ISSN: 2641-6816
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2018
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Biopreservation and Sensory Quality of Soymilk (Glycine Max) By Using Essential Oil from Cymbopogon Citratus (DC) Stapf in Burkina Faso
Agbémébia Yawovi Akakpo1,4*, Marius K. Somda1,2, Abdoul-latif Fatouma Mohamed3, Donatien Kabore5, Essodolom Taale2, Henriette B. Mihin1, Cheik A. T. Ouattara1, Souleymane Sanon6, Alfred S. Traore2, Aboubakar S. Ouattara1
1Départment de Biochimie Microbiologie, Université Joseph KI-ZERBO, Centre de Recherche en Sciences Biologiques Alimentaires et Nutritionnelles (CRSBAN), Laboratoire de Microbiologie et de Biotechnologies Microbienne (LAMBM), Ouagadougou, BFA/03BP:7021, Burkina Faso
2Départment de Biochimie Microbiologie, Université Joseph KI-ZERBO, Laboratoire de Technologie Alimentaire, Ouagadougou, BFA/03BP:7021, Burkina Faso
3Institut de Recherches Médicinales, Centre d’Etudes et de Recherche de Djibouti, Route de l’aéroport, BP: 486 Djibouti, Djibouti
4Institut Togolais de Recherche Agronomique (ITRA), Laboratoire de Contrôle Qualité et Normalisation (LCQN), Lomé, BP: 1163, Togo
5Institut de Recherche en Sciences Appliquées et Technologies (IRSAT), Département Technologie Alimentaire (DTA), Ouagadougou, BFA/03BP:7047, Burkina Faso
6Centre National de Recherche et Formationsur le Paludisme (CNRFP), Ouagadougou, BFA/01 BP: 2208, Burkina Faso
Received Date: 06 May, 2019; Accepted Date: 31 May, 2019; Published Date: 16 November, 2019
*Corresponding Author: Agbémébia Yawovi AKAKPO, 1Départment de Biochimie Microbiologie, Université Joseph KI-ZERBO, Centre de Recherche en Sciences Biologiques Alimentaires et Nutritionnelles (CRSBAN), Laboratoire de Microbiologie et de Biotechnologies Microbienne (LAMBM), Ouagadougou, BFA/03BP:7021, Burkina Faso (and) 3Institut Togolais de Recherche Agronomique (ITRA), Laboratoire de Contrôle Qualité et Normalisation (LCQN), Lomé, BP: 1163, Togo. Tel : +22666746905 Email: akakpo07@hotmail.fr
Citation: Akakpo AY, Somda MK, Kabore D, Taale E, Mihin HB, Ouattara CAT, Sanon S, Traore AL, Ouattara AS (2019) Biopreservation and Sensory Quality of Soymilk (Glycine Max) By Using Essential Oil from Cymbopogoncitratus (DC) Stapf in Burkina Faso. Adv Nutri and Food Sci: ANAFS-131.
Abstract
The use of essential oil in foods has attracted great interest, due to their antimicrobial and aromatic properties. The aim was to evaluate essential oil from Cymbopogon citratus for the biopreservation of soymilk. Thirty samples of soymilk were collected and the essential oil was extracted from Cymbopogon citratus leaf. Physicochemical and microbiological parameters were carried out by using Official Methods of Analysis. Three concentrations (v/v) of 0.15%; 0.25% and 0.3% of essential oil were tested. Soymilks were stored at 4°C and 26±1°C. The result showed that with 0.15% of essential oil, pH was 6.5 to 6.4 and titratable acidity was 0.138 to 0.141% of lactic acid for three weeks under refrigerating (4°C) with no significance difference. There was decrease of total viable and fungal count load in soymilk added essential oil comparatively to soymilk without essential oil. The overall acceptability of essential oil in soymilk was 0.15%. It can be concluded that essential oil from Cymbopogon citratus is able to preserve soymilk and could be used to prevent biodeterioration.
Keywords: Bio-activities; Biopreservation; Cymbopogon citratus; Food safety; Soymilk
Practical Applications of This Study
In the present study, essential oil from Cymbopogon citratus was used to preserve self-life of soymilk due to it antimicrobial substances content. So, essential oil has worked as natural soymilk preservative compared to synthetic and chemical preservatives. The overall acceptability of this essential oil in soymilk was 0.15%. In this study, the use of essential oil allowed to preserve food matrix (soymilk) against deterioration and to prolong self-life for 3 weeks at refrigerating condition. In conclusion practical application of this study will concern essentially the safety and preservation of soymilk or beverage.
