Publication Information
ISSN 2691-8803
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
The Fermentation of Sugar Beet in Boiled and Unboiled State
Raymond Jagessar1*, Sarita Ragoonanan2
1Department of Chemistry, Faculty of Natural Sciences, University of Guyana, South America
2Final Year Research student, Department of Chemistry, University of Guyana, South America
Received Date: December 15, 2024; Accepted Date: December 31, 2025; Published Date: Febuary 11, 2025;
*Corresponding author: Raymond Jagessar; Department of Chemistry, Faculty of Natural Sciences, University of Guyana, South America; Email: raymond.jagessar@uog.edu.gy
DOI: 10.37722/ESPRAM.2025102
Abstract
There is an urgent serious need to further curb global warming, considering its ongoing catastrophic effects. One way to do this is to use greener/fuels such as bioethanol, to eventually substitute fossil fuels. Bio-ethanol is a cleaner burning fuel, than fossil fuel and will pump less carbon dioxide into the atmosphere. It is one form of renewable energy sources. Bio-ethanol can be obtained via the fermentation of sugar rich sources, such as fruits or the acid hydrolysis of lignocellulosic material, followed by subsequent fermentation of the hydrolyzates. The focus of this study is to determine the feasibility of sugar beet as a fermentable feedstock to produce a high yield of ethanol. The researcher examined sugar beet in two (2) environments: Boiled and Un-boiled with no additives in either of the conditions it was conducted in. The yeast used in this experiment was Saccharomyces cerevisiae and the experiment were carried out in triplicates. Various conditions were monitored including pH, alkalinity, temperature, etc. A mean of 2.36% v/v of ethanol in 100ml of the boiled sample and 1.72% v/v of ethanol in 100ml of the un-boiled sample.
Keywords: global warming, catastrophic effects, bioethanol, fossil fuels, fruit and fruit peels
Introduction
With a view to decrease dependence on fossil fuel, as a result of depletion, increasing global fuel price, increasing population and increasing global warming, there has been increased interest in the use of renewable energy sources of which bioethanol is one [1,2,3]. Bioethanol (b.p: 78.5°C) can be used for a variety of purposes, of which blending with gasoline to produce gas alcohol to power automobiles is of current utilization [1,2,3, 24-25]. In addition, ethanol is a clean burning renewable energy source4 and thus can act as a fuel in many dimensions. Ethanol of varying percentages is also an important component of alcoholic beverages such as wine, beer, cider, vodka, gin. whisky, brandy etc. Thus, production of bioethanol will lead to an increase production of beer and other alcoholic beverages. Bioethanol is also an important starting material for aldehydes, ketones, carboxylic acid, carboxylic acid derivatives and the hydroxyl group is a component of many pharmaceutical drugs [5]. Ethanol can be used in the perfume, disinfectant, tincture, biological and biofuel industries. Bioethanol can be used in the manufacture of sanitisers and was used significantly to clean surfaces during the COVID-19 era. Ethanol production through Fermentation has been one of the world most significant approaches to aid in the advancement of commercial Industries which are listed above.
Ethanol doesn’t have significant environmental impact as fossil fuel combustion [3]. It has low air polluting effect and low atmospheric photochemical reactivity, further reducing impact on the ozone layer [6]. It contributes little net CO2 accumulation to the atmosphere and thus should curb global warming [6-9].
Ethanol can be used in three primary ways as biofuel, namely, E10 which is a blend of 10% ethanol and 90% unleaded gasoline, a component of reformulated gasoline both directly and or as ethyl tertiary butyl ether (ETBE) and as E85 which is 85% ethanol and 15% unleaded gasoline. When mixed with unleaded gasoline, ethanol increases octane levels, decreases exhaust emissions and extends the supply of gasoline. In Latin America and the Caribbean, bioethanol is combined with gasolene to produce gas alcohols which are used to power automobiles. Bio-ethanol is made by fermenting almost any material that contain starch or sugar. Grains such as corn and sorghum are good sources, but fruits that are high in sugar concentration are excellent sources as well, since they contain ready to ferment sugars [10].
