Publication Information
ISSN 2692-1529
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Optimizing Pacific White Shrimp Penaeus vannamei Nursery Phase in Super-Intensive Culture Through Probiotic Mix Application and Management
Aline Bezerra1, Luis Poersch1, Dariano Krummenauer2, Dionéia Cesar3, Wilson Wasielesky1*
1Laboratory of Marine Shrimp Culture, Institute of Oceanography, Federal University of Rio Grande-FURG, C. P. 474, Rio Grande CEP 96201-900, RS, Brazil; aline.bezerra09@gmail.com (A.B.); lpoersch@gmail.com (L.H.P.), manow@mikrus.com.br (W.W.)
2Laboratory of Ecology of Microorganisms Applied to Aquaculture, Institute of Oceanography, Federal University of Rio Grande-FURG, C. P. 474, Rio Grande CEP 96201-900, RS, Brazil; darianok@gmail.com (D.K.)
3Laboratory of Ecology and Molecular Biology of Microorganisms, Department of Biology, Federal University of Juiz de Fora, UFJF, Juiz de Fora, Minas Gerais CEP 36036-900, Brazil; dioneia.cesar@icb.ufjf.br (D.C.)
ORCID: Aline Bezerra (0000-0002-0303-3057), Luis Poersch (0000-0002-1663-6252), Dionéia Cesar (0000-0001-9289-0335), Dariano Krummenauer (0000-0002-2454-3822), Wilson Wasielesky Jr (0000-0002-7267-4755).
Received Date: September 28, 2024; Accepted Date: October 02, 2024; Published Date: October 21, 2024;
*Corresponding author: Wilson Wasielesky Jr.; Laboratory of Marine Shrimp Culture, Institute of Oceanography, Federal University of Rio Grande-FURG, C. P. 474, Rio Grande CEP 96201-900, RS, Brazil. E-mail: manow@mikrus.com.br; wasielesky@gmail.com
Citation: Bezerra A, Poersch L, Krummenauer D, Cesar D, Wasielesky W (2024) Optimizing Pacific White Shrimp Penaeus vannamei Nursery Phase in Super-Intensive Culture Through Probiotic Mix Application and Management. Jr Aqua Mar Bio Eco: JAMBE-134.
DOI: 10.37722/JAMBE.2024204
Abstract
In biofloc shrimp production systems, probiotics are crucial for managing microorganisms, outcompeting pathogens, colonizing the gut of shrimp, and enhancing their immune systems. The aim of this study was to investigate the efficacy of using a probiotic mix (composed of multi-species Bacillus subtilis, Lactobacillus plantarum and Pediococcus acidilactici) in marine shrimp nursery in biofloc system through different applications and its relationship with the microbial community, water quality and zootechnical performance. The FISH (fluorescence in situ hybridization) technique was used to analyze the bacterial abundance present in the water and in the gut of the shrimps, as well as the analysis of the microorganisms present in the system. The treatments were divided into two systems, clear water (CW) and biofloc (BFT), where the probiotics were added only to the feed (PF), only to the water (PW) and both feed and water (PFW), and controls where probiotics were not included. Then, the experiment was carried out with eight treatments. The nursery experiment lasted 35 days, with a stocking density of 2,000 shrimps/m2. No significant differences were found for water quality data (p>0.05) among treatments. The diversity and abundance of microorganisms was higher in the treatments with biofloc and two routes of probiotic application, as was the bacterial abundance in the water and in the gut of shrimp. The colonization of the shrimp gut was evidenced by the presence of hybridized, quantified, and classified probiotic bacteria, which were directly related to the rearing water. Zootechnical performance data were significantly (p<0.05) better in the treatment with the addition of probiotics in the feed and water in the biofloc system (BFT-PFW), where all of the indices were higher than the other treatments. Survival rates were over 89%, except for the control treatment (79%). Other treatments with at least one way of application showed satisfactory zootechnical performance compared to the control, including the clear water system which came close to the biofloc system without the addition of probiotics (BFT-CTL). The use of the probiotic mix was efficient and showed a positive effect on the culture of Penaeus vannamei in the nursery phase.
