Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Highlighting the Competitive Interaction between Pearl Millet and Acacia senegal in Sahelian Agroforestry Systems
ISSOUFOU Hassane Bil-Assanou1*, ELHADJI SEYBOU Djibo2, MOUSSA Alio Adamou, ABDOU Maman Manssour3, LAOUALI Sitou1, ASSOUMANE Aïchatou2, ALZOUMA MAYAKI Zoubeirou3
1Département des Sciences et Techniques de Productions Végétales, Faculté d’Agronomie et des Sciences de l’Environnement, Université Dan DickoDankoulodo, BP 465 Maradi, Niger
2Département de Biologie, Faculté des Sciences et Techniques, Université Abdou Moumouni BP 10662, Niamey Niger
3Faculté des Sciences Agronomiques et de l’Environnement, Université de Tillabéry, Niger
Received Date: December 31, 2019; Accepted Date: January 10, 2020; Published Date: January 21, 2020
*Corresponding author: ISSOUFOU Hassane Bil-Assanou, Département des Sciences et Techniques de Productions Végétales, Faculté d’Agronomie et des Sciences de l’Environnement, Université Dan DickoDankoulodo, BP 465 Maradi, Niger. Tel: +22791188574; Email: hassanebil-assanou.issoufou@ird.fr
Citation: Bil-Assanou IH, Djibo ES, Adamou MA, Manssour AM, Sitou L, et al. (2020) Highlighting the Competitive Interaction between Pearl Millet and Acacia Senegal in Sahelian Agroforestry Systems. Adv Agri Harti and Ento: AAHE-108.
Abstract
The scaling up of the agroforestry requires the evaluation of its agronomic and environmental performances. An experimental study was undertaken in Acacia senegal parkland in Sahelian region of Niger. The effects of tree cover on pearl millet above and below-ground biomass production as well as on its water status were studied. The experimental design was implemented during rainy season and was consisted of concentric circles around the tree trunk of A. Senegal determining four zones (Z1, Z2, Z3 and Z4). Ten plots (T) were also set up far from the trees and used as a control. Predawn leaf water potential (ΨPD) was measured on pearl millet plants located in these zones and on A. senegal. Yield components (ear weight, grain weight and below-ground dry biomass weight) of pearl millet were assessed in the delimited zones. Soil matric potential (ΨM) was measured under and outside the A. Senegal tree crown. Results showed that ΨPD measured on A. Senegal leaves was not different from that of pearl millet in Z1 but was lower than the ΨPD measured on millet in the remaining three zones. We also found that ΨM was lower crown under its outside. Due to tree competition for water, grain yield as well as biomass were lower in Z1than in zonesZ2, Z3, Z4 and T far from the tree trunk, thus zone Z1 can be used for the association with a third crop of C3plant which tolerates tree shade.
Keywords: Agro Forestry; Semi-Arid; Tree-Crop Interactions; Water Status
Introduction
Combination of trees to rain fed cropping systems is a secular in sub-Saharan Africa. This system is today an alternative to the problems of land degradation and loss of organic soil fertility for subsistence farmers [1-3]. The tree-crop association became an important means of building resilience of rain-fed farming systems in the face of strong climatic variability [4] because its led to improve and diversify the production in the Sahelian context of high demographic growth (3% / year).
In most Sudano-Sahelian farming areas, there is a diversity of agroforestry parks whose specific composition arise from fallow recruits during its recultivation [4], but also recently the practice of assisted natural regeneration [5]. However, in Niger most of Acacia Senegal parklands arose from the policy of re-launching gum Arabic sector [6]. The lack of success of certain plantations for the production of gum has led farmers to practice cereal intercropping. Now, both natural stands and plantations have today become beyond, over gum production, to agroforestry vocation thus offering a very important agronomic potential [7-9].