To solve the above problem, emanating from fossil fuel, one alternative is to produce bioethanol from fruits, other grown organic matter or waste [3,4,6-8,9]. Bioethanol can be obtained via the fermentation of glucose, fructose or sucrose under the influence of Saccharomyces cerevisiae at room temperature [4,6-35]. Also, acid hydrolysis of lignocellulose material followed by subsequent fermentation [7-21]. Sugar rich sources include ripe fruits 8-18 etc. Other sources include biodegradable fraction of products, waste and residues from agriculture like vegetables, fruit wastes and animal origin [11-12, 17, 20-21] etc. The percentage yield of ethanol, ranging from 4.0 -10.0 v/v) have been reported [3-12]. Fruits that are high in sugar concentration are favourable to the fermentation process, since they can produce high percentage volume of ethanol
Fermentation is the process of energy production in a cell in an anerobic environment with the lack of an external electron acceptor [22]. Sugars are the common substrate of fermentation and the products include ethanol, lactic acid and hydrogen. In some instances, compounds such as butyric acid and acetone are produced [22].
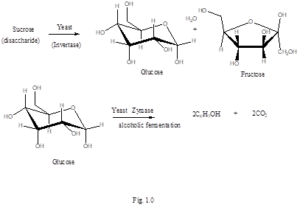
The fermentation process begins with the yeast breaking down the different forms of sugar in any fermenting matrix. Saccharomyces cerevisiae contains two enzymes that is very important for the yeast enzyme activity in the fermentation process. These two enzymes are called Invertase and Zymase and they functions are similar but somewhat prerequisite to each other. Invertase aids in converting any sucrose sugar that is present in any biomass that is used in fermentation to glucose and fructose while zymase aids in the conversion of glucose to ethanol 22., Fig. 1.0.
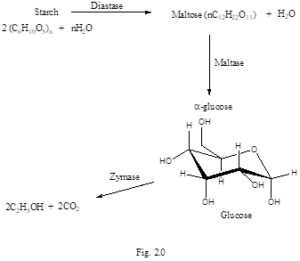
During Fermentation, starch is first hydrolysed to maltose by the action of the enzyme diastase. This enzyme is obtained from germinating barley seeds or malt. Maltose is converted to glucose by the enzyme maltase. Glucose is then fermented to ethanol via the enzyme zymase 23, Fig. 2.0. Once the sugars are broken down into monosaccharides, the yeast can now use them. Saccharomyces cerevisiae is able to perform both aerobic and anaerobic respiration.
This paper reports the fermentation of sugar beet juice in its boiled and unboiled state with a view to compare the % yield of bio-ethanol. Sugar beet is a plant whose roots contain a high concentration of sucrose. Its usually grown in cold climates in Europe and in the USA. However, recently it has been found that certain soil in Guyana supports its growth. Thus, providing the opportunity for its investigation. The fermentation of sugar rich substrates, notably fruits is ongoing research [24-30]. The production of bioethanol from sugar beet will allow for an increase in their production and productivity via agricultural best practices which is expected to be sustainable. Sustainable smart agriculture practices include growing sugar beets in shade houses Guyana, has lots of land spaces that can be utilized for the growing of orchards which can provide the quantum of fruits and sugar beets needed for bioethanol production. This will provide more jobs for persons in the agriculture field. The bioethanol obtained from sugar beet can be used as a source of biofuels, in gas alcohols of varying blends to drive automobiles, manufacturing of perfumes, disinfectant, tincture, sanitisers etc. The use of gas alcohols as a fuel is lacking in the Caribbean. To date, the USA and Brazil are the leading producers of gas alcohol and countries in the Caribbean, Latin America and rest of the world should follow likewise. As a biofuel, it will help Guyana remain below zero CO2 emission, since it will decrease dependence on fossil fuel like other non-fossil fuel sources. Guyana is listed as one country that has a net zero emitter of CO2.
A literature review was done to identify short comings. There are few reports on fermentation of sugar beet juice. For sugar beet, most articles focused on the fermentation of sugar beet pulp and lignocellulosic wastes. Thus, there exist needs to study the fermentation of sugar beet juice [31- 32].