Introduction
Soybean (Glycine max Linn Merrill) is a legume widely consumed as skewers, pulp, stock milk, yoghourt [1, 2, 3]. It plays an important role in traditional diets of human and feed animal due to their nutritional properties such as proteins with a high nutritive value, carbohydrate, lipid, vitamins, minerals, essential amino acid profile and the beneficial characteristics of their content [4]. Soybean also contains many bioactive compounds such as polyphenol, flavonoid, vitamin E, vitamin C, phytochemical [5]. Many researches showed that the presence of many bioactive compounds in soybean can help people to prevent heart disease, cancer, obesity, osteoporosis, cardiovascular diseases and coronary risk [6]. The beverage elaborated from soybeans is called soymilk [7]. It is nutritive and is composed of 44.90 to 48.36% of proteins [1]. Soymilk is deprived of cholesterol, contains flavonoids and lipids. So lipids are constituted mainly of poly unsatured fatty acids and presented dietetic interest [7].
In Burkina Faso, as other developing countries, local beverage is flourishing rapidly due to important socio-economic benefits derived from it [8]. Soymilk is produced by local companies and consumed only by 11.91% of households comparing to 94.47% as skewers [3]. This beverage could play a significance part in healthy diet but it could be potential source of bacterial pathogens notably: pathogenic Escherichia coli, Salmonella spp., Shigella spp. and Staphylococcus aureus [9].
Manufacturing of beverage strongly implicate manual operation without significance pasteurization process to prevent microbial contamination. In fact, this traditional processing method might be a source of foodstuff pathogens growth [10]. During soymilk storage, chemical reactions could occur due to the presence of microorganisms, which induce deterioration of nutritional and organoleptic qualities and reducing the duration of preservation. Spoilage and contamination may occur in the soymilk chain because of poor hygiene, long periods of transportation and lack of appropriate storage facilities [11]. The instability of soymilk could create it deterioration and economical lost. Thus rapid solution to preserve beverage is to use synthetics preservation which are not without consequences on the nutritional and organoleptic quality of milk. The alternative to preserve soymilk is to employ natural bioactive molecules from plants [12]. However one of efficient way of beverage preservation is a process combining addition of essential oil and heat treatment [13]. In this regard, several studies aim new sources of plants with antimicrobial activity, with fewer side effects, low cost, greater safety and efficacy for drink preservation. Essential oil of plants known for aromatizing, antioxidant and antimicrobial properties is generally used for food conservation [14]. Among plants used, Cymbopogon citratus (DC) Stapf (C. citratus) is a prominent herb; mainly for it essential oil [15]. Several studies have reported the antimicrobial activities of its oil against different Gram positive and Gram negative pathogenic bacteria, yeasts and fungi [16]due to their major bioactive component[17,18]. Based on biological properties and attributes of essential oil from C. citratus, it has used to preserve deterioration cause by microorganisms or grass oxidation and it was used to prolong the shelf-life of food [19]. The present study was performed to preserve soymilk by using essential oil from Cymbopogon citratus.
Materials and Methods
Materials
Soymilk and essential oil from C. citratus (DC) Stapf are used in this study. The essential oil was extracted from C. citratus leaf dried at room temperature for five days.
For the antimicrobial activity of essential oil, microorganisms such as Escherichia coli, Micrococcus luteus LMG 3293, Rhyzopus nigricans and Aspergillus niger were used due to their capacity of food deterioration and toxin-infection. The microorganisms were obtained from Research Center in Biological, Food and Nutrition Sciences (CRSBAN), University Joseph KI-ZERBO, Burkina Faso.
Samples Collection
The fresh leaf of C. citratus were collected early morning at 6 to 7 am in agronomic experimentation station of the University of Lomé (Togo).For soymilk samples, thirty samples of soymilk were randomly collected (five samples by batch) from a local company (Agribusiness Association) in Ouagadougou (Burkina Faso). Each batch corresponding five bags of 500 mL of soy milk per sample was collected at the end of the manufacturing. The collection was realized in six different period of high production in the company. Samples were packaged in a thermo-ice containing icebox to maintain refrigerated conditions and transported to the laboratory. The samples were preserved at refrigerating (4°C) condition on standby of analyses.