A. senegal is a tree of Leguminosae family (legumes) widely distributed in Sudano-Sahelian agro-pastoral areas where it is used for the production of gum arabic, fodder, wood and in agroforestry.A. senegal has a dimorphic root system with both lateral and pivoting deep roots [10], which allows it to maximize water and nutrients uptake from superficial and deep layers of soil. A. senegal forms symbiotic root nodules with a wide variety of microorganisms (bacteria and fungi) which allowing it to take up atmospheric nitrogen [11-13] as well to improve water supply [14]. The importance of its root system and the symbiotic potential allows A. senegal to survive severe droughts and nutritional stress but also to be associated with cereals in order to improve soil nitrogen level and thus to increase the production.
A. senegal is a deciduous long-leafed specie that shed its leaves at the beginning of the dry season (September) and continues gradually throughout it, but leaves bud burst occurs before the onset of rains with (April-May) [15-16]. The maximum leafing stage is centered in the rainy season; which is the growing period of intercropping cereals. In a seasonal environment, it is well-known that deciduous trees have high photosynthetic and water uptake efficiency related to their broadleaved traits [17].
Pearl millet (Pennisetumglaucum) is the major staple crop in soudano-sahelian belt. Thank to it important root systems, pearl millet tolerates drought and is well-adapted to the low level of soil fertility [18]. For the most part in soudano-sahelian areas, pearl millet is not produced in monocropping but it is often associated to other cereals (cowpea, sorghum, groundnut…). Recently, with the scaling up of agroforestry in Niger leading to the densification of trees cover, research on functioning of pearl millet-based production systems should consider the presence of trees.
Due to the limitation of water resources, we therefore hypothesize that a competitive interaction would exist between A. senegal and intercrops. The aim of this study was to (i) study the water states of millet and A. senegal trees (ii) and determine their impacts on the production of millet biomass (above and below ground) in order to characterize the nature of interaction between A. senegal and millet cultivation. The results would help improve our understanding of the functioning mechanisms of an agroforestry species in the Sahelian agricultural environment, where such knowledge is still scarce, in order to optimize the production of intercrops.
Material and Methods
Site and Experimental Design Description
The study was carried out at Dakoro (14°30′38″N and 6°45′54″E) located in Maradi region, Central-south of Niger. It consisted ofA. Senegal-based parkland with intercropped pearl-millet. It is an agro-pastoral area with a Sahel-type bioclimate where the total annual rainfall varied from 350 to 400 mm/year, with rain falling from June to September. The average annual temperature is 28.3 °C. Soils are mainly sandy with tropical ferruginous types poor in organic matters.
Material is composed of A. Senegal tree stands and an early variety of pearl-millet (HKP) selected by IRAT (Institute for Tropical Agronomic Researc h) and released by INRAN (National Institute of Agronomic Research of Niger). This variety of pearl-millet is considered to be resistant to drought [19]. A total of ten (10) adult trees of A. senegal were chosen for this study. Each tree was isolated from its neighbors by a distance at least equal to twice the radius of its crown regardless of the direction considered. The characteristics of the 10 selected trees are showed in (Table 1).
Sampled Trees
Trunk Diameter at 1.30 m (cm)
Height (m)
Crown Diameter (m)
Geographic Coordinates
P1
21.56
4.60
7.27
N 14°31’42,4’’ E 6°42’54,7’’
P2
20.21
4.65
7.35
N 14°31’43,4’’ E 6°42’54,5’’
P3
19.74
4.50
5.30
N 14°31’43,8’’ E 6°42’53,6’’
P4
27.06
5.75
8.07
N 14°31’46,8’’ E 6°42’52,2’’
P5
11.14
4.20
5.86
N 14°31’47,1’’ E 6°42’51,3’’
P6
19.74
5.50
9.30
N 14°31’41,7’’ E 6°42’50,3’’
P7
15.60
4.30
6.41
N 14°31’40,5’’ E 6°42’50,0’’
P8
14.64
5.50
8.95
N 14°31’39,0’’ E 6°42’48,0’’
P9
25.46
5.10
7.70
N 14°31’37,4’’ E 6°42’48,1’’
P10
26.10
6.80
8.56
N 14°31’35,4’’ E 6°42’45,8’’
Means
20.12
5.09
7.48
CV (%)
25.97
15.90
17.62
CV (%): Coefficient of variation
The experimental design was consisted of concentric circles around of each selected tree trunk comprising four (4) delimited zones: one circle and three concentric rings (see Figure 1, [20]). The radius of concentric circles was determined based on the average crown diameter of each tree. The first circle covering half (½) of the radius of the crown of the tree, represents zone Z1; the second covering the rest of the tree crown represents zone Z2, the third zone Z3 enveloping Z2 on the ½ radius of the tree crown and the fourth Z4 wrapping Z3 on ½ radius of the crown constituting the zone outside crown represents the zone Z4 (Figure 1). Ten 10*10m plots away from the trees were delimited serving as a control.