It has been reported that bioethanol from sugar beet has been reported using two fermentation techniques: fed-batch and multi-stage batch conditions. The fed-batch method was found to be the most effective in producing the maximum ethanol concentration. With proper control of the operational parameters (starting volume and feeding rate), 15.2% (v/v) ethanol could be produced in 53 hours with no residual sucrose and an ethanol productivity of 2.3 g L h1[33].
Tan et al., 2015, reported that sugar beet juice’s high concentration of fermentable sugars and high energy output/input ratio make it a suitable feedstock for ethanol production. Batch ethanol fermentation of thick juice and raw juice demonstrated that adding mineral supplements could speed up fermentation rather than boost ethanol concentration. The ethanol concentration, ethanol yield, and productivity were all 70.7 g L-1, 89.8%, and 21.2 g L-1 h-1, respectively, after the continuous ethanol fermentation of raw juice was carried out at 35 °C with a dilution rate of 0.3 h-1 [34].
Zabed et al., 2014, investigated the feasibility and cost-effectiveness of producing ethanol from sugar beet juices that are free of added sugar. This experiment is a split-slot design. The fermentation method (batch) is used to create ethanol from these sugar liquids. Although Zymomonas mobilis was probably utilized, yeast, particularly Saccharomyces cerevisiae, is the most frequent microbe used in fermentation. The fermentation process is significantly influenced by a variety of variables, and their optimization is essential for effective ethanol production from this feedstock. At 30oC and at a pH of 6.5, 72.4–86.1% of ethanol was produced at an Agitation rate of 200rpm [35].
Sugar beet pulp was used for the production of bioethanol due to its high content of cellulose, hemicelluloses, and pectin. Its structural and chemical robustness limits the yield of fermentable sugars obtained by hydrolyzation and represents the main bottleneck for bioethanol production. Physical (ultrasound and thermal) pretreatment methods were tested and combined with enzymatic hydrolysis by cellulase and pectinase to evaluate the most efficient strategy. The optimized hydrolysis process was combined with a fermentation step using a Saccharomyces cerevisiae strain for ethanol production in a single-tank bioreactor. Optimal sugar beet pulp conversion was achieved at a concentration of 60 g/l (39% of dry weight) and a bioreactor stirrer speed of 960 rpm. The maximum ethanol yield was 0.1 g ethanol/g of dry weight (0.25 g ethanol/g
total sugar content), the efficiency of ethanol production was 49%, and the productivity of the bioprocess was 0.29 g/l·h, respectively 36.
Methodology
Materials and Method
Fermentation of the pulp of sugar beet
Sugar beet was purchased from a vendor at Bourda market, Georgetown. The fruits were washed with distilled water, dried, weighed and the volume determine. The volume of the fruit was determined with help of graduating measuring cylinder. The fruit was introduced in a cylinder filled three quarter of the way with water. The difference of volume before and after total immersion of the fruit corresponds to the volume of the fruit (Vf). Specific gravity of the fruits is calculated as the ratio between the mass of the fruits (Mf) and its volume (Vf). The beet was cut into smaller sizes and the seed removed and then added to the blender. The blended materials were filtered using Whatman size filter paper. The filtrate was used as the fermentation matrix. Experiments was done in triplicates. The experiment was repeated. However, the collected filtrate was boiled. The inoculated Yeast, S. Cerevisiae (6 grams), wine strain, capable of existing up to a temperature of 35-45 degree Celsius in the presence of 30% sugar and 18% of ethyl alcohol was added to the boiled and unboiled filtrate. The requisite common pH 4.5 was maintained using citric acid. The fermentation process was monitored at specific intervals. At the end of 72 hours, the contents of the mixture was filtered and the filtrate distilled using a vigreux column. The composition of the filtrate was tested for the percentage yield of ethanol using a pictometer and HPLC at Demerara Distilleries Limited.
In addition to the above, a control and reference experiment was conducted to validate research results. All results collected was expressed as mean values.