Keywords: Fluorescence in situ hybridization, microbial community, bacterial abundance, Bacillus subtilis, Lactobacillus plantarum, Pediococcus acidilactici.
Introduction
The intensification of aquaculture farming systems ensures higher yields in less space and higher profitability. However, super-intensive systems require regular and precise control of water quality and the production environment [1]. The nursery phase is considered a management tool between the first larval stages and the final grow-out phase. Intensification through nurseries can lead to rapid growth and enable high stocking densities, provided that appropriate management is carried out at this phase [2,3]. The emergence of pathogens and infectious diseases caused by viruses, bacteria and parasites poses major challenges to the aquaculture industry and is associated with the increasing intensification of production [4].
The microbial community plays an essential role in aquaculture, influencing productivity, nutrient cycling, feed efficiency for farmed animals, water quality, disease control and the environmental impact of effluents [5–7]. Biofloc-based production systems include various groups of microorganisms, mainly bacteria, but also feed residues, detritus from cultured animals, and organic and inorganic particles. These systems are considered efficient in controlling and balancing the microbial community [8]. Its composition is influenced by various factors such as the production system, the target species, the feed formulation and environmental, physical and chemical conditions [9,10].
Microorganisms are effective in fighting pathogens because they compete with potential pathogens and possess probiotic properties. They promote growth, digestion, metabolism and disease resistance in aquatic organisms [11–14]. In terms of relative abundance, bacteria make up the majority of bioflocs. They are capable of converting organic material, removing nitrogen-containing compounds and serving as a food source within the trophic food chain in aquaculture systems [15–17].
In marine shrimp production systems that utilize Biofloc technology, the diverse composition of microorganisms includes probiotics, which serve as a crucial management tool. Probiotics can outcompete pathogenic bacteria, colonize the gastrointestinal tract of shrimp, and enhance their immune system [18–21]. Probiotic bacteria can be added to aquaculture systems through various methods, and their mechanisms of action are diverse and essential for the establishment of a resilient microbiota capable of competing with potential disease outbreaks [22–24].
Management of probiotic application may vary and should be based on the specific needs of each production system and target species. Probiotics can be added directly to water, added to feed with oils or binders, applied via animal immersion baths, or introduced into soil or sediment. Each method has its advantages and disadvantages in terms of storage, handling and use in large quantities [25–27]. The use of probiotics with different bacterial composition is currently being discussed in detail and tested in various studies. These are called probiotic blends, multi-species probiotics or multi-strain probiotics [28,29].
The vast majority of commercial products consist of bacteria of the genus Bacillus sp. with the most commonly used species being B. subtilis and B. licheniformis. Bacillus sp. is considered the most studied and commercially exploited genus worldwide [30,31]. Lactic acid bacteria such as Lactobacillus plantarum and Pediococcus acidilactici are used in conjunction with bacilli and exhibit synergistic beneficial effects on growth, nutrition, strengthening the immune system of animals, fighting disease, colonizing the host gut and stimulating immune responses to stressful conditions [32–39].
Monitoring of microbial communities in super-intensive systems should be carried out regularly and in a timely manner. This is essential as rapid and efficient responses to the occurrence of pathogen outbreaks, are critical to ensuring system integrity and productivity [40,41]. Microbial density can influence how pathogenicity and resistance manifest. In addition, fluorescence in situ hybridization (FISH) is a useful method for tracking changes in the density of microorganisms [42]. This method uses specific probes for each target bacteria to be identified [43,44]. The effectiveness of probiotics has been confirmed by numerous studies using this molecular biology technique in aquaculture systems [19,45].
The microbiota present in the gut of marine shrimp plays a crucial role in their immunity, disease resistance and increased production. This microbiota is directly related to the water in the culture environment [46,47]. In a stable cultivation environment with favorable water quality conditions, probiotic bacteria are likely to dominate other groups and reduce the pathogenic load in both the water and gut of aquatic species farmed in biofloc systems [48]. Probiotic bacteria exhibit various capabilities, including direct modulation of bacterial communities, occupation of binding sites, and competition with harmful bacteria. They also contribute to the production of digestive enzymes and antagonistic substances. Probiotics are able to survive and proliferate under the adverse conditions in the animal gut while modulating and adapting the host microbiota [49–52]. Biotechnological advances must keep pace with the demand for necessary information, considering that there are still significant gaps in the literature that need to be filled and addressed regarding the characterization of gut microbes [53,54].