Figure 1: from left to right: overview of study site and on-farm experimental design.
Pearl-Millet above-and Belowground Biomass Production: Pearl-millet production parameters such as ear weight (kgha-1), grain weight (kgha-1) and below-ground dry biomass weight (kgMSha-1) were recorded in each zone at maturity.
A semi-destructive method a root auger with a volume of 0.74 dm3 (Eijkelkamp Academy, The Netherlands) was used to sample the roots. In each zone around each tree, four holes were drilled along the cardinal points and in each hole, soil cores were taken at depths of 0-25, 25-50, 50-75, 75-100, 100 -125 and 125-150 cm. Roots were extracted from the soil samples by sieving under a water jet with a fine mesh sieve and dried at70 °C for at least 3 days. After drying, samples were weighed on a G & G JJ224BC balance (accuracy 0.1 mg).
Water Status of Soil, Tree and Crop
Predawn leaf water potential (ΨPD) were monitored using a membrane press (HP, Objectif K model, France) on pearl-millet and A. Senegal trees selected in different areas. Soil matrix potential was determined on samples collected under -and outside of A. Senegal trees crown using a dew point potential meter (WP4-T Decagon Devices, Inc.). The closure of the stomata having allowed a rebalancing of the two potentials overnight [21], we considered the basic leaf water potential as an estimate of the soil water potential in the root zone.
Data analysis
Analysis of variance was performed to test the effects of zone on A. senegal trees and pearl-millet predawn leaf water potential (ΨPD) and on pearl-millet yield components (ear weight, grain weight and dry biomass weight) using R64 3.4.4 software (R Core Team). The means were compared by the Fisher test at the threshold of 5%.
Results
Value ranges of matric potential in the soil (ΨM) and predawn leaf water potential of A. senegal trees and pearl-millet (ΨPD) are shown in Figure 2. Predawn leaf water potential of pearl-milletin zone Z1 (-0.97 MPa) was not significantly different from that of the tree (-1.02 MPa) (Figure 2a). However, it is significantly different from that measured on pearl-milletin zones Z2, Z3 and Z4 which is around -0.7 MPa. Similarly, the matric potential of the soil measured under the crown close to the tree is lower (-0.73 MPa) than that measured outside the crown (-0.58 MPa) (Figure 2b).
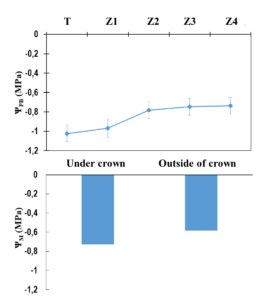
Figure 2: (a) Predawn leaf water potential on A. senegal trees (Zone T) and pearl mil sur les plants at maturity in Z1, Z2, Z3, et Z4. (b) Soil matric potential under –and outside of A. senegal trees in the study of Dakoro in the southern Sahel (Niger).
The variation of pearl-millet root density according to soil depth in the different delimited areas is illustrated in (Figure 3). In all zones, pearl-millet root density decreased exponentially with soil depth (R2 between 0.69 and 0.87) as expected in sandy soils. However, in the upper horizons of soil, pearl-millet root density was higher in zone (Z1) closer to the A. senegal tree than those far away. In all zones pearl-millet roots were found to a depth of 150 cm.