Experimental Design
The evaluation of essential oil from C. citratus on the stability of soymilk was realized by it addition in soymilk collected from a local company. Base on the proportion of essential oil from C. citratus reported by [20], half of each dose was used. It was the concentrations (v/v) of 0.15%; 0.25% and 0.3% of essential oil were tested. A control positive was realized. The storage was under 26±1°C (temperature of the laboratory) and at 4°C (refrigerating condition). The pH, titratable acidity and microbial were determined for quality control of soymilk samples and during the storage conditions. Sensory quality was evaluated on soymilk added essential oil.
Extraction of Essential Oil from Cymbopogoncitratus
The extraction of essential oil was carried out by hydro distillation in adapted Clevenger apparatus from dried leaves of C. citratus according to method used by [21]. The essential oil obtained was transferred in a bottle, packed with aluminum and was stored at 4°C in refrigerator.
Antioxidant Activity of Essential Oil from Cymbopogoncitratus
The antioxidant activity of the essential oil samples and standards was determined by the radical scavenging activity method using DPPH (2, 2-diphenyl-1-picrylhydrazyl radical) [22]. A volume of 50 µL of methanolic solutions of C. citratus essential oil or standards (ascorbic acid) at different concentrations (2, 4, 6, 8 and 10 µg/mL) was each added to 5 mL of 2,2- diphenyl-1-picrylhydrazyl methanolic solution 0.004% (p/v). The tests were carried out in duplicate. The antioxidant activity was expressed as the antioxidant activity index (AAI), calculated as [23].
Antimicrobial Activity of Essential Oil from Cymbopogoncitratus
Preparation of Inoculums
Each bacteria, yeast and mould were pre-enriched in nutritive broth. Bacteria were then sowed on nutritive agar-agar and incubated at 37°C (24 hours). Yeast and mould were sowed on Sabouraud agar and incubated at 25°C (3 days). After incubation, bacteria were selected and sowed in each growth conditions. Afterwards, 0.5 Mac Farland standard of each bacteria inoculum were prepared according to [24].
Determination of Minimum Inhibitory and Bactericidal Concentrations
Minimum inhibitory concentration of C. citratus essential oil was determined by the microdilution method in Microtiter-plates of 96-wells [25]. 50 µL Mueller Hinton broth addition with 0.5% of tween 80 were inoculated in all the wells of A1 to A11 column and 100 µL in the wells of A12 column. 50 µL of C. citratus essential oil were addition to all the wells of A1 column. After mix contents of wells A1, 50 µL of this wells were used for dilutions in the wells A2 to A11. 50 µL of bacteria inoculum were inoculated in the wells except well A11 which contained only C. citratus essential oil and Mueller Hinton broth, well A12 contained only Mueller Hinton broth. Wells A11 and A12 are the control. C. citratus essential oil concentration used are 500 ; 250 ; 125 ; 62.5 ; 31.25 ; 15.62 ; 7.81 ; 3.91 ; 1.95 et 0.98 µL/mL. Microtiter-plate was incubated at 37°C/ 24 hours for bacteria and 30°C/ 24 hours for yeast and mould. After incubation, 100 μL of bacterial suspension subculture from each wells of microtiter-plate were inoculating on nutrient agar plates (for bacteria) and Sabouraud (for yeast and mould). Minimum inhibitory concentration was defined as the lowest concentration where no change was observed, indicating no growth of bacteria. Minimum inhibitory concentration (MIC) was defined as the lowest concentration of C. citratus essential oil at which no colony was observed after 24 hours incubation at 37°C. Minimum Bactericidal Concentration (MBC) for bacteria and Minimum Fungicidal Concentration (MFC) were determined as the lowest concentration of C. citratus essential oil at which no colony was observed after 5 incubation days at 37°C [26]. The bactericidal and bacteriostatic capacity of C. citratus essential oil on bacterial was characterized by minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The ratio MBC/MIC was used to evaluate antibacterial activity. If the ratio MBC/MIC = 1 or 2, the effect is considered as bactericidal; but if the ratio MBC/MIC = 4 or 16, the effect was defined as bacteriostatic [27].
Physicochemical Analysis of Soymilk
In order to follow the evolution of soymilk under storage conditions, the pH and titratable acidity of soymilk were determined. The pH was measured using pH-meter HANNA according to AFNOR NF T 90-008 (2001). The titratable acidity was determined according to NF EN 12147 V 76-130 (1997).