Preparation of 12% yeast solution
44 ml of deionized water was added to a 100ml beaker. 6g of dry yeast was added and left to incubate during 90 minutes at 27 degree Celsius to achieve a solution of 12% yeast.
Results
Table 1: Fermentation of Sugar beet in the Boiled condition
| Sugar beet
Boiled |
Initial
o Brix |
Final
o Brix |
Initial
pH |
Final
pH |
Final
Specific Gravity |
Total
Acid |
Alcohol
Content % |
Ethanol
Content % |
| Jar 1 | 6.25 | 0.885 | 4.23 | 4.27 | 0.98704 |
0.15 |
9.03 | 1.73 |
| Jar 2 | 7.02 | -3.687 | 4.07 | 4.12 | 0.98594 | 10.57 | 2.84 | |
| Jar 3 | 6.85 | 0.236 | 4.40 | 4.56 | 0.98613 | 10.26 | 2.50 | |
| Average | 6.71 | -0.855 | 4.23 | 4.32 | 0.98637 | 9.95 | 2.36 |
Table 2: Fermentation of Sugar beet in the Un-boiled condition
| Sugar beet
Un-boiled |
Initial
o Brix |
Final
o Brix |
Initial
pH |
Final
pH |
Final
Specific Gravity |
Total
Acid |
Alcohol
Content % |
Ethanol
Content % |
| Jar 1 | 4.133 | 0.106 | 4.90 | 5.20 | 1.01619 |
0.15 |
5.68 | 1.73 |
| Jar 2 | 4.120 | 0.194 | 4.85 | 5.02 | 1.01614 | 5.40 | 1.70 | |
| Jar 3 | 2.874 | 0.111 | 4.03 | 4.30 | 1.01115 | 0.90 | – | |
| Average | 3.709 | 0.137 | 4.59 | 4.84 | 1.01449 | 3.99 | 1.72 |
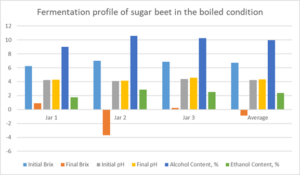
Graphs: Fermentation profile of sugar beet in the boiled condition

Graph 2: Fermentation profile of sugar beet in the unboiled condition

Figure: 3.0
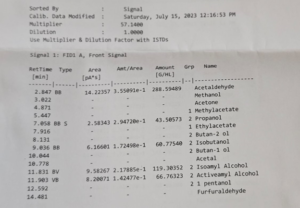
Figure: 4.0
Discussion
Table 1.0 shows the fermentation of sugar beet in the boiled condition, whereas Table 2.0 shows the fermentation of the sugar beet in the unboiled condition. From Table 1.0, it can be seen that the average Brix decrease from 6.71 to (-0.855), indicating that all of the reducing sugar has been utilized in the fermentation process and the fermentation was indeed a success. In addition, the average pH seems to increase from 4.23 to 4.32. The average specific gravity was noted at 0.98637. It was also noted that the average alcoholic content was found to be 9.95, whereas the average ethanolic content was found to be 2.36. From Fig. 4.0, other alcohols observed on the chromatogram were: methanol, propanol, 2-butan-2-ol, 2-Isobutanol, 2-butan-1-ol, 2-Isoamyl alcohol, 2-activeamyl alcohol, 2-1-pentanol.
For the fermentation of sugar beet in the unboiled condition, the average Brix content decrease from 3.709 to 0.137. This again demonstrated the success of the fermentation process. The pH also increase, showing an increase from 4.59 to 4.84. The total alcoholic content was found to be 3.99, with an ethanolic content of 1.72 %. Low values were obtained for the ethanolic content in both cases. Fig. 3.0. shows the chromatogram of the ethanol standard. Bar Graphs 1.0 and Graph 2.0 show the fermentation profile of sugar beet in the boiled and unboiled state and reflect results shown in Tables 1.0 and Table 2.0.
Conclusion
The production of green fuel such as bio-ethanol is the way forward to environmentally save our planet from global warming and its catastrophic consequences. The production of greener fuels, should be one of the world orders. Bio-ethanol production from sugar rich sources, such as pulp of fruits will promote the agroindustry. Our country has vast empty spaces for the planting of fruit trees. The establishment of a fruit-based bioethanol plants is necessary, considering the fruitful results. Fruit pulp and peel can act as one of the main bioethanol feedstocks.