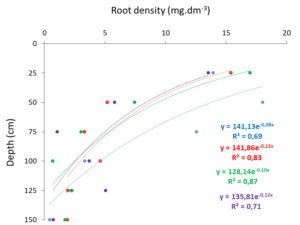
Figure 3: Variation of pearl-millet root density according to soil depth in the different delimited areas in the study of Dakoro in the southern Sahel (Niger) (blue: Z1, red: Z2, green: Z3 and violet: Z4).
(Table 2) indicates yield components (ear weight, grain weight and dry biomass weight) in different delimited zones. Yield components were significantly greater in zones (Z3 and Z4) far from the A. Senegal tree and in control zone (T) than in those which are close (Z1 and Z2). Zones
Yield components
Earyield (kg/ha)
Grain yield (kg/ha)
Dry biomass yield (kgMS/ha)
Z1
530,40c
310c
658,47b
Z2
926, 65b
638,33bc
1449,21a
Z3
1373,40a
961,04a
1712,61a
Z4
1286,54a
900,25a
1958,28a
T
1153,50ab
891,74ab
1399,01ab
Means in the same row followed by the same letter are not significantly different (α =0.05%; Fischer test)
Discussion
Impact de l’arbre sur les États Hydriques du sol et des Plants du Mil
The objective of the study was to analyze the water status of soil, intercropped cultures and trees in an A. senegal-based parkland. The soil and intercropped plant water status improvement due to the presence of trees in semi-arid agroforestry systems have been widely documented [22-27]. Indeed, the woody cover mitigates aridity effects by increasing the infiltration and recycling of precipitation by a deeper root extraction while declining the direct evaporation from the soil. Furthermore, several studies have shown that species with a dimorphic root system have an ability to passively up well water from deeper moist soil layers to drier surface layers which would favor intercropping and other shaded species [28-32].
Our results showed that in environments close to the A. senegal tree, the soil was drier and pearl-millet plants growing there were more stressed than those located in the zones outside the crown. The water stress observed on the pearl-millet plants located near the tree may be explained by the canopy phenology of A. Senegal. Indeed, in semi-arid agroforestry systems in Kenya, Muthuri et al. (2009) [25] observed that the rate of transpiration is higher in Paulownia fortunei, a deciduous species, than in Alnusacuminata and Grevillea robusta respectively a semi-deciduous and an evergreen species. This author has shown that young mature leaves of Paulownia fortunei have a very high transpiration rate and photosynthetic efficiency. A. senegal buds before the start of the first rain [15, 16] and its leaves become mature when crops are installed, which would increase it competitive capacity against other species.
Impact de l’arbre sur la Production de Biomasse des Plants du Mil
In our study conditions, the variation in the root density of pearl millet as a function of the depth of the soil is characterized by an exponential relationship. Similar relationships have been observed in other arid ecosystems in both herbaceous and woody plants. For example, in the north-Sahelian past oral rangelands of Senegal, more arid than our site, Grouzis and Akpo (1997) [33] reported that the root density of herbaceous plants varied exponentially as a function of depth, both outside and under tree crown. Similarly, in the South Sahelian agroforestry systems, Kizito et al. (2006) [24] found an exponential relationship between root density and depth in Guierasenegalensis and Piliostigmareticulatum. On the other hand, we have found that pearl-millet roots can reach significant depths. Our results are consistent with those of Brück et al. (2003) [34] which found roots up to 200 cm.
One of the morphological mechanisms of arid environments plants to response to water and nutritional constraints is the development of an important root system allowing of extracting water and the reduction of above-ground biomass to reduce its losses [35-37]. Our results showed that cumulated root density of pearl-millet in the 0-150 cm depth interval was higher in zone Z1 (51 mg / dm3), from which level the soil contains less water (ѰM = -0.73 MPa and ѰPD = -0.97 MPa), than in the other zones (on average 30 mg / dm3). These results corroborate those of Santucci et al. (1990) [38] who showed an increase in the root density of pearl-millet, in response to the imposition of water stress under controlled experimental conditions in the Sahelian environment. In the Mediterranean environment in Morocco, El Fakhri et al. (2010) [39] showed that the imposition of water stress led to an increase of 40 to 80% in the root density on ten genotypes of durum wheat (Triticum Durum Desf). In the semi-arid region of Sudan, Abaker et al. (2018) [40] showed that soil humidity is higher under grasslands than under A. senegal parklands. This phenomenon has been explained by a presence of the fine roots of A. senegal in the upper horizons. However, highlighting the increase of pearl-millet root biomass due to the competition for soil water related to the presence of trees in agroforestry parks as an adaptive response is new.