Microbiological analysis of soymilk
The microbial quality of soymilk was appreciated by the numeration of total viable count on Plate Count Agar at 30°C/ 72 hours according to AFNOR NF V08-051 (1999). Yeasts and mould were enumerated on Sabouraud Agar at 30°C/ 5 days according to AFNOR XP V 059 (1996). The spores were numerated on liver meat agar at 37°C/ 48h by using norm standard XP V 08-061. Salmonella spp. were numerated according to ISO 6579/A1 (2007). The total coli form and thermo tolerant 44°C were numbered by using Violet Red Bile Agar (VRBG) according to NF V08-054. E. coli and Staphylococcus aureus were numbered using respectively AFNOR NF V08 017 and AFNOR NF V08-057 (1996). All results were given according to norm AFNOR.
Sensory Quality of Soymilk
Thirty (30) member panelists consisting of staff and students from the university community were selected for the test. The hedonic test according to recommendation ISO-ICS 67.240 and rang test were used. For hedonic test, they were asked to assess the samples based on the following attributes: colour, smell, bitter flavour, prickly flavour, sweet flavour, acid flavour and overall acceptability. Panelists rating were based on a 9-point hedonic scale. All samples were presented simultaneously to each panelist. For rang test, they were asked to rang all samples of soymilk by attributing 1 for the first sample which was most acceptable, 2 for the second and 3 for the last one.
Statistical Analysis
Data were expressed as the mean ± Standard deviation, group means were compared by one way ANOVA and Tukey test to identify significance (p < 0.05) among groups using GraphPad.Prism.v5.0. Sensory rang test was analyzed by using Friedman test (p < 0.01).
Results and Discussion
Results
The present study investigated the biological properties of C. citratus essential oil and it use for soymilk preservation. In order to follow the quality of soymilk under storage conditions, titratable acidity, pH and bacteria load were determined during the conservation test.
Biological Activities of Essential Oil From C. citratus
The Antioxidant and antimicrobial activities of essential oil from C. citratus was presented in (Table 1). The value of index IC50 and AAI of essential oil from C. citratus showed that the Index IC50, concentration to inhibit 50% of free radical was 7.47. The Antioxidant activity index was 5.3. This result is low than the control positive (ascorbic acid) used witch was 7.4. So, the antioxidant activity index of 5.3 obtained showed that the essential oil from C. citratus presents a very highly antioxidant activity. The Minimal inhibition concentration (MIC) of this essential oil obtained were respectively 62.5 µL/mL for Micrococcus luteus LMG 3293; 7.81 µL/mL for Rhyzopus nigricans; 15.62 µL/mL for Aspergillus niger and higher than 500 µL/mL for E. coli. Nevertheless, this essential oil from C. citratus had an antimicrobial activity, so an inhibiting capacity on the microbial growth.
| Minimal Inhibition Concentration (µL/mL) | |||
| MIC | MBC or MFC | MBC/MIC or MFC/MIC | |
| Escherichia coli | > 500 | > 500 | – |
| Micrococcus luteus LMG 3293 | 62.5 | 125 | 2 |
| Rhizopus nigricans | 7.81 | 15.62 | 2 |
| Aspergillus niger | 15.62 | 31.25 | 2 |
| Antioxidant Activity Index With [DPPH] = 39.6 µg/mL | |||
| IC50 (µg/mL) | AAI | ||
| Essential oil from C. citratus | 7.47 | 5.3 | |
| Ascorbic acid | 5.35 | 7.4 | |
| (-): none-given; MIC: Minimum inhibitory concentration; MBC: minimum bactericidal concentration; MFC: Minimum Fungicidal Concentration; AAI: Antioxidant activity index; IC50: concentration providing 50% inhibitions; [DPPH]: final concentration of DPPH in the reaction. | |||
Titratable Acidity and pH of Soymilk
The pH indicates the level of acidity (H+) and alkalinity (OH–) in the milk and is used to determine the wholesomeness of the milk. The level of titratable acidity in all soymilk samples ranged between 0.133 to 0.136% of lactic acid, no significance difference was observed (p = 0.678). The pH was between 6.55 – 6.57 for batches of soymilk with no significance difference (p > 0.05) (Table 2). During the storage conditions of soymilk at 26°C and 4°C (Table 3, 4), it was observed a decrease of pH and increase of titratable acidity of soymilk. A rapidly decrease was observed in three days at ambient temperature (26 ± 1°C) of storage. In refrigerating (4°C) condition, the fact that the decrease of pH and acidity, no significance different was observed up to 21 days of storage. According to each concentration of essential oil used, a significance different was noted at 21 days of storage. A concentration of 0.15% has no significance different. The pH varied slightly under refrigerating (4°C) until the 9thday. The same observation was made for titratable acidity. However, soymilk without addition essential oil showed the highest acidity (0.16 – 0.162% of lactic acid) from 13th to 21st day under refrigeration at 4°C. The used of 0.15% of essential oil has stabilize pH and titratable acidity of soymilk at refrigerating condition.