References
- Demirbas AH, Demirbas I (2007). “Importance of rural bioenergy for developing countries”. Energy Conversion Management. 48, 2386-2398.
- Demirbas A (2008). Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Conversion and Management. 49, 2106-2116.
- Yu Z., Zhang H. (2004). Ethanol fermentation of acid-hydrolysed cellulosic pryolysate with Saccharomyces cerevisiae. Biores.Technol. 93, 199-204.
- Reddy V.L., O.V.S, Reddy. (2007). Production of Ethanol from Mango (Mangifera indica I) fruit Juice Fermentation, Research Journal of Microbiology, 2(10): 763-769.
- G.W.T, Fryhle, CB., Organic Chemistry. (2008). 9th Edition, John Wiley and Sons, Inc.2008.
- Martin M, Galbe M., Wahlborn C.F, Hahn-Hagerdal B, Jonsson, L J. (2002). “Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme Micro.Technol. 31: 274-282.
- Graham, R.W., Reynolds T.W., Hsu Y. (1976). Preliminary assessment of systems for deriving liquid and gaseous fuels from waste or grown organics. US Department of Commerce, National Technical Information Service. 1-40.
- Dutta A, Mukherjee A. (2010). Comparison of alcohol production in batch culture using different substrates by Saccharomyces cerevisiae. Biomedical and Pharmacology Journal, 3(1), 23-26.
- Reddy V.L, Reddy O.V.S. (2009). Production, optimization and characterization of wine from Mango (Mangifera indica Linn Natural Product Radiance. 8(4), 426-435.
- Naik S.N., Goud V.V., Rout P.K., Dalai A.K. (2010). “Production of first- and second-generation biofuels: a comprehensive review, “Renewable and Sustainable Energy Reviews, 14 (2), 578-597.
- Hossain A.B.M.S., Ahmed S.A., Ahmed M.A., Adnan F.M.A., Annuar M.S.M., Mustafa H, Hammad N. (2011). “Bioethanol fuel production from rotten banana as an environmental waste management and sustainable energy, African Journal of Microbiology Research. 5(6), 586-598.
- Ingale S., Joshi S, Gupte A. (2014). Production of bioethanol using agricultural waste: banana pseudo stem. Braz J. Microbiol. 45(3): 885–892.
- Reddy V., Reddy, O.V.S. (2009). Production, optimization and characterization of wine from Mango (Mangifera indica Linn Natural Product Radiance. 8(4), 426-435.
- Massengo V, Loumouamou B.W., Diakabana P, Silou T. (2014). Ethanol production by fermentation of the pulp of the “BOKO” mango”. International Journal of Chemical Science and Technology. 4(4), 71-77.
- Wairagu N.W., Kiptoo J, Githiomi J.K. (2013). Nutritional assessment of Sclerocarya birrea (amarula) fruit from Kenya. International Journal of Current Research. 5(5):1074–1078.
- Chanprasartsuk O, Pheanudomkitlert K, Toonwai D. (2012). Pineapple wine fermentation with yeasts isolated from fruit as single and mixed starter cultures. J. Food Ag-Ind. 5(02), 104-111
- Mohammed S. et al, (2014). “Bioethanol Production from Mango Waste (Mangifera indica L.): Biomass as Renewable Energy”
- Chowdhury P, Ray R.C. (2007). Fermentation of Jamun (Syzgium cumini L.) Fruits to Form Red Wine. ASEAN Food Journal. 14 (1): 15-23.
- Patil S.S, Thorat R.M, Rajasekaran P. (2012). Fermentation of Jamun (Syzgium cumini L.) Fruits to Form Red Wine, Journal of Advanced Laboratory Research in Biology. 3(3): 234-238.
- Tropea A, Wilson D, Giovanna Loredana La Torre, Lo CURTO Rosario, Saugman P, Davies, P.T. (2014). Bioethanol Production from Pineapple Wastes. Journal of Food Research; Published by Canadian Centre of Science and Education. 3 (4); 60-70.