Inversely to the observed impacts on root biomass, we found the highest pearl-millet aboveground biomass in zones far away from the tree crown (Z2, Z3 and Z4). However, the presence of A. senegal in crop fields really creates an agro-ecological environment favorable to the production of pearl-millet because production is statistically higher in Z3 and Z4 compared to the control. Although this results confirm a competitive interaction for water resources and probably nutriments between A. senegal tree and pearl-millet in zone Z1, the average grain yields in zones Z4, Z3, Z2 and in the absolute control are clearly higher than the national one (450 kg/ha; [41]. The low yields observed in zone Z1 suggested that, in this zone, pearl-millet reallocates more energy in the production of root biomass than in the production of seeds.
Conclusion
Unlike the agroforestry parklands of Faidherbiaalbida [42] and Guierasenegalensis [2, 43], in the A. senegal system, seedlings must be densified in zones Z4 and Z3 and secondarily in the zone Z2. On the other hand, pearl-millet is a C4 plant that is well adapted to high temperature [44] but which has significant photosensitivity. Due to the shade and lower temperatures, zone Z1 can be used for an association with a third crop of type C3 which tolerates the shade of the tree.
Acknowledgments
The authors are grateful to AIRD for funding the research program of the AVACLI team (Agriculture Adaptation to Climate Change), which supported the realization of the study. They are also grateful to the Nigerien Ministry of Agriculture through its Departmental Directorate of Dakoro and to the research team of Dan Dicko Dankoulodo University of Maradifor technical support.
References
- Kessler JJ, Breman H (1991) The potential of agroforestry to increase primary production in the Sahelian and Sudanian zones of West Africa. Agroforestry Systems 13: 41-62.
- Wezel A (2000) Scattered shrubs in pearl millet fields in semiarid Niger: Effect on millet production. Agroforestry Systems 48: 219-228.
- Félix G.F., Scholberg J.M.S., Clermont-Dauphin C., Cournac L., Tittonell P. 2018. Enhancing agroecosystem productivity with woody perennials in semi-arid West Africa. A meta-analysis. Agronomy for Sustainable Development 38: 57.
- Boffa J-M (1999) Agroforestry parklands in Sub-Saharan Africa. FAO Conservation Guide 34. Food and Agriculture Organization of the United Nations, Rome, Italy.
- Larwanou M, Saâdou M (2011) The role of human interventions in tree dynamics and environmental rehabilitation in the Sahel zone of Niger. J Arid Environ 75:194-200.
- Ministère de l’Hydraulique, de l’Environnement et de la Lutte contre la Désertification (2003) Stratégie Nationale de Relance de la production et de la commercialisation de la gomme Arabique au Niger. République du Niger. 118 p.
- Fadl KEM, SE El sheikh (2010) Effect of Acacia senegal on growth and yield of groundnut, sesame and roselle in an agroforestry system in North Kordofan state, Sudan. Agroforest Syst 78: 243-252.
- Fadl KEM (2013) Influence of Acacia senegal agroforestry system on growth and yield of sorghum, sesame, roselle and gum in north Kordofan State, Sudan. Journal of Forestry Research 24: 173−177.
- Abdoulkadri A, Assoumane A, Abdou M M, Issoufou HB-A, El Hadji Seybou D, MayakiAlzouma Z (2018) Improvement of the productivity of millet (Pennisetumglaucum (L.) R. Br.) Intercropped with the Arabic gum tree (Acacia senegal (L.) Willd.) in agroforestry parkland in Niger. Advances in Agricultural Science 7: 74-84.