| Samples 0f Soymilk (n = 5)/ Batch | Titratable Acidity (% lactic acid) | pH |
| Batch 1 | 0.136 ± 0.004a | 6.55 ± 0.01a |
| Batch 2 | 0.135 ± 0.005a | 6.57 ± 0.02a |
| Batch 3 | 0.133 ± 0.003a | 6.56 ± 0.01a |
| Batch 4 | 0.133 ± 0.003a | 6.56 ± 0.01a |
| Batch 5 | 0.133 ± 0.004a | 6.56 ± 0.02a |
| Batch 6 | 0.136 ± 0.005a | 6.56 ± 0.01a |
| Mean | 0.134 ± 0.002 | 6.56 ± 0.01 |
| n: number of soymilk samples; Batch: represent five samples of soymilk; the values represent the mean of the five samples by batch ± Standard deviation; The same letter (a) in the same column indicated no statistical difference according to ANOVA and Tukey test (p ≥ 0.05). | ||
| Period of storage | pH | Titratable acidity (% lactic acid) | |||||||
| SM | SM+0.15% EO | SM+0.25% EO | SM+0.3% EO | SM | SM+0.15% EO | SM+0.25% EO | SM+0.3% EO | ||
| 1 day | 6.56 ± 0.00a,e | 6.55 ± 0.00a,e | 6.54 ± 0.00a,f | 6.54 ± 0.00a,f | 0.140 ± 0.000a,e | 0.138 ± 0.005a,e | 0.138 ± 0.005a,e | 0.140 ± 0.000a,e | |
| 2 day | 6.55 ± 0.00a,e | 6.55 ± 0.0a,e | 6.55 ± 0.01a,e | 6.55 ± 0.01a,e | 0.140 ± 0.000a,e | 0.135 ± 0.006a,e | 0.133 ± 0.005a,e | 0.141 ± 0.007a,e | |
| 3 days | 6.55 ± 0.00a,e | 6.55 ± 0.01a,e | 6.55 ± 0.01a,e | 6.55 ± 0.01a,e | 0.143 ± 0.003a,e | 0.138 ± 0.005a,e | 0.139 ± 0.006a,e | 0.141 ± 0.002a,e | |
| 9 days | 6.55 ± 0.00a,e | 6.55 ± 0.01a,e | 6.55 ± 0.01a,e | 6.55 ± 0.01a,e | 0.144 ± 0.002a,e | 0.141 ± 0.002a,e | 0.140 ± 0.000a,e | 0.141 ± 0.002a,e | |
| 12 days | 6.55 ± 0.00a,e | 6.55 ± 0.01a,e | 6.55 ± 0.01a,e | 6.55 ± 0.01a,e | 0.144 ± 0.002a,e | 0.141 ± 0.002a,e | 0.140 ± 0.000a,e | 0.141 ± 0.002a,e | |
| 15 days | 6.53 ± 0.00b,e | 6.54 ± 0.00a,e | 6.54 ± 0.00a,e | 6.54 ± 0.00a,e | 0.144 ± 0.002a,e | 0.141 ± 0.002a,e | 0.140 ± 0.000a,e | 0.150 ± 0.000ab,e | |
| 18 days | 6.20 ± 0.00c,e | 6.50 ± 0.01b,f | 6.50 ± 0.01b,f | 6.49 ± 0.00b,f | 0.160 ± 0.000b,e | 0.141 ± 0.002a,f | 0.149 ± 0.003b,g | 0.151 ± 0.003b,g | |
| 21 days | 6.17 ± 0.01d,e | 6.41 ± 0.01c,f | 5.83 ± 0.03c,gh | 5.79 ± 0.02c,h | 0.163 ± 0.005b,e | 0.154 ± 0.003b,f | 0.155 ± 0.004b,f | 0.151 ± 0.003b,f | |
Table 3: pH and titratable acidity of soymilk stored at refrigerating (4°C).