- Mishra J. Kumar D, Samanta S, Vishwakarma M. (2012). “A comparative study of ethanol production from various agro residues by using Saccharomyces cerevisiae and Candida albicans. Journal of Yeast and Fungal Research. 3(2), 12 – 17.
- Khan Z, Dwivedi A.K. (2013). Fermentation of Biomass for the production of ethanol: Universal Journal of Environmental Research and Technology. 3 (1), 1-13.
- Robinson, J., (2006). The Oxford Companion to Wine. 3rd Oxford University Press. 268-780.
- Jagessar, R.C, Fraser, C.F. (2016). Fermentation of Pineapple, Ananas comosus, mash in the absence and presence of additives, American Journal of Research Communications, 4 (3), 131-140, 2016
- Jagessar, R.C, Meusa, T. (2016). “The fermentation capacity of the pulp of Magnifera indica, Carica papaya and the peel of Magnifera indica in the absence and presence of additives. The highest % yield of ethanol, furnished by a fruit, Carica papaya, American Journal of Research Communication, 6 (10) 68-86.
- Jagessar*, R.C, Lynch, J. (2017). “The Fermentation of the Pulp of Watermelon, Citullus lanatus in the absence and presence of additives with a view to obtain ethanol for commercial use”, American Journal of Research Communication. 5(9), 1-24.
- Jagessar*, R.C., Collins. (2018). “The Ethanolic Content of Papaya Carica papaya verses Sapodilla, Manilkara zapota via Fermentation, American Journal of Research Communication, 6 (9),
- Jagessar*, R.C, Douglas, L. (2020). “The fermentation of sugar rich fruits: jamun (Syzigium cumini), soursop (Annona muricata) and papaya, Carica papaya, with and without additives, in order to produce optimum ethanol yield for commercial purposes. 8(3), 2020.
- Jagessar, R.C, George, K. “Unpublished results, 2020.
- Jagessar, R.C, Phillips, M. “Unpublished results, 2020.
- Berłowska, J., Pielech-Przybylska, K., Balcerek, M., Dziekońska-Kubczak, U., Patelski, P., Dziugan, P., & Kręgiel, D. (2016). Simultaneous Saccharification and Fermentation of Sugar Beet Pulp for Efficient Bioethanol Production. BioMed Research International. 1–10. https://doi.org/10.1155/2016/3154929
- Günan Yücel, H., & Aksu, Z. (2015). Ethanol fermentation characteristics of Pichia stipitis yeast from sugar beet pulp hydrolysate: Use of new detoxification methods. Fuel, 158, 793–799. https://doi.org/10.1016/j.fuel.2015.06.016.
- Joannis-Cassan, C., Riess, J., Jolibert, F., & Taillandier, P. (2014). Optimization of very high gravity fermentation process for ethanol production from industrial sugar beet syrup. Biomass and Bioenergy, 70, 165–173. https://doi.org/10.1016/j.biombioe.2014.07.027).
- Tan, L., Sun, Z. Y., Okamoto, S., Takaki, M., Tang, Y. Q., Morimura, S., & Kida, K. (2015). Production of ethanol from raw juice and thick juice of sugar beet by continuous ethanol fermentation with flocculating yeast strain KF-7. Biomass and Bioenergy. 81, 265–272. https://doi.org/10.1016/j.biombioe.2015.07.019).
- Zabed, H., Faruq, G., Sahu, J. N., Azirun, M. S., Hashim, R., & Nasrulhaq Boyce, A. (2014). Bioethanol Production from Fermentable Sugar Juice. The Scientific World Journal. 1–11. https://doi.org/10.1155/2014/957102
- Ton i Rezi Damir Oros, Iva Markovi , Daniel Kracher , Roland Ludwig, and Božidar Šantek, J. (2013). Integrated Hydrolyzation and Fermentation of Sugar Beet Pulp to Bioethanol. Microbiol. Biotechnol. 23(9), 1244–1252 http://dx.doi.org/10.4014/jmb.1210.10013.