The aim of this study was to evaluate the effect of using a commercial probiotic mixture via different application routes in different nursery systems within a super-intensive system. The assessment included water quality, zootechnical performance, microorganisms, bacterial community and gut colonization of the shrimp.
Materials and methods
Experimental conditions
The experiment was conducted at the Marine Station of Aquaculture (EMA) from the Institute of Oceanography, Federal University of Rio Grande (FURG), Rio Grande/RS, Brazil (32°110S; 52°100W). The study lasted for 35 days. Shrimps of the species Penaeus vannamei, sourced from Aquatec® (Rio Grande do Norte), were obtained at the nauplius stage and underwent hatchery phase at the Marine Shrimp Culture Laboratory at EMA. After this period, post-larvae with an average weight of 0.012g (±0.001) were stocked in the experimental units at a stocking density of 2000 shrimps m-2. The experiment was conducted in an experimental room with temperature and photoperiod control. Submersible water heaters with thermostats (Stealth, ETP250, USA, 250W) were used for temperature control. Light intensity was controlled using an analog light timer, maintaining a 12-hour dark and 12-hour light cycle. The aeration system consisted of a blower-type air pump, where atmospheric oxygen was distributed in each experimental unit through two microperforated hoses (Aerotubes®) measuring 30 cm.
Clear water system and BFT system
The tanks had a bottom area of 0.49 m2, with a useful volume of 150 liters. The tanks were filled with saltwater (salinity 30). To initially disinfect the water, a solution of sodium hypochlorite (10ppm) was applied, followed by the application of ascorbic acid (1ppm) to neutralize the sodium hypochlorite residues. The study was conducted in triplicate and included two different systems: a clear water system (CW) and a biofloc system (BFT), each consisting of twelve (12) tanks, for a total of twenty-four (24) tanks. To maintain suitable water quality in the clear water system, 50% water renewals (75 liters) were performed every three days or based on ammonia and nitrite concentrations throughout the experimental period to maintain ammonia and nitrite below safety concentrations.
For the tanks with biofloc systems, 10% of their volume was inoculated with a mature biofloc water inoculum, with values of ammonia and nitrite close to zero, nitrate at 30 mg/L, phosphate at 1.10 mg/L, and total suspended solids at 460 mg/L [55]. Organic fertilizations with sugarcane molasses were conducted based on ammonia concentrations to maintain the carbon-nitrogen (C:N) ratio of the water. [56,57]. To replenish the volume lost through evaporation, additions of dechlorinated freshwater were made. Additionally, to maintain pH and alkalinity values in the biofloc system, doses of hydrated lime were added according to the decline in pH and alkalinity values [58].
Experimental design
The experiment was conducted in triplicate, utilizing twenty-four (24) tanks, with three tanks assigned to each treatment. The treatments were distributed as follows:
- Clear water system (CW):
- CW-CTL (Without addition of probiotics)
- CW-PF (Probiotics added in feed)
- CW-PW (Probiotics added in water)
- CW-PFW (Probiotics added in both feed and water)
- Biofloc sysem (BFT):
- BFT-CTL (Without addition of probiotics)
- BFT-PF (Probiotics added in feed)
- BFT-PW (Probiotics added in water)
- BFT-PFW (Probiotics added in both feed and water)
- Probiotic application and feed management
The commercial probiotic mixture contains: Bacillus subtilis (3.4×109 CFU g-1), Lactobacillus plantarum (1.2×109 CFU g-1) and Pediococcus acidilactici (1.2×109 CFU g-1), using lactose as a vehicle. The recommended dosages by the manufacturer were applied daily in the feed (2g of probiotic/kg of feed), and in the water, daily doses of 1g/ton of water were used. In treatments with two application routes (feed and water), the doses were doubled. The probiotic was mixed with water from each experimental unit to be sprinkled on the feed and pipetted into the culture water. The shrimps were fed three times daily (08:00; 14:00 and 16:00 h) with commercial feed Active 40% crude protein (Guabi®), following feeding tables according to the biomass of each experimental unit [59,60].