| Period of storage | pH | Titratable acidity (% lactic acid) | ||||||||
| SM | SM+0.15% EO | SM+0.25% EO | SM+0.3% EO | SM | SM+0.15% EO | SM+0.25% EO | SM+0.3% EO | |||
| 0 | 6.56 ± 0.00a,d | 6.55 ± 0.00a,d | 6.54 ± 0.00a,e | 6.54 ± 0.00a,e | 0.140 ± 0.000a,d | 0.138 ± 0.005a,d | 0.138 ± 0.005a,d | 0.133 ± 0.005a,d | ||
| 1 day | 6.03 ± 0.02b,d | 6.46 ± 0.03b,d | 6.34 ± 0.03b,d | 6.538± 0.04b,d | 0.356 ± 0.203a,d | 0.155 ± 0.004b,d | 0.159 ± 0.003b,d | 0.165 ± 0.006a,d | ||
| 2 days | 4.26 ± 0.04c,d | 4.43 ± 0.03c,d | 4.26 ± 0.00c,d | 2.69 ± 0.01c,d | 0.765 ± 0.010b,d | 0.468 ± 0.010c,e | 0.405 ± 0.010c,e | 0.405 ± 0.058b,e | ||
Table 4: pH and titratable acidity of soymilk stored at ambient temperature (26 ± 1°C).
Microbial Load of Soymilk Under Storage Conditions
Microbiological quality of soymilk samples (Table 5) showed the absence of pathogenic microorganisms such us sulfite-reducing anaerobe, S. aureus, E. coli and Salmonella in soymilk confers to the product a good hygienic and commercialized quality. The mean of total viable count was 13×103 ± 3.9×103 CFU/ mL. No significance different was observed. For yeasts and moulds count the mean was 10 CFU/ mL. The microbial quality of soymilk stored at ambient temperature (Table 6) showed that the load of total counts, total coli forms and thermo tolerant, yeasts and moulds of various soymilks were decrease in soymilk added essential oil. The best decrease was observed fort the use of 0.3% of essential oil. However, soymilk without essential oil presented a high value of total viable count compare to soymilk added essential oil. However, soymilk added essential oil had acceptable microbiological quality.
| Samples of Soymilk
(n = 5)/ Batch |
TF | TC | TTC | YM | SRA | S. aureus | E. coli | Salmonella spp. |
| Batch 1 | 13000 ± 2700a | ˂ 10 | ˂ 1 | 9 | Absent | ˂ 1 | Absent | Absent |
| Batch 2 | 14000 ± 3800a | ˂ 10 | ˂ 1 | 10 | Absent | ˂ 1 | Absent | Absent |
| Batch 3 | 13000 ± 3300a | ˂ 10 | ˂ 1 | 10 | Absent | ˂ 1 | Absent | Absent |
| Batch 4 | 14000 ± 5200a | ˂ 10 | ˂ 1 | 9 | Absent | ˂ 1 | Absent | Absent |
| Batch 5 | 13000 ± 4800a | ˂ 10 | ˂ 1 | 10 | Absent | ˂ 1 | Absent | Absent |
| Batch 6 | 10000 ± 2100a | ˂ 10 | ˂ 1 | 10 | Absent | ˂ 1 | Absent | Absent |
| Mean | 13000 ± 3900 | ˂ 10 | ˂ 1 | 10 | Absent | ˂ 1 | Absent | Absent |
| n: number of soymilk samples; Batch: represent five samples of soymilk; the values represent the mean of the five samples by batch ± Standard deviation. TF: total viable count; TC: Total Coliform; TTC: Thermotolerant Coliform; YM: Yeasts and Moulds; SRA: Sulfito-reducing Anaerobe; CFU: Colony Forms Unit. The same letter (a) in the same column indicated no statistical difference according to ANOVA and Tukey test (p ≥ 0.05). | ||||||||
| Soymilk Samples | Period of Storage | Load Microbial (UFC/mL)/ (n = 2) | ||||
| TF | TC | TTC | YM | SRA | ||
| SM | t0 | 5700 ± 350 | ˂ 10 | ˂ 1 | 2 | Absent |
| 1 day | 100000 ± 4200 | ˂ 10 | ˂ 1 | 2 | Absent | |
| 2 days | 169000 ± 4800 | ˂ 10 | ˂ 1 | 3 | Absent | |
| SM+0.15% EO | t0 | 5400 ± 420 | ˂ 10 | ˂ 1 | 2 | Absent |
| 1 day | 510 ± 14 | ˂ 10 | ˂ 1 | 1 | Absent | |
| 2 days | 500 ± 7 | ˂ 10 | ˂ 1 | 1 | Absent | |
| SM+0.25% EO | t0 | 5200 ± 350 | ˂ 10 | ˂ 1 | 1 | Absent |
| 1 day | 480 ± 14 | ˂ 10 | ˂ 1 | 1 | Absent | |
| 2 days | 420 ± 14 | ˂ 10 | ˂ 1 | ˂ 1 | Absent | |
| SM+0.3% EO | t0 | 5400 ± 280 | ˂ 10 | ˂ 1 | 1 | Absent |
| 1 day | 350 ± 49 | ˂ 10 | ˂ 1 | ˂ 1 | Absent | |
| 2 days | 350 ± 21 | ˂ 10 | ˂ 1 | ˂ 1 | Absent | |
| n: test numbers; the values represent the mean ± Standard deviation; SM: soymilk; EO: essential oil; TF: Total viable count; TC: Total Coliform; TTC: Thermotolerant Coliform; YM: Yeasts and Molds; SRA: Sulfito-reducing Anaerobe; CFU: Colony Forms Unit. | ||||||
Sensory Quality of Soymilk
Two tests such us hedonic and rang tests were used to evaluate the appreciation and acceptability of essential oil from C. citratus in soymilk.
Data of Hedonic Test
The appreciation of the organoleptic characteristics of soymilk showed that soymilk added 0.15% of essential oil was the more appreciated by all panelist (Figure 1). A significance difference between the three kind of soymilk compared to the taste pricking and the general appreciation (p < 0.05) were recorded. The soymilk added 0.25% and 0.3% of essential oil showed the strongest odor, acid taste and the prickly taste. From this result, it arises that soymilk added 0.15% of essential oil has the overall acceptability.
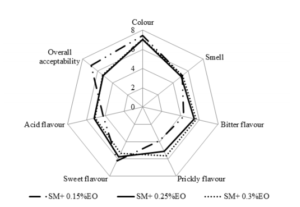
Figure 1: Organoleptic characteristics of different soymilk added essential oils.
SM : Soymilk
EO : Essential Oils
Results Classification Test
This test aimed to appreciate the use of essential oil from C. citratus in soymilk. According to acceptability, the panelists attributed to each sample a mark of 1; 2 and 3. The most acceptable sample related to the mark 1, the second to mark 2 and 3 for the last. The results obtained (Table 7) showed the critical value calculated for p-value ≤ 0.01 of the 30 panelists and 3 soymilk samples was 19. The analysis of the results obtained from Friedman test showed no significance difference between soymilk samples incorporated with 0.25% and 0.3% of essential oil (difference < 19). The difference was significance (Difference between total of pair classification ≥ 19) between the three soymilk samples (p-value < 0.01). The panelist classified the three kind of soymilk by order preferably by allotting the first rang to the soymilk added 0.15% of essential oil.
| Panelist Score of Classification | ||||||
| SM+0.15% EO (A) | SM+0.25% EO (B) | SM+0.3% EO (C) | ||||
| 1st group | 2nd group | 1st group | 2nd group | 1st group | 2nd group | |
| 1 | 1 | 2 | 3 | 3 | 2 | |
| 1 | 1 | 2 | 3 | 3 | 2 | |
| 1 | 1 | 3 | 3 | 2 | 2 | |
| 1 | 3 | 3 | 2 | 2 | 1 | |
| 3 | 2 | 2 | 3 | 1 | 1 | |
| 2 | 1 | 1 | 2 | 3 | 3 | |
| 3 | 1 | 1 | 3 | 2 | 2 | |
| 1 | 2 | 3 | 3 | 2 | 1 | |
| 1 | 1 | 3 | 3 | 2 | 2 | |
| 1 | 3 | 3 | 1 | 2 | 2 | |
| 1 | 1 | 3 | 3 | 2 | 2 | |
| 3 | 1 | 2 | 2 | 1 | 3 | |
| 1 | 1 | 3 | 3 | 2 | 2 | |
| 1 | 1 | 2 | 3 | 3 | 2 | |
| 1 | 1 | 3 | 3 | 2 | 2 | |
| Total of classification | A = 43 | B = 76 | C = 61 | |||
| Rang | 1 | 3 | 2 | |||
| Difference between total of pair classification | B – A = 33 | B – C = 15 | C – A = 18 | |||
| A, B and C represent the total of the score classification of thirty panelists of each soymilk samples; SM: soymilk; EO: essential oil. | ||||||
Discussion
The use of essential oil has a great interest in Food technology and traditional medicine [17]. Other importance was accorded to this essential oil because of it high inhibiting activity on the growth fungical strains [28], antioxydant and aromatics properties [17; 18]. Before it use in food matrix, the minimal concentration must be known, so that the antioxidant and/or antimicrobial effect do not exceed the acceptability organoleptic levels [29]. This essential oil of C. citratus with antioxidant properties (antioxidant activity index of 5.3) and antimicrobial activity was obtained (62.5 µl/mL for Micrococcus luteus LMG 3293, 7.81 µl/mL for Rhyzopus nigricans, 15.62 µl/mL for Aspergillus niger). The difference between MIC could result from the chemical composition of essential oil [30]. In addition, minimum inhibitory concentration founded was lower than 250 µl/mL, showing interesting inhibitory capacity of C. citratus essential oil against Micrococcus luteusLMG329, Rhyzopus nigricans and A. niger [31]. Nevertheless, this essential oil from C. citratus had an antimicrobial activity, so an inhibiting capacity on the microbial growth, so can be used as conservative of foodstuffs [14]. The same results were conclude from the essential oil of C. citratus in the stabilization of beer produced from starch-based matter, milk, the fruit juices [20¶, ¶18]; for that, essential oil from C. citratus can be used for soymilk preservation. So, as