- Poupon H (1980) Structure et dynamique de la strate ligneuse d’une steppe sahélienne du Nord-Sénégal. Trav. Orstom, Paris, DOC.no 115. 351 p.
- Dommergues Y, Duhoux E, Diem HG (1988) Les arbres fixateurs d’azote en foresterie et agroforesterie tropicales. Bull Soc bot. Fr., 135, Actuel. bot., 3, 49-64.
- Bala K, Rao AV, Tarafdar JC, (1989) Occurance of VAM associations in different plant species of the Indian desert. Arid Soil Reasearch and Rehabilitation, 3, 391-396.
- Bakhoum N, Fall D , Fall F, Diouf F, Hirsch AM, Balachandar D, Diouf D (2018) Senegaliasenegal (synonym: Acacia senegal), its importance to sub-Saharan Africa, and its relationship with a wide range of symbiotic soil microorganisms. South African Journal of Botany 119: 362–368.
- Laminou Manzo O, Ibrahim D, Campanella B, Paul R (2009) Effects of mycorrhizal inoculation of the substrate on growth and water stress tolerance of five sand dune fixing species: Acacia raddianaSavi ; Acacia nilotica (L.) Willd. Ex Del. var. adansonii; Acacia senegal (L.) Willd; Prosopischilensis andBauhinia rufescens Lam.. Geo-Eco-Trop. 33: 115-124.
- Hiernaux P, Cisse MI, Diarra L, De Leew PN (1994) Fluctuations saisonnières de la feuillaison des arbres et des buissons sahéliens. Conséquences pour la quantification des ressources fourragères. Revue d’Elevage et de Médecine Vétérinaire des Pays tropicaux 47: 117-125.
- De Bie S, Ketner P, Paase M, Geerling C (1998) Woody plant phenology in the West Africa savanna. Journal of Biogeography: 883-900.
- Givnish TJ (2002) Adaptive Significance of Evergreen vs. Deciduous Leaves: Solving the Triple Paradox. Silva Fennica 36(3): 703-743.
- Winkel T, Do F (1992) Caractères morphologiques et physiologiques de résistance du mil, Pennisetumglaucum (L.) R. Br., à la sécheresse. L’Agronomietropicale46: 339-351.
- Daouda Ousmane S (1991) Rôle du système racinaire dans la résistance du mil à la sécheresse:Mémoire de fin de première année de formation à la recherche. Niamey: IRI, 51 p. multigr.
- Bayala J, Sanou J, Teklehaimanot Z, Ouedraogo SJ, Kalinganire A, Coe R, van Noordwijk M (2015) Advances in knowledge of processes in soil–tree–crop interactions in parkland systems in the West African Sahel: A review. Agriculture, Ecosystems and Environment 205: 25-35.
- Ritchie GA, Hinkley TM (1975) The pressure chamber as an instrument for ecological research. AdvEcolRes 9: 165-254.
- Charreau Claude, Vidal Pierre (1965) Influence de l’Acacia albida sur le sol, nutrition minérale et rendements des mils Pennisetum au Sénégal. L’Agronomie Tropicale. Série 3, Agronomie Générale. Etudes Scientifiques20: 600-626.
- Brenner AJ, Jarvis PG, Vandenbeldt RJ (1991) Transpiration from a neem windbreak in the Sahel. Soil Water Balance in the Sudano-Sahelian Zone. In: Proceedings of the Niamey workshop, February 1991.
- Kizito F, Dragila M, Sène M, Lufafa A, Dick RP, Diedhiou I, Dossa E, Khouma M, Ndiaye S, Badiane A (2006) Seasonal soil water variation and root patterns among two semi-arid shrubs coexisting with Pearl millet in Senegal, West Africa. J. Arid Environ. 67: 436-455.
- Muthuri CW, Ong CK, Craigon J, Mati BM, Ngumi VW, Black CR (2009) Gas exchange and water use efficiency of trees and maize in agroforestry systems in semi-arid Kenya. Agriculture, Ecosystems and Environment 129: 497-507.