Water quality variables
Parameters such as temperature, pH, and dissolved oxygen were monitored twice daily (08:00 and 17:00 h) using a YSI® multiparameter instrument (model 556). Salinity was checked weekly using an optical refractometer (ATC, RTP-20ATC, Brazil). Total ammonia nitrogen (TA-N) and nitrite (NO–2-N) levels were analyzed every two days following methodologies described in [61,62]. Concentrations of nitrate (NO3 -N), phosphate (PO4-3-P) followed methodologies from [63] and alkalinity [64] were measured weekly. Total suspended solids (TSS) were measured at the beginning and end of the experiment, based on the method of [62].
Shrimp growth
The growth of shrimp in all experimental units was monitored through weekly biometrics using a digital scale with a precision of 0.001g. At the end of the experiment, the following parameters were evaluated:
Survival rate: ((final biomass / final mean individual weight) / number of stocked individuals) x 100.
Apparent feed conversion ratio (FCR): feed offered / biomass increment.
Yield: (final biomass / volume of experimental unit).
The specific growth rate [(LnWf – LnWi) × 100 / days], where Wf is the final weight, Wi is the initial weight, and days of culture.
Phytoplankton and zooplankton community assessment
For the quantification of microorganisms present in the culture water, water samples (20 mL) were collected at the end of the experimental period. The samples were fixed in 4% formalin (final concentration) and kept in amber bottles for subsequent counting and identification of the main groups of microorganisms present. The microorganisms were classified into different groups: flagellates, ciliates, rotifers, nematodes, and microalgae. An Olympus IX51 inverted microscope with a final magnification of 200x was used, where aliquots of 2.1 mL of sample were placed in a sedimentation chamber, and 30 fields were randomly counted. [65]. The counts were performed at the Laboratory of Ecology of Microorganism Applied to Aquaculture (LEMAQUI) at the Federal University of Rio Grande (FURG).
Bacteria abundance by Fluorescence In Situ Hybridization (FISH)
Final water samples from each experimental unit and shrimp gut were collected, both fixed in 2% paraformaldehyde (final concentration), and stored under refrigeration for subsequent performance of fluorescence in situ hybridization (FISH) analysis to identify bacteria in the probiotic mix (Table 1). Prior to the start of the FISH protocol [44,45] the gut samples were weighed and sonicated (Vibra Cell VCX 130PB, Sonics Materials®) with an amplitude of 110.7 mm for 60s (three times). After sonication, the samples were centrifuged at 500 g for five minutes, and the supernatant was removed, repeating this step twice with the addition of ultrapure water for content washing. The three portions of the supernatant were combined in the same flask, centrifuged again, and then filtered through white polycarbonate filters (Nuclepore® 0.2 mm) and kept refrigerated at 4°C until the FISH protocol was performed. Water samples were filtered directly through white polycarbonate filters (Nuclepore® 0.2 mm).
Subsequently, the samples were evaluated through epifluorescence microscopy using oligonucleotide probes directed towards rRNA to identify and quantify the target bacterial groups of the study (Table 1). All probes were labeled with Cy3 fluorochrome. Probes from the same bacterial group were mixed for hybridization. Additionally, each specific probe was added with DAPI to determine the total bacterial abundance. A negative control (NON) was used with a probe without any specific bacterial marker, serving as a test of the hybridization process efficiency. Bacterial abundance was obtained through direct counting at 1000x magnification using an epifluorescence microscope (Olympus® BX-60), equipped with the filters: Chroma U-N41007, U-MWU2, U-MWB2 and U-MWG2, at the Laboratory of Ecology and Molecular Biology of Microorganisms (LEBIOMM), at the Federal University of Juiz de Fora (UFJF).