concern soymilk samples quality at first, it is noted that, the mean value of pH obtained (6.56) is slightly higher than that reported by [1] in soymilk (pH: 6.08 – 6.22). Moreover, titratable acidity mean value (0.134% lactic acid) obtained corroborate with that obtained by [1] which varied between 0.135 to 0.145% of lactic acid. The titratable acidity and pH of soymilk samples were in conformity with the criteria respectively 0.15 % lactic acid and 6.5 set by [32]. Microbiological quality of soymilk samples was in conformity with microbiological criteria according to Soy Foods Association of America (SFAA) and French legislative and lawful guide N8155 (2000). The absence of pathogenic microorganisms in soymilk confers to the product a good hygienic and commercialized quality. From soymilk stored during the bioprservation test, a rapidly decrease of acidity or pH was observed at ambient temperature in soymilk without added essential oil. This is attribute to the actions of certain microorganisms which is referred to as biological acidity [33]. Consequence of the increase of the bacterial load refers to acidification and influence of organoleptic properties [34]. By contrast to this, for soymilk added essential oil, the decrease of microbial load was observed. This could be explained by the effect of essential oil on the bacteria. Similar results were reported by [20] on the stabilization of beer produced from starch-based matters against the effects of deterioration by the use of C. citratus essential oil at 1 mL by liter of beer. Furthermore, the total inhibition of fungal in soymilk added essential oil was registered. This corroborates the idea for what essential oil from C. citratus has effective inhibition on the growth of fungal [28]. It observe that high concentration has more inhibition on total count and fungal, but the high concentration has not appreciated by the most of the panelist.
Conclusion
The stabilization of soymilk through the conservation property of essential oil from C. citratus was evaluated. For the dairy products, the lawful limiting date of conservation is two weeks in refrigeration (4°C), so, the use of essential oil from C. citratus made it possible to prolong for one week more. It arise from the present study that stabilization of soymilk by addition of essential oil from C. citratus highlighted stabilization at refrigerating (4°C) with conservation of organoleptic properties up to three weeks against two weeks in absence of the essential oil. The sensory quality showed that the overall acceptability is according to soymilk added 0.15% of essential oil. It can be concluded that essential oil from C. citratus has properties of stabilization of soymilk and could be used as a bio-conservator.
Acknowledgements
We thank DJIGUI ESPOIR, a local company (Agribusiness Association) where soymilk samples were collected; Research Center in Biological, Food and Nutrition Sciences (CRSBAN), tallied of this study as well as the purse of the Agricultural Program of Productivity in West Africa (PPAAO-Togo) for the funds placed for the realization of the study. Authors need also to thank the International Sciences Program (Sweden).
Author Contributions Conflict of Interest: Authors have declared no conflict of interest. References
Agbémébia Y. AKAKPO
Has done data acquisition, field and lab work, data analysis and manuscript writing.
Marius K. SOMDA
Contributed to draft work design plan, analysis of data and revising manuscript.
Donatien KABORE
Contribute for the manuscript writing
Essodolom TAALE
Has revised critically organoleptic data and the proposal manuscript.
Henriette B. MIHIN
Contributed to lab work and data collection.
Cheik A. T. OUATTARA
Help to analysis and interpretation of data.
Souleymane SANON
Has revised the proposal manuscript.
Alfred S. TRAORE
Has given the final approval of the version to be published.
Aboubakar S. OUATTARA
Has revised and the draft work of the proposal manuscript.