- Siriri D, Wilson J, Coe R, Tenywa MM, Bekunda MA, Ong CK, Black CR (2013) Trees improve water storage and reduce soil evaporation in agroforestry systems on bench terraces in SW Uganda. Agroforest Syst 87: 45-58.
- Issoufou HB.A, Demarty J, Cappelaere B, Allies A, Moussa R, Velluet C, Maïnassara I, Chazarin JP, Oï M, Seghieri J (2019) Contribution des arbustes au fonctionnement hydrique et carboné des parcs agroforestiers à GuierasenegalensisF Gmel : observations et modélisation. In: Seghieri J. & Armand J-M. (eds). Agroforesterie et services écosystémiques en zone tropicale. Montpellier, France: Editions Quæ, 205-215.
- Caldwell MM, Dawson TE, Richards JH (1998) Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113: 151-161.
- Burgess SSO (2011) Can hydraulic redistribution put bread on our table? Plant Soil 341: 25-29.
- Moreira MZ, Scholz FG, Bucci SJ, Sternberg LS, Goldstein G, Meinzer FC, Franco AC (2003) Hydraulic lift in a neotropicalsavanna. Functional Ecology 17: 573-581.
- Muñoz MR, Squeo FA, León MF, Tracol Y, Gutiérrez JR (2008) Hydraulic lift in three shrub species from the Chilean coastal desert. Journal of arid Environment 72: 624-632.
- Burgess SSO, Pate JS, Adams MA, Dawson TE (2000) Seasonal water acquisition and redistribution in the Australian woody phreatophyte, Banksia prionotes. Annals of Botany 85: 215-224.
- Grouzis M, Akpo LE (1997) Influence of tree cover on herbaceous above- and below-ground phytomass in the Sahelian zone of Senegal. Journal of Arid Environments 35: 285-296.
- Brück H, Piro B, Sattelmacher B, and Payne WA (2003) Spatial distribution of roots of pearl millet on sandy soils of Niger. Plant Soil 256, 149–159.
- Levitt J (1980) Drought avoidance. In: Responses of Plants to Environmental Stress. Lelitt, J. (ed). PhysiologicalEcology. II: 93-128.
- Turner NC (1986) Crop water deficit: a decade of progress. Adv. Agron. 39: 1-51.
- Collin P (2001) Plant adaptations to environmental conditions. AnnéeBiologique40: 21-42.
- Santucci P, Thiery J, Daouda Ousmane S, Do F, Marini P (1990) Contribution à l’étude des profils racinaires du Mil et méthode de calcul de la masse racinaire sous le poquet.
- El Fakhri M, Mahboub S, Benchekroun M, Nsarellah N (2010) Effet du stress hydrique sur les caractéristiques d’enracinement du blé dur (TriticumdurumDesf). Nature et Technologie 3: 6-12.
- Abaker WE, Berninger F, Starr M (2018) Changes in soil hydraulic properties, soil moisture and water balance in Acacia senegal plantations of varying age in Sudan. Journal of Arid Environments 150: 42–53.
- Ministère de l’Agriculture (2005) Résultats définitifs de la campagne agricole 2014 et Perspectives alimentaires 2014-2015, Niger. 32 pp.
- Maï Moussa KA (1996) Environnement de FaidherbiaalbidaA; chev. Caractérisation, exploitation et perspective d’optimisation dans les zones soudano-sahéliennes de l’Afrique de l’Ouest du Niger. Thèse de doctorat 3e cycle. Université Nationale de Côte d’Ivoire, 147 P.
- Issoufou HB.A, Rambal S, Le Dantec V, Oï M, Laurent JP, Saadou M, Seghieri J (2015) Is the WBE model appropriate for semi-arid shrubs subjected to clear cutting? Tree Physiology 35: 197-208.
- Breman H, Kessler J (1995) Woody plants in agro-ecosystems of semi-arid regions, with an emphasis on the Sahelian countries. Berlin Springer-Verlag, Berlin.