Table 1 – Oligonucleotide probes for identification of different bacterial groups used in this study. All probes were labeled with Cy3 fluorochrome.
| Probe | Specificity | Sequence (5’-3’) | %FA* | Reference |
| NON | Negative Control | TAGTGACGCCGTCGA | 30 | [66] |
| Bacil1 | Bacillus | GCCGCCTTTCAATTTCGAAC | 35 | [67] |
| Bmy843 | Bacillus | CTTCAGCACTCAGGTTCG | 35 | [68] |
| Bsub | B. subtilis | CGTTCAAACAACCATCCGG | 35 | [69] |
| BsubC | B. subtilis group | AAGCCACCTTTTATGTTTGA | 35 | [69] |
| Lacto15 | Lactobacillus | CCGTCAACCCTTGAACAGTT | 30 | [70] |
| Lacto39 | Lactobacillus | TCTGTTTAGTTCCGCTCGTTC | 30 | [70] |
| LGC354A | Firmicutes | TGGAAGATTCCCTACTGC | 35 | [71] |
| LGC354B | Firmicutes | CGGAAGATTCCCTACTGC | 35 | [71] |
| LGC354C | Firmicutes | CCGAAGATTCCCTACTGC | 35 | [71] |
* Percentage of formamide (FA) in the in situ hybridization solution.
Data analysis
The data were subjected to one-way analysis of variance, considering the assumptions of homoscedasticity and normality through the Levene and Shapiro-Wilk tests, respectively. The Tukey test was applied when significant differences were detected (p<0.05), and survival, microorganism, and bacterial abundance data were transformed (arcsine square root) before analysis [72].
Results
Water quality
No significant differences were found among treatments for temperature, dissolved oxygen, and salinity. However, significant differences were observed among treatments for pH, alkalinity, ammonia, nitrite, nitrate, phosphate, and total suspended solids. All water quality results are presented in Table 2.
Table 2 – Mean (± standard deviation) of the average values found for physical and chemical parameters of water quality monitored during 35 days of P. vannamei nursery in super-intensive clear water system and biofloc system under different treatments.
| Variables | Treatments | |||||||
| CW-CTL | BFT-CTL | CW-PF | BFT-PF | CW-PW | BFT-PW | CW-PFW | BFT-PFW | |
| Temperature (°C) | 28.44±0.76 | 28.74±0.69 | 28.61±0.51 | 28.87±0.78 | 28.64±0.29 | 28.86±0.54 | 28.58±0.62 | 28.84±0.72 |
| DO (mg L-1) | 5.94±0.20 | 5.80±0.20 | 5.91±0.24 | 5.79±0.23 | 5.98±0.18 | 5.77±0.20 | 5.87±0.22 | 5.79±0.22 |
| pH | 8.28±0.03a | 7.96±0.05b | 8.20±0.03a | 8.00±0.06b | 8.24±0.03a | 7.98±0.05b | 8.23±0.04a | 7.95±0.03b |
| Alkalinity (mg CaCO3 L-1) | 207.33±8.66a | 153.33±8.60b | 205.00±17.29a | 159.67±18.27b | 207.00±5.21a | 159.00±7.15b | 202.00±13.34a | 155.67±7.83b |
| Ammonia (TA-N mg L-1) | 1.47±0.30a | 0.11±0.06b | 1.18±0.29a | 0.11±0.05b | 1.50±0.30a | 0.13±0.06b | 1.51±0.38a | 0.11±0.04b |
| Nitrite (NO2- – N mg L-1) | 0.93±0.27ab | 0.30±0.09c | 1.26±0.43a | 0.28±0.04c | 1.03±0.16ab | 0.34±0.08c | 1.33±0.28a | 0.38±0.11bc |
| Nitrate (NO3- – N mg L-1) | 0.94±0.54b | 26.73±2.34a | 1.47±0.23b | 23.25±2.67a | 1.01±0.23b | 25.28±2.29a | 1.14±0.23b | 26.45±2.12a |
| Phosphate (PO4-3-P mg L-1) | 0.07±0.02b | 0.35±0.03a | 0.07±0.02b | 0.30±0.09a | 0.08±0.04b | 0.32±0.06a | 0.10±0.01b | 0.31±0.09a |
| Salinity | 31.06±1.53 | 32.78±2.10 | 30.67±1.29 | 32.67±1.71 | 30.50±1.19 | 32.67±1.29 | 30.83±2.29 | 32.94±1.55 |
| TSS (mg L-1) | 86.40±23.56b | 352.53±22.92a | 129.40±32.78ab | 363.67±25.69a | 85.07±20.27b | 363.53±23.47a | 103.40±37.82ab | 388.33±35.38a |
Different letters on the same line represent significant differences (p<0.05) among treatments throughout the experimental period after one-way ANOVA followed by Tukey’s test.
The temperature values averaged 28.5°C, with no variations throughout the experimental period. Similarly, the concentrations of dissolved oxygen remained at average concentrations above 5.7 mg/L across all treatments. Salinity values were maintained between 30 and 32, with no difference among treatments (p>0.05).
The pH values varied according to the systems, with clear water systems maintaining averages above 8.2, while in the biofloc system, the values found were not lower than 7.95 (Figure 1). The same trend was observed for alkalinity, with average values above 200 mg CaCO3/L in clear water treatments, and above 150 mg CaCO3/L in biofloc system treatments (Figure 3c).
Ammonia concentrations were higher in the clear water systems in all treatments compared to the biofloc system, with the highest value found in the CW-PFW treatment (1.51), and the lowest concentration found in the biofloc system treatments BFT-CTL, BFT-PF, and BFT-PFW (0.11) (Figure 2a). The mean concentrations of nitrite were significantly higher in the treatments of the clear water system, with the highest mean value in the CW-PFW (1.33) and CW-PF (1.26) treatments. The lowest mean values were found in the biofloc system treatments BFT-PF (0.28), BFT-CTL (0.30), and BFT-PW (0.34) (Figure 2b).
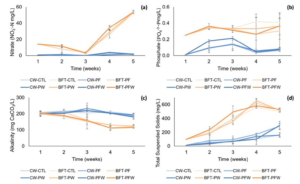
Nitrate concentrations were significantly higher in the biofloc system, with no difference among treatments within this system. Mean values of nitrate concentrations in the biofloc system treatments ranged from 26.7 to 23.2 in the BFT-CTL and BFT-PF treatments, respectively. For the clear water system, mean values remained between 0.94 and 1.47 in the CW-CTL and CW-PF treatments, respectively (Figure 3a). Phosphate concentrations remaining at near-zero concentrations in the clear water system, the highest mean values were found in the BFT-CTL treatment (0.35), and the lowest mean value was in the CW-CTL treatment (0.07) (Figure 3b). Mean concentrations of total suspended solids were higher in the biofloc system treatments, ranging from 352.5 to 388.3 in the BFT-CTL and BFT-PFW treatments, respectively. In the clear water system, the lowest mean value was found in the CW-PW treatment (85.0), and the highest mean value was in the CW-PF treatment (129.4) (Figure 3d).
Figure 1: Temporal variation of pH during the 35-day nursery period in the clear water system (CW) and biofloc system (BFT) under different treatments.
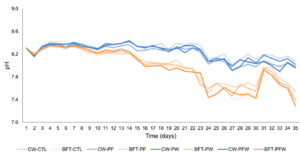
Figure 2: Temporal variation of total ammonia nitrogen (TA-N) (a) and nitrite (NO2-N) (b) during the 35-day nursery period under different treatments.
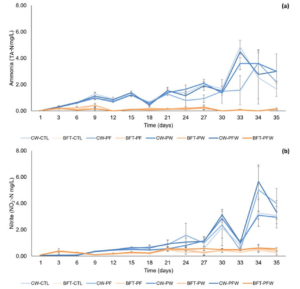
Figure 3: Temporal variation of nitrate (NO3-N) (a), phosphate (P-PO4-3) (b), alkalinity (CaCO3) (c), and total suspended solids (mg/L) (d) over the 5-week nursery period under different treatments.
Shrimp growth
The BFT-PFW treatment showed the best zootechnical performance for all evaluated parameters, including the highest final weight (1.22 g), the second-highest survival rate (96.5%), the highest specific growth rate (13.2%), the highest final biomass (354.1 g), and consequently, the highest yield per square meter (0.72 kg) (Table 3). The BFT-PF, BFT-PW, and CW-PFW treatments were statistically equivalent for mean final weight values, with 1.03 g, 1.01 g, and 1.01 g, respectively. The CW-PF treatment (0.98 g) was similar to the BFT-CTL treatment (0.94 g). The second lowest final weight was found in the CW-PW treatment (0.80 g), and the lowest final weight was found in the CW-CTL treatment (0.62 g), presenting as the treatment with the least satisfactory performance in the present study.
Table 3 – Mean (± standard deviation) of the average values of zootechnical performance parameters at the end of a 35-day nursery period of P. vannamei in a super-intensive system in clear water and biofloc system among different treatments.
| Zootechnical performance | Treatments | |||||||
| CW-CTL | BFT-CTL | CW-PF | BFT-PF | CW-PW | BFT-PW | CW-PFW | BFT-PFW | |
| Initial weight (g) | 0.012±0.001 | 0.012±0.001 | 0.012±0.001 | 0.012±0.001 | 0.012±0.001 | 0.012±0.001 | 0.012±0.001 | 0.012±0.001 |
| Final weight (g) | 0.62±0.05d | 0.94±0.04bc | 0.98±0.11bc | 1.03±0.01b | 0.80±0.13c | 1.01±0.04b | 1.01±0.01b | 1.22±0.09a |
| Survival (%) | 79.00±1.86b | 90.89±2.17a | 91.89±4.68a | 96.89±0.84a | 89.00±4.58a | 91.22±4.53a | 91.33±2.65a | 96.56±1.84a |
| SGR (%) | 11.26±0.24c | 12.46±0.12b | 12.56±0.33b | 12.71±0.02b | 11.99±0.45b | 12.66±0.11b | 12.68±0.01b | 13.20±0.21a |
| FCR | 3.01±0.37a | 2.85±0.22ab | 2.23±0.59ab | 2.20±0.32ab | 2.05±0.18b | 2.56±0.17ab | 2.11±0.31ab | 2.85±0.22ab |
| Final biomass (g) | 146.74±11.38d | 256.21±9.14bc | 269.15±32.62bc | 298.42±2.92ab | 213.65±28.52c | 276.26±13.65b | 277.81±8.20b | 354.15±29.63a |
| Yield (kg/m2) | 0.29±0.02d | 0.52±0.02bc | 0.54±0.07bc | 0.60±0.01ab | 0.43±0.06c | 0.56±0.03b | 0.56±0.02b | 0.72±0.06a |
Different letters on the same line represent significant differences (p<0.05) among treatments throughout the experimental period after one-way ANOVA followed by Tukey’s test.
The strategy of applying the probiotic mix through two application methods in the biofloc system (BFT-PFW) showed significantly better performance compared to all other treatments. Additionally, the treatment without probiotics in the clear water system (CW-CTL) exhibited poorer zootechnical performance across all evaluated parameters and was significantly inferior to all other treatments. This treatment obtained a lower mean final weight, lower survival rate (79%), lower specific growth rate (11.2%), higher apparent feed conversion ratio (3.0), lower final biomass (146.7 g), and consequently, a lower mean yield value (0.29 kg) (Table 3).
Survival rates were significantly higher in all treatments except for the treatment without probiotic addition in clear water (CW-CTL). The biofloc system treatments, including the control (BFT-CTL), when compared to treatments in the clear water system, except for the control (CW-CTL), showed statistically similar zootechnical performance. This means that treatments in the clear water system with at least one probiotic mix application method exhibited similar performance. For example, for the zootechnical parameter of specific growth rate (%), treatment BFT-PF obtained a value of 12.7, and treatment CW-PW had a value of 11.9, both statistically equal. The apparent feed conversion ratio (FCR) in treatment CW-PFW was 2.11, and in treatment BFT-CTL, the value found was 2.85, both statistically identical. The final biomass found in treatment BFT-PF was 298.4 g, similar to treatment CW-PFW with 277.8 g. The mean values for yield in kilograms per square meter were 0.56 (BFT-PW) and 0.56 (CW-PFW), as shown in Table 3.
Composition of phytoplankton and zooplankton community
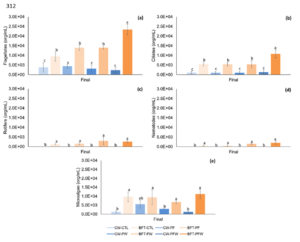
At the end of the experimental period, flagellates and ciliates were found in greater abundance in the biofloc system treatments, with treatment BFT-PFW having the highest mean values for flagellates (2.34E+04), ciliates (1.08E+04), nematodes (2.04E+03), and microalgae (1.12E+04). The treatments in the clear water system did not differ from each other, and the lowest mean abundance values were found for flagellates (2.32E+03) in treatment CW-PFW and ciliates (9.60E+02) in treatment CW-PF.
Protozoa such as rotifers and nematodes were found only in the biofloc system treatments with no difference between treatments, with variations in mean values in treatment BFT-CTL (1.27E+03) and treatment BFT-PW (2.96E+03) for rotifers, and for nematodes in treatment BFT-PF (4.86E+02) and treatment BFT-PFW (2.04E+03). Microalgae were found in all systems and treatments, with the highest mean values found in treatment BFT-PFW (1.12E+04) and the lowest mean value found in treatment CW-PFW (1.27E+03) (Figure 4).
Figure 4: Abundance of microorganisms (mean ± standard deviation) present in the clear water system and biofloc system at the end of the 35-day nursery period. Divided into flagellates (a), ciliates (b), rotifers (c), nematodes (d), and microalgae (e). Different letters indicate significant differences (p <0.05).
Composition of probiotic bacteria
The data for the abundance of total bacteria and archaea present in the culture water and shrimp gut are expressed in Figure 5 (a), where we find similar total values of bacterial community in both the water and the in the gut of the shrimp. Total values of Bacillus subtilis (b) and total lactic acid bacteria (c) exhibited similar trends, with higher bacterial prevalence in the water than in the gut, but with non-significant difference in the quantity present in the water or in the gut of the shrimp.
Figure 5: Total bacterial abundance (a), Bacillus subtilis (b), and lactic acid bacteria (c) present in the culture water and gut of the shrimp at the end of the 35-day nursery period.
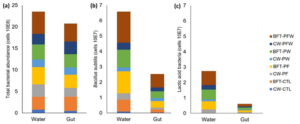
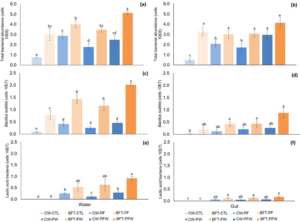
Through the total count of bacteria found in the culture water, the highest mean values are observed in the biofloc system in the BFT-PFW treatment (5.13E+08), standing out as the treatment with the highest total bacterial abundance (Figure 5). The BFT-PF treatment (4.00E+08) represents the second highest bacterial abundance, followed by the BFT-PW (3.47E+08) and BFT-CTL (3.01E+08) treatments. In the clear water systems, mean values are lower compared to the biofloc system, where the CW-PF treatment (2.88E+08) shows the highest abundance among the treatments in clear water, followed by the CW-PFW (2.49E+08) and CW-PW (1.77E+08) treatments. The CW-CTL treatment (0.75E+08) exhibits the lowest total bacterial abundance (Figure 6a).
The total count of bacteria present in the gut of the animals in the biofloc system treatments is statistically equal in the BFT-PFW (4.14E+08), BFT-CTL (3.29E+08), BFT-PF (3.01E+08), BFT-PW (3.07E+08), and CW-PFW (2.97E+08) treatments, with the latter being the only treatment in the clear water system that equals the treatments in the biