinetic model described the performed procedure which thermodynamically was confirmed to be an endothermic and spontaneous process [62].
In a later study of Tuzen et al. (2018) the enhanced adsorption of the dye Rhodamine B was accomplished after the improvement and alteration of the activated carbon produced from pieces of waste tires by magnetic nanoparticles of combined Fe and Ce, finally yielding in adsorption capacity of 324.6 mg g−1 [61]. Moreover, the developed materials could be re- utilized after the desorption of previously adsorbed Rhodamine B molecules with an alcoholic solution (ethanol) until 10 times (regeneration). Based on the acquired data obtained by the conducted thermodynamic tests, the endothermic adsorption process was indicated that could be well described by pseudo-second-order equations of kinetics and Langmuir isotherm model [61].
Overall, several of the found and reviewed developed methods that were designed and evaluated for their efficiency in the removal of specific synthetic dyes from aquatic samples gave very promising and reliable results, and hence could be applied in large-scale adsorption cleaning and remediation systems.
Pharmaceuticals
A wide variety of synthetic and semisynthetic organic molecules are classified in the category of pharmaceuticals or drugs or medicines that are worldwide used for the cure or prevention of human or animal diseases. Several classifications of pharmaceuticals can be done based on different criteria such as their mode of action or pharmacological activity, their chemical characteristics or their therapeutic effects and results.
Their detection in marine-, surface-, ground-water and wastewater samples has raised scientific concerns and prompted the relevant research for the development of efficient methods of their partial or total removal from environmental matrices [109]. Their removal from water and wastewater matrices needs the application of new techniques with higher efficiencies than the ones of conventional treatment methods due to their toxic and persistent nature [109].
In a recently published survey of Phasuphan et al. (2019) pulverized waste tire crumb rubber (of 300 μm median diameter) was prepared and after its surface modification with adsorbed polymeric chitosan the obtained adsorbent was applied for the elimination of contamination caused by three anti-inflammatory drugs [110]. Specifically, under the optimum operating parameters the evaluated removal capacities of the novel sorbent material were found to be 2.3 mg g-1 for naproxen, 17.7 mg g-1 for diclofenac, and 70.0 for ibuprofen. The results acquired after the application of the developed method to real samples showed a more enhanced adsorption process for diclofenac compared to the adsorption of the other two (naproxen and ibuprofen). This fact was attributed to the chemical structure of the substance (-NH- and - COOH functional groups) which allowed the molecule to be adsorbed via electrostatic interactions and hydrogen bonds on chitosan [110].
Based on the results found during the current review it is observed that extended research regarding the decontamination caused by antibiotics via waste tires produced sorbent materials has been conducted [32, 40, 111-112]. Tetracycline [32, 40], ciprofloxacin, danofloxacin, enrofloxacin [111], and sulfamethoxazole [112] are some of the antibiotic agents that are included in the list of pharmaceuticals with antimicrobial action which have been successfully adsorbed onto materials prepared and developed from tire end-of-life tires (figure 7).
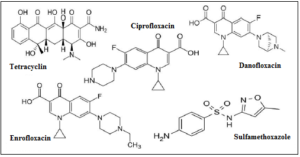
Figure 7. Skeletal structural formulas of selected antibiotics found in the reviewed scientific literature concerning their removal from aquatic solutions via adsorption onto activated waste tire-derived materials has been investigated.
Acosta, et al. (2016) [32] studied the adsorption of tetracycline, which is according to published data the second most widely used antibiotic worldwide, onto activated carbons produced by KOH activation of tire pyrolysis char [32]. Several proportions of KOH to the mass of pyrolyzed tire char (into the range of 0.5-6.0) and temperatures of thermal activation (in the range of 600-800°C) were tested to prepare the adsorbent with the best adsorption capacity. Manufactured activated sorbents possessed specific surface areas comparable to those of commercial activated carbons (as high as 814 m2 g-1). According to the conclusion of the same survey, the spontaneous and efficient removal of tetracycline was accomplished, while the equilibrium of the adsorption process was achieved at 25oC after the duration of 15 h (maximum adsorption 312 m2 g-1). A large number of various isotherm models were used for the kinetic analysis of obtained data (such as Freundlich, Langmuir, Dubinin-Radushkevich, Sips, Temkin, Dubinin-Astokov, Redlich-Peterson, Radke-Prausnitz and Toth) and finally, pseudo-second-order kinetic equations described kinetic data well [32].
According to previously conducted investigation performed by Lian, et al. (2013) aiming to understand the mechanism by which tetracycline is adsorbed on tire powder and pyrolyzed chars prepared from the waste tire and how the process is affected by the presence of Cu2+ ions in the reaction solution and pH it was assumed that the observed affinity of the hydrophobic substance tetracycline towards the tested graphite surfaces could be explained by intermolecular forces and attractions originating from π-π electron-acceptor-donor– interactions [40]. Furthermore, the combination of the tetracycline and Cu2+ in a broad-range pH-solution values resulted in a mutual positive impact of their adsorption phenomena which was explained by mechanisms of bridging-surface and/or metallic complexation pathways [40].
Activated carbon obtained from waste tires was employed for the development and application of a simple and low-cost solid-phase technique used for the removal of three selected fluoroquinolones from wastewater samples [111]. Particularly, nanofibers of polymers (polyacrylonitrile) were decorated with waste tire chars of activated adsorbents materials and afterward were utilized for the extraction of ciprofloxacin, danofloxacin, and enrofloxacin antibiotics from aqueous samples (figure 7) [111]. The adsorption ability of the developed nanofibers was characterized by the authors as satisfactory because of the evaluated recoveries of removal which varied from 90% to 99% after their application to real wastewater samples [111].
With the same concept and with similar logic and purpose Dimpe, et al. (2018) used tire- derived activated carbon as solid-phase adsorbent for the microwave-assisted extraction of sulfamethoxazole contained in wastewater matrices [112]. After the optimization of the crucial parameters affecting the method (such as pH, mass of adsorbent, extraction time, microwave power, etc) the optimum value of adsorbent efficiency was equal to 138 mg g−1 for the studied antibiotic sulfamethoxazole and its highly efficient removal was achieved [112].
The highly efficient removal of aspirin via an environmentally friendly and economic adsorption process that occurred onto tire-prepared adsorbent surfaces has been accomplished and reported by Azman, et al (2019) [113]. More specifically, the carbon black material that is previously prepared from pyrolyzed waste tires (at 800°C) undergoes chemical-thermal (acid treatment, HNO3, 6 M at 90 °C for 0.5 h duration) and thermal activation (600°C for 1 h duration). The effect of the crucial factors affecting the adsorption process has been also investigated such as the temperature (3 tested values: 30, 50 and 70°C); the initial pH value of the solution (3 tested values: 3, 7, and 11); the initial concentration of aspirin (tests in the range 10-100 mg L-1) and the dosage of the adsorbent (3 tested values: 0.1, 0.5, and 1.0 g) [113]. Under the optimum experimental conditions that were pH=3, temperature =30°C, initial concentration of aspirin=100 mg L-1 and adsorbent dosage =0.02g a maximum removal capacity of the tire-derived adsorbent towards aspirin was observed that was evaluated equal to 40.40 mg g-1 [113].
Pesticides
Pesticides are defined as all the compounds and ingredients (individual or mixtures) that are used to control pests which are unwanted plant or animal species (weeds also included) and therefore are designed to protect the cultured crops either before or after harvest. Several different chemical classes of synthetic organic pesticides are being applied worldwide so as to ensure the best quality and quantity of obtained agricultural products and hence improve crop yields. However, and despite the advantages gained by their use, there are some serious drawbacks that have raised global concerns regarding their potential environmental effects on non-target species of the exposed ecosystems.
The persistence, toxicity and environmental fate of each pesticide applied to terrestrial areas and can reach aquatic systems are depended on their physicochemical properties. In figure 8 are illustrated the skeletal structural formulas of some selected pesticides that have been found in the scientific literature and reviewed in the current study regarding their removal from aquatic solutions via adsorption onto activated waste tire-derived materials [2, 50, 65- 66, 114-115].
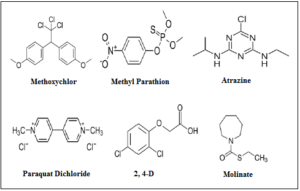
Figure 8. Skeletal structural formulas of selected pesticides found in the reviewed scientific literature concerning their removal from aquatic solutions via adsorption onto activated waste tire-derived materials has been investigated.
The removal of three selected pesticides that belong to three different chemical groups from wastewater by utilizing the adsorption strategy onto activated carbon obtained from the discard rubber tire was surveyed by Gupta, et al. (2011) [2]. More particularly, the organochlorine insecticide methoxychlor, the organophosphorus insecticide methyl parathion, and the triazine insecticide atrazine were selected by the aforementioned authors as model pesticides of high toxicity. Although the application of all three compounds has been banned in developed countries they are still used in the developing countries of the third world. After the performance of the appropriate chemical and thermal pretreatment stages that are described in detail in (table 2), the carbonaceous adsorbent that is obtained has better adsorption characteristics among which the higher mesopore, macropore content and the improved surface chemistry (attributed to the presence of oxygen-containing functional groups, carbonyl and hydroxyl) are included [2]. Both batch and fixed-bed columns studies were performed to investigate the kinetics of the process. The obtained results from conducted batch adsorption experiments showed that under the optimum operating conditions (which were: initial pesticide concentration, 12 mg L-1; contact time, 60 min; particle adsorbent size of 200–250 μm; and pH=2), the decreasing order of adsorption capacities of the adsorbents were for methoxychlor 112.0 mg g-1, for atrazine 104.9 mg g-1, and finally for methyl parathion 88.9 mg g-1 ; this trend was indicative of the influence of the pesticide solubility. The equilibrium data followed the Langmuir kinetic model and well fitness of the pseudo-first-order model was revealed. Furthermore, physisorption and pore diffusion of the molecules of the adsorbates (pesticides) were highlightened as the most important characteristics of the surveyed adsorption system which was evaluated as an efficient technique for the removal of the tested pesticide compounds [2].
Paraquat dichloride (figure 8) is a heterocyclic organic salt known for its herbicidal properties and thus one of the most widely used herbicides, despite the fact that it has been classified as "of restricted use" or in other words used by licensed applicators only. Due to its severe toxic effects (both acute and chronic toxicity) towards organisms of several levels of the trophic chain, even humans, its removal from environmental matrices is a necessity. Hamadi, et al. (2004) have investigated the decontamination of wastewater from paraquat dichloride through the application of adsorption technology that was based on the use of car tire-derived activated carbon [65]. The performance of pyrolyzed and activated adsorbents prepared from discarded tires was evaluated and compared with the ones of commercial activated carbons. Adsorbate’s adsorption was not significantly affected by changes in the pH value, while instead, the process was strongly dependent on other examined parameters of the initial concentration of paraquat, temperature, particle size and dosage of the adsorbent [65]. Adsorption equilibrium was reached very fast in the first 5 min when almost 90% of the target pesticide has been sorbed onto the surface of the tire-obtained carbon via the physical adsorption mechanism. Kinetics studies revealed that the data obeyed best the pseudo- second-order reaction model and least pseudo-first-order or pseudo-second-order reaction models [65].
Rubber granules obtained from waste tires were examined for their ability in sorption and desorption procedures of atrazine and 2,4-D (their chemical structures are shown in figure 8 from aqueous environmental matrices [114]. According to the results reported by Alam, et al. (2004), the achieved values for the removal of atrazine at two tested concentration levels of initial spiking into the solution that were 0.5 and 1 mg L-1 were observed to be 84.2% and 87.6%, correspondingly. The relevant removal of 2, 4-D pesticide was 83.2% in the case of its initial concentration 0.5 mg L-1 and 87% in the case of its initial concentration 1 mg L-1 [114]. Moreover, the feasibility of regenerating the used adsorbent-granules and reusing them in a number of cycles was evaluated. The addition of the organic solvents ethyl alcohol and acetone (15% in both cases) in 2, 4-D and atrazine solutions, respectively caused a successful regeneration of the studied adsorbents for more than three times [114].
Molinate is a selective pre- and early post-emergent thiocarbamate herbicide which is widely known as the most extensively applied herbicide to rice fields all over the planet. Based on our findings during the current review, molinate has been successfully removed from polluted environmental aquatic compartments through the adsorption treatments utilizing recycled granular tire rubber [115]. Specifically, Carvalho, et al., (2010) reported the completely reversible adsorption of this organic herbicide. Equilibrium adsorption measurements were conducted by employing granules whose nominal original particle size was between the range of 0.18–0.60 mm (provided by a recycling company of Portugal), while for column breakthrough tests (fixed bed runs) that were also performed a fraction of smaller particles was engaged with particle sizes between the range of 0.35–0.50 mm (after sieving phase). The acquired column efficiencies of the studied lab-scale system were approximately 40% and the subsequent regeneration of a saturated bed was accomplished with water [115].
On the whole, and on the basis of the found and reviewed literature concerning the removal of extremely bulky molecules of pesticides belonging to several different chemical classes it can be concluded that their adsorption onto activated tire-obtained materials is an effective method for their removal.
PAHs
Polycyclic aromatic hydrocarbons (PAHs), also known by the name polyaromatic hydrocarbons are organic compounds containing multiple aromatic rings (more than two) and they can be found in the environment either by natural or anthropogenic sources. In other words, they can be produced geologically or they can be formed by man-made combustion processes. This class of organic pollutants may disperse widely in the environment from their point and non- point sources through several pathways and mas transference phenomena and finally be distributed and deposited in several environmental compartments and ecosystems. They are considered as environmental pollutants, which are capable to enter into various water and wastewater systems.
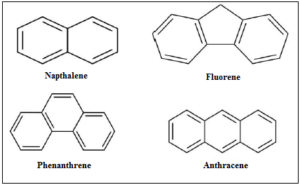
Figure 9. Skeletal structural formulas of selected PAHs found in the reviewed scientific literature concerning their removal from aquatic solutions via adsorption onto activated waste tire-derived materials has been investigated.
A number of scientific research projects aiming to remove PAHs from aquatic solutions by converting highly available waste tires into adsorbent materials has been published [41-42, 116]. In figure 9 the structural formulas of four molecules of PAHs for which their successful adsorptive removal has been reported in the available scientific literature.
According to the results of the work conducted by Gupta, et al. (2016), good adsorption and desorption efficiencies were demonstrated by the produced activated carbon towards the elimination of naphthalene, fluorene and phenanthrene [41]. The preparation of the vehicular derived adsorbents involved the stages of cut of raw tires (into smaller parts), washing (with ultrapure H2O), oven-drying (at 100 °C, for 2 h), carbonization (at 500°C, for 5 h), oxidation (with 30% H2O2, at 60°C for 24 h), cleaning (with ultrapure H2O), oven-dry (at 110°C for 2 h), thermal and alkali treatment (2 g of obtained material and 8 g KOH, at 900°C for 2 h, acid treatment (with 1 M HCl), final wash and dry at 100°C [41]. The adsorption characteristics of the produced carbon were: BET surface area of 643.86 m2 g-1; total pore volume of 0.4270 cm3 g-1, and pore diameter of 2.65 nm, representing its mesoporous nature. The evaluated adsorption capacities (after 120 min, at 20°C) towards the three studied PAHs were 86.20, 79.36, and 68.49 mg g-1 for naphthalene, fluorene, and phenanthrene, respectively. Calculated thermodynamic data (ΔG°, ΔH°, and ΔS°) revealed a spontaneous and endothermic process [41].
The removal of naphthalene from aqueous solutions via the batch equilibration techniques by utilizing ground discarded tires has also been studied by Gunasekara, et al. (2000) [42]. The sorption process that occurred in the surveyed and proposed adsorption system was observed to be a relatively fast procedure that reached equilibrium within 30 min. Based on the calculated adsorption coefficient through the linear isotherm model that was equal to 1340 mg L-1, the method was evaluated as an environmentally friendly and efficient method for the removal of the selected PAH pollutant from aquatic matrices [42].
Good results have been also reported for the case of anthracene that has been removed >99% via the adsorption phenomenon onto activated adsorbents prepared from discard automobile tires [116]. The same authors examined all the experimental factors that influence the process of the adsorption system and found that the increase of anthracene’s concentration in the aquatic phase decreased the adsorption efficiency, while the opposite result was revealed by the contact time that enhanced the whole procedure. In addition, the decrease in the pH- value of the solution led to an increase in adsorption capacity. The equilibrium was reached after 45 min of contact duration in the co-presence of 14-20 mg of the adsorbate and 8 mg of the adsorbent. Better applicability of the kinetic data was observed for the second-order kinetics, whereas the investigation of the adsorption process mechanism and intra-particle diffusion were performed by the application of the Boyd-Reichenberg model [116].
Conclusions
Unquestionably, the development of low-cost, environmentally friendly, and simultaneously effective techniques that can be applied for the removal of toxic and persistent organic and inorganic pollutants by utilizing activated carbons as adsorbent materials prepared from waste vehicular tires is a topic of great importance and a priority for the global research community. For that reason, the adsorption of a wide variety of persistent pollutants via the use of end-of-life-automobile waste tires in adsorption technology is a subject on which scientific interest has been focused on.
Based on the data found during the current survey it was observed that a variety of alternative and different preparation methods for the production of activated carbonaceous adsorbent materials obtained from waste rubber tires has been applied, optimized and evaluated for their adsorption efficiencies. According to the findings of the present review it has been discovered that many studies have reported the fact that the relative fractions of non- carbonized and carbonized organic component materials of pyrolyzed rubber adsorbents are affected by the employed pyrolytic conditions. Numerous techniques that are different either in the employed conditions of pyrolysis (in terms of temperature and duration) or activation stage (thermal or/and chemical) have been investigated. As a consequence, various adsorption materials (powder, chars, nanofibers, etc.) have been produced exhibiting different adsorption properties and characteristics such as surface area, adsorption capacity, and adsorption behavior.
The results of the present review showed that the majority (≈33% of the total found reports) of the relevant published articles have investigated and displayed the high adsorption affinities of a variety of waste tire adsorption materials towards several different heavy metals. Specifically, the corresponding decreasing order of surveyed metals according to their relative scored frequencies of reported articles was: lead (≈19%), mercury (≈19%), copper (≈18%), chromium (≈14%), cadmium (≈11%), zinc (≈5.5%), iron (4.4%), nickel (≈3.3%), arsenate (≈2.2%), manganese (≈1%), and cobalt (≈ 1%). Studies regarding the surveys on the application of activated carbon derived from scrap tires for the adsorption of dyes like Methylene Blue, Methyl Orange, Rhodamine B, and many other substances of that class was in the second place (≈21% of the total found reports), followed by the studies on the sorption of organic solvents in aqueous solutions by recycled tires crumb rubber (≈10% of the total found reports).
Apart from the aforementioned classes of organic pollutants, the adsorption removal of many other organic groups of synthetic contaminants has been also examined by the researchers, such as pesticides, pharmaceuticals, PAHs, petroleum oils, etc. However, the sorption behavior of some “emerging contaminants” by tire sorbents and the mechanisms through which the process occurs are still largely unknown and many research gaps still remain in this direction.
In addition, the forthcoming experimental adsorption systems that are designed to be conducted in the future for the evaluation of their adsorption efficiency in order to simulate better the real environmental conditions should take into account that many of these polluting compounds under realistic conditions are contained in cocktails and not in individual aquatic solutions prepared in ultrapure water. In other words, a target contaminant is simultaneously co-present with another organic and inorganic chemical components of a complex mixture that may influence its adsorption process. Finally, the cost analysis study of each one of the proposed adsorption systems utilizing waste-tire-adsorbents should be conducted as well.
References
- Sienkiewicz M, Kucinska-Lipka J, Janik H, Balas A (2012) Progress in used tyres management in the European Union: a review. Waste Management 32: 1742-1751.
- Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayak A (2011) Pesticides removal from waste water by activated carbon prepared from waste rubber tire. Water Research 45: 4047-4055.
- Ariyadejwanich P, Tanthapanichakoon W, Nakagawa K, Mukai SR, Tamon H (2003) Preparation and characterization of mesoporous activated carbon from waste tires. Carbon 41: 157-164.
- Aisien FA, Amenaghawon NA, Adeboyejo R (2013) Potential application of recycled rubber from scrab tire in the removal of phenol from aqueous solution. Acta Technica Corviniensis-Bulletin of Engineering Tome VI, Fascicule 4 [October-December]: 127-132.
- https://en.wikipedia.org/wiki/Tire_recycling
- Fernández AM, Barriocanal C, Alvarez R (2012) Pyrolysis of a waste from the grinding of scrap tyres. Journal of Hazardous Materials 203-204:236-243.
- Seng-eiad S, Jitkarnka S (2016) Untreated and HNO3-treated pyrolysis char as catalysts for pyrolysis of waste tire: In-depth analysis of tire-derived products and char characterization. Journal of Analytical and Applied Pyrolysis 122: 151-159.
- Acevedo B, Barriocanal C, Alvarez R (2013) Pyrolysis of blends of coal and tyre wastes in a fixed bed reactor and a rotary oven. Fuel 113: 817-825.
- Bunthid D, Prasassarakich P, Hinchiranan N (2010) Oxidative desulfurization of tire pyrolysis naphtha in formic acid/H2O2/pyrolysis char system. Fuel 89: 2617-2622.
- Chan OS, Cheung WH, McKay G (2011) Preparation and characterization of demineralized tyre derived activated carbon. Carbon 49: 4674-4687.
- Zabaniotou Α, Madau P, Oudenne PD, Jung CG, Delplancke M-P, et al. (2004) Active carbon production from used tire in two-stage procedure: industrial pyrolysis and bench scale activation with H2O–CO2 mixture. Journal of Analytical and Applied Pyrolysis 72: 289-297.
- Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayaka A (2011) A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-Acid Blue 113. Journal of Hazardous Materials 186: 891-901.
- Wagner S, Hüffer T, Klӧckner P, Wehrhahn Μ, Hofmann Τ, et al. (2018) Tire wear particles in the aquatic environment - A review on generation, analysis, occurrence, fate and effects. Water Research 139: 83-100.
- Baumann W, Ismeier M (1998) Rubber, Data and Environmental Facts Volume 1/2(Kautschuk und Gummi, Daten und Fakten zum Umweltschutz Band 1/2), 1st Ed Springer-Verlag Berlin Heidelberg, Berlin Heidelberg.
- Wik A, Dave G (2009) Occurrence and effects of tire wear particles in the environment-a critical review and an initial risk assessment. Environmental Pollution 157: 1-11.
- Grigoratos T, Martini G (2014) Non-exhaust traffic related emissions Brake and tyre wear PM, JRC Science and Policy Reports. Luxembourg.
- Castells XE (2000) Reciclaje de Residuos Industriales, vol. 495 Diaz Santos, Madrid.
- Morillo R, Aylon E, Navarro MV, Callen MS, Aranda A (2006) The application of thermal processes to valorize waste tyre. Fuel Processing Technology 87:143-147.
- Giacomin H, Unno M, Eichbauer K, Atkins C (2019) Automotive wastes. Water Environment Research 91: 1223-1228.
- Mukherjee K, Mishra AK (2019) Hydraulic and mechanical characteristics of compacted sand–bentonite: tyre chips mix for its landfill application. Environment Development and Sustainability 21: 1411-1428.
- Rupali J, Sharma S, Sharma A, Verma S (2012) Study of non-conventional low-cost adsorbents for treatment of phenolic waste: A mini review. Pollution Research 31: 277-287.
- Saleh TA, Gupta VK (2014) Processing methods, characteristics and adsorption behavior of tire derived carbons: A review. Advances in Colloid and Interface Science 211: 93-101.
- Labaki M, Jeguirim M (2017) Thermochemical conversion of waste tyres—a review. Environmental Science and Pollution Research 24: 9962-9992.
- Saleh TA, Gupta VK (2014) Processing methods, characteristics and adsorption behavior of tire derived carbons: A review. Advances in Colloid and Interface Science 211: 93-101.
- Williams PT (2013) Pyrolysis of waste tyres: a review. Waste Management 33: 1714-1728.
- Helleur R, Popovic N, Ikura BY, Stanciulescu M, Liu D (2001) Characterization and potential applications of pyrolytic char from ablative pyrolysis of used tires. Journal of Analytical and Applied Pyrolysis 58: 813-824.
- Lehmann CMB, Rostam-Abadi M, Rood MJ, Sun J (1998) Reprocessing and reuse of waste tire rubber to solve air-quality related problems. Energy and Fuels 12: 1095-1099.
- Knocke WR, Hemphill LH (1981) Mercury (II) sorption by waste rubber. Water Research 15: 275-282.
- San Miguel G, Fowler GD, Sollar CJ (2003) A study of the characteristics of activated carbons produced by steam and carbon dioxide activation of waste tyre rubber. Carbon 41: 1009– 1016.
- Nakagawa K, Namba A, Mukai SR, Tamon H, Ariyadejwanich P, et al. (2004) Adsorption of phenol and reactive dye from aqueous solution on activated carbons derived from solid wastes. Water Research 38: 1791-1798.
- Lin YR, Teng H (2002) Mesoporous carbons from waste tire char and their application in wastewater discoloration. Microporous Mesoporous Materials 54: 167-174.
- Acosta R, Fierro V, Martinez de Yuso A, Nabarlatz D, Celzard, A (2016) Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 149: 168-176.
- Al-Rahbi AS, Williams PT (2016) Production of activated carbons from waste tyres for low temperature NOx control. Waste Management 49: 188-195.
- Han L, Liu J, Lou X, Qiu M, Song J, Li P (2016) Adsorption of dye in aqueous solution by the waste polymer activated carbon. Nature Environment and Pollution Technology 15: 1227-1230.
- Lian F, Liu C, Li G-G, Liu Y-F, Li Y, Zhu L-Y (2012) Adsorption and desorption of dyes by waste-polymer-derived activated carbons. Huanjing Kexue/Environmental Science 33: 147-155.
- Benjamin SE, Sajjid MA (2017) Factors affecting the adsorption of trivalent chromium ions by activated carbon prepared from waste rubber tyres. Advances in Science, Technology and Engineering Systems 2: 1660-1664.
- Joseph CG, Hoon GG, Sharain-Liew YL, Krishnaiah D, Massuanna M (2013) Preparation and characterization of activated carbon derived from waste rubber tire via chemical activation with ZnCl2: Surface area and morphological studies. Developments in Sustainable Chemical and Bioprocess Technology 371-380.
- Manchón-Vizuete E, MacÍas-García A, Nadal Gisbert A, Fernández-González C, Gómez- Serrano V (2005) Adsorption of mercury by carbonaceous adsorbents prepared from rubber of tyre wastes. Journal of Hazardous Materials 119: 231-238.
- Hüffer T, Wehrhahn M, Hofmann T (2020) The molecular interactions of organic compounds with tire crumb materials differ substantially from those with other Environmental Science: Processes and Impacts 22: 121-130.
- Lian F, Song Z, Liu Z, Zhu L, Xing B (2013) Mechanistic understanding of tetracycline sorption on waste tire powder and its chars as affected by Cu (2+) and pH. Environmental Pollution 178: 264-270.
- Gupta H, Gupta B (2016) Vehicular tire as potential adsorbent for the removal of Polycyclic Aromatic Hydrocarbons, Polycyclic Aromatic Compounds 38: 354-368.
- Gunasekara AS, Donovan JA, Xing B (2000) Ground discarded tires remove naphthalene, toluene, and mercury from water. Chemosphere 41: 1155-1160.
- Danmaliki GI, Saleh TA (2016) Influence of conversion parameters of waste tires to activated carbon on adsorption of dibenzothiophene from model fuels. Journal of Cleaner Production 117: 50-55.
- Prpich GP, Rehmann L, Daugulis AJ (2008) On the use, and reuse, of polymers for the treatment of hydrocarbon contaminated water via a solid-liquid partitioning bioreactor. Biotechnology Progress 24: 839-844.
- Shahrokhi-Shahraki R, Kwon PS, Park J, O'Kelly BC, Rezania S (2020) BTEX and heavy metals removal using pulverized waste tires in engineered fill materials. Chemosphere 242: 125281.
- Lu Q, de Toledo RA, Xie F, Li J, Shim H (2015) Combined removal of a BTEX, TCE, and cis- DCE mixture using Pseudomonas sp. immobilized on scrap tyres. Environmental Science and Pollution Research 22: 14043-14049.
- Lu Q, de Toledo RA, Xie F, Li J, Shim H (2017) Reutilization of waste scrap tyre as the immobilization matrix for the enhanced bioremoval of a monoaromatic hydrocarbons, methyl tert-butyl ether, and chlorinated ethenes mixture from water. Science of the Total Environment 583: 88-96.
- Alamo-Nole LA, Perales-Perez O, Roman-Velazquez FR (2011) Sorption study of toluene and xylene in aqueous solutions by recycled tires crumb rubber. Journal of Hazardous Materials 185: 107-111.
- Oh DI, Song J, Hwang SJ, Kim JY (2009) Effects of adsorptive properties of biofilter packing materials on toluene removal. Journal of Hazardous Materials 170: 144-150.
- Lian F, Chang C, Du Y, Zhu L, Xing B, et al. (2012) Adsorptive removal of hydrophobic organic compounds by carbonaceous adsorbents: A comparative study of waste-polymer- based, coal-based activated carbon, and carbon nanotubes. Journal of Environmental Sciences (China) 24: 1549-1558.
- Saleh TA, Alhooshani KR, Abdelbassit MSA (2015) Evaluation of AC/ZnO composite for sorption of dichloromethane, trichloromethane and carbon tetrachloride: Kinetics and isotherms. Journal of the Taiwan Institute of Chemical Engineers 55: 159-169.
- Trubetskaya A, Kling J, Ershag O, Attard TM, Schröder E (2019) Removal of phenol and chlorine from wastewater using steam activated biomass soot and tire carbon black. Journal of Hazardous Materials 365: 846-856.
- Tanthapanichakoon W, Ariyadejwanich P, Japthong P, Nakagawa K, Mukai SR, et al. (2005) Adsorption–desorption characteristics of phenol and reactive dyes from aqueous solution on mesoporous activated carbon prepared from waste tires. Water Research 39: 347- 355.
- Mukherjee T, Rahaman M, Ghosh A, Bose S (2019) Optimization of adsorbent derived from non-biodegradable waste employing response surface methodology toward the removal of dye solutions. International Journal of Environmental Science and Technology 16: 8671- 8678.
- Daraei H, Mittal A (2017) Investigation of adsorption performance of activated carbon prepared from waste tire for the removal of methylene blue dye from wastewater. Desalination and Water Treatment 90: 294-298.
- Quek A, Vijayaraghavan K, Balasubramanian R (2011) Methylene blue sorption onto oxygenated pyrolytic tire char: Equilibrium and kinetic studies. Journal of Environmental Engineering 137: 833-841.
- Mui ELK, Cheung WH, McKay G (2010) Tyre char preparation from waste tyre rubber for dye removal from effluents. Journal of Hazardous Materials 175: 151-158.
- Mui ELK, Cheung WH, Valix M, McKay G (2010) Mesoporous activated carbon from waste tyre rubber for dye removal from effluents. Microporous Mesoporous Materials 130: 287-294.
- Sainz-Diaz CI, Griffiths AJ (2000) Activated carbon from solid wastes using a pilot-scale batch flaming pyrolyzer (2000) Fuel 79:1863-1871.
- Chennouf-Abdellatif Z, Cheknane B, Zermane F, Gaigneaux EM, Mohammedi O, et al. (2017) Equilibrium and kinetic studies of methyl orange and rhodamine B adsorption onto prepared activated carbon based on synthetic and agricultural wastes. Desalination and Water Treatment 67: 284-291.
- Tuzen M, Sarı A, Saleh TA (2018) Response surface optimization, kinetic and thermodynamic studies for effective removal of rhodamine B by magnetic AC/CeO2 nanocomposite. Journal of Environmental Management 206: 170-177.
- Li L, Liu S, Zhu T (2010) Application of activated carbon derived from scrap tires for adsorption of Rhodamine B. Journal of Environmental Sciences 22: 1273-1280.
- Khudhair AB, Musa M, Mohd Jaafar MS, Hadibarata T (2015) Cresol red dye removal using recycled waste tire rubber. International Journal of Engineering Research in Africa 16: 57-63.
- Hajizadeh Y, Onwudili JA, Williams PT (2011) Removal potential of toxic 2378-substituted PCDD/F from incinerator flue gases by waste-derived activated carbons. Waste Management 31: 1194-1201.
- 65. Hamadi NK, Swaminathan S, Chen XD (2004) Adsorption of Paraquat dichloride from aqueous solution by activated carbon derived from used tires. Journal of Hazardous Materials 112: 133-141.
- Lian F, Huang F, Chen W, Xing B, Zhu L. (2011) Sorption of apolar and polar organic contaminants by waste tire rubber and its chars in single- and bi-solute systems. Environmental Pollution 159: 850-857.
- Chang N-B, Houmann C, Lin K-S, Wanielista M (2016) Fate and transport with material response characterization of green sorption media for copper removal via desorption process Chemosphere 154: 444-453.
- Chang N-B, Houmann C, Lin K-S, Wanielista M (2016) Fate and transport with material response characterization of green sorption media for copper removal via adsorption process. Chemosphere 144: 1280-1289.
- Deng Y, Morris C, Rakshit S, Landa E, Punamiya P, et al. (2016) Water treatment residuals and scrap tire rubber as green sorbents for removal of stormwater metals. Water Environment Research 88: 500-509.
- Song M, Wei Y, Yu L, Tang X (2016) The application of prepared porous carbon materials: Effect of different components on the heavy metal adsorption. Waste Management and Research 34: 534-541.
- Ramola S, Mishra T, Rana G, Srivastava RK (2014) Characterization and pollutant removal efficiency of biochar derived from baggase, bamboo and tyre. Environmental Monitoring and Assessment, 186: 9023-9039.
- Feroze N, Kazmi M, Iqbal W, Muhammad S. (2013) Heavy metal on waste tire crumb. Journal of Environmental Protection and Ecology 14: 85-98.
- Shahtalebi A, Sarrafzadeh MH, McKay G (2013) An adsorption diffusion model for removal of copper (II) from aqueous solution by pyrolytic tyre char. Desalination and Water Treatment 51: 5664-5673.
- Quek A, Balasubramanian R (2009) Low-energy and chemical-free activation of pyrolytic tire char and its adsorption characteristics. Journal of the Air and Waste Management Association 59: 747-756.
- Oladoja N.A, Ofomaja A, Ebare E. (2006) Evaluation of sorption capacity of scrap tyre in the removal of copper (II) ion from aqua system. Pakistan Journal of Scientific and Industrial Research, 49 (6): 400-406.
- Gupta VK, Ali I, Saleh TA, Siddiqui MN, Agarwal S (2013) Chromium removal from water by activated carbon developed from waste rubber tires. Environmental Science and Pollution Research 20: 1261-1268.
- Hamadi NK, Chen XD, Farid MM, Lu MGQ (2001) Adsorption kinetics for the removal of chromium (VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chemical Engineering Journal 84: 95-105.
- Dimpe MK, Ngila CJ, Nomngongo PN, Xiaoliang Wei X (2017) Application of waste tyre-based activated carbon for the removal of heavy metals in wastewater, Cogent Engineering 4:1-11.
- Alexandre-Franco M, Fernández-González C, Alfaro-Domínguez M, Gómez-Serrano V (2011) Adsorption of cadmium on carbonaceous adsorbents developed from used tire rubber. Journal of Environmental Management 92: 2193-2200.
- Entezari MH, Ghows N, Chamsaz M (2006) Ultrasound facilitates and improves removal of Cd (II) from aqueous solution by the discarded tire rubber (2006) Journal of Hazardous Materials 131: 84-89.
- Liu X, Wang J, Gheni A, ElGawady MA (2018) Reduced zinc leaching from scrap tire during pavement applications. Waste Management 81: 53-60.
- Solano L, Ristvey AG, Lea-Cox JD, Cohan SM (2012) Sequestering zinc from recycled crumb rubber in extensive green roof media. Ecological Engineering 47: 284-290.
- Saleh TA, Gupta VK, Al-Saadi AA (2013) Adsorption of lead ions from aqueous solution using porous carbon derived from rubber tires: Experimental and computational study. Journal of Colloid and Interface Science 396: 264-269.
- Mousavi HZ, Hosseynifar A, Jahed V, Dehghani SAM (2010) Removal of lead from aqueous solution using waste tire rubber ash as an adsorbent. Brazilian Journal of Chemical Engineering 27: 79- 87.
- Lin H-Y, Yuan C-S, Chen W-C, Hung C-H (2006) Determination of the adsorption isotherm of vapor-phase mercury chloride on powdered activated carbon using thermogravimetric analysis. Journal of the Air and Waste Management Association 56: 1550-1557.
- Lin H-Y, Yuan C-S, Wu C-H, Hung C-H (2006) The adsorptive capacity of vapor-phase mercury chloride onto powdered activated carbon derived from waste tires. Journal of the Air and Waste Management Association 56: 1558-1566.
- Imyim A, Sirithaweesit T, Ruangpornvisuti V (2016) Arsenite and arsenate removal from wastewater using cationic polymer-modified waste tyre rubber. Journal of Environmental Management 166: 574-578.
- Siddiqui MN, Redhwi HH, Al-Saadi AA, Rajeh M, Saleh TA (2016) Kinetic and computational evaluation of activated carbon produced from rubber tires toward the adsorption of nickel in aqueous solutions. Desalination and Water Treatment 57: 17570-17578.
- Belgacem A, Rebiai R, Hadoun H, Khemaissia S, Belmedani M (2014) The removal of uranium (VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environmental Science and Pollution Research 21: 684-694.
- Nieto-Márquez A, Atanes E, Morena J, Fernández-Martínez F, Valverde JL (2016) Upgrading waste tires by chemical activation for the capture of SO2. Fuel Processing Technology 144: 274-281.
- Hossain F, Chang N-B, Wanielista M (2010) Modeling kinetics and isotherms of functionalized filter media for nutrient removal from stormwater dry ponds. Environmental Progress and Sustainable Energy 29: 319-333.
- Krayzelova L, Lynn TJ, Banihani Q, Bartacek J, Jenicek P, et al. (2014) A Tire-Sulfur Hybrid Adsorption Denitrification (T-SHAD) process for decentralized wastewater treatment. Water Research 61: 191-199.
- Lin C, Hong Y-J, Hu AH (2010) Using a composite material containing waste tire powder and polypropylene fiber cut end to recover spilled oil. Waste Management 30: 263-267.
- Aisien FA, Hymore FK, Ebewele RO (2003) Potential application of recycled rubber in oil pollution control. Environmental Monitoring and Assessment 85: 175-190.
- Aisien FA, Hymore FK, Ebewele RO (2003) Potential application of recycled rubber in oil pollution control. Environmental Monitoring and Assessment 85: 175-190.
- Aisien FA, Hymore FK, Ebewele RO (2006) Comparative absorption of crude oil from fresh and marine water using recycled rubber. Journal of Environmental Engineering 132: 1078-1081.
- Lin C, Huang CL, Shern CC (2008) Recycling waste tire powder for the recovery of oil spills. Resources, Conservation and Recycling 52: 1162-1166.
- Mashile PP, Dimpe MK, Nomngongo PN (2019) Application of waste tyre-based powdered activated carbon for the adsorptive removal of cylindrospermopsin toxins from environmental matrices: Optimization using response surface methodology and desirability function. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering 54: 679-685.
- Mashile PP, Mpupa A, Nomngongo, PN (2018) Adsorptive removal of microcystin-LR from surface and wastewater using tyre-based powdered activated carbon: Kinetics and isotherms. Toxicon 145: 25-31.
- Mashile GP, Mpupa A, Nqombolo A, Dimpe MK, Nomngongo PN (2020) Recyclable magnetic waste tyre activated carbon-chitosan composite as an effective adsorbent rapid and simultaneous removal of methylparaben and propylparaben from aqueous solution and wastewater. Journal of Water Process Engineering 33: 101011.
- Gupta VK, Ganjali MR, Nayak A, Bhushan B, Agarwal S (2012) Enhanced heavy metals removal and recovery by mesoporous adsorbent prepared from waste rubber tire. Chemical Engineering Journal 197: 330-342.
- Nasyrov IA, Terentyeva VV, Mavrin GV (2018) Investigation of heavy metals emission from pyrolysis product of rubber wastes treated with ashing. International Journal of Green Pharmacy 12:S751-S755.
- Al-Asheh S, Banat F (2000) Adsorption of copper ions on to tyre rubber. Adsorption Science and Technology 18: 685-700.
- Adathodi L, Murugadoss RJ, Gaddam K (2018) Application of a novel adsorbent- aircraft tyre rubber ash for the removal of chromium from wastewaters. Rasayan Journal of Chemistry 11: 1204-1210.
- Li G, Shen B, Lu F (2015) The mechanism of sulfur component in pyrolyzed char from waste tire on the elemental mercury removal. Chemical Engineering Journal 273: 446-454.
- Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. Journal of Environmental Management 113: 170-183.
- Gupta VK (2009) Application of low-cost adsorbents for dye removal-A review". Journal of Environmental Management 90: 2313-2342.
- Elmaslar Özbaş E, Balçık B, Ozcan HK (2019) Preparation of activated carbon from waste tires, and its use for dye removal. Desalination and Water Treatment 172: 78-85.
- Petsas AS, Vagi MC (2019) Trends in the bioremediation of pharmaceuticals and other organic contaminants using native or genetically modified microbial strains: A review. Current Pharmaceutical Biotechnology 20: 787-824.
- Phasuphan W, Praphairaksit N, Imyim A (2019) Removal of ibuprofen, diclofenac, and naproxen from water using chitosan-modified waste tire crumb rubber. Journal of Molecular Liquids 294: 111554.
- Dimpe MK, Nomngongo PN (2019) Application of activated carbon-decorated polyacrylonitrile nanofibers as an adsorbent in dispersive solid-phase extraction of fluoroquinolones from wastewater. Journal of Pharmaceutical Analysis 9:117-126.
- Dimpe MK, Mpupa A, Nomngongo PN (2018) Microwave assisted solid phase extraction for separation preconcentration sulfamethoxazole in wastewater using tyre based activated carbon as solid phase material prior to spectrophotometric determination. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 188: 341-348.
- Azman A, Ngadi N, Zaini DKA, Jusoh M, Mohamad Z, et al. (2019) Effect of adsorption parameter on the removal of aspirin using tyre waste adsorbent. Chemical Engineering Transactions 72: 157-162.
- Alam JB, Dikshit AK, Bandyopadhyay M (2004) Sorption and desorption of 2,4-D and atrazine from water environment by waste tyre rubber granules and its management. Global Nest Journal 6: 105-115.
- Carvalho D, Mendes A, Magalhães FD, Nunes OC (2010) Treatment of waters containing the thiocarbamate herbicide molinate through an adsorption/bio-regeneration system using a low-cost adsorbent. Water, Air, and Soil Pollution 207: 289-298.
- Gupta H (2018) Anthracene removal from water onto activated carbon derived from vehicular tyre. Separation Science and Technology (Philadelphia) 53: 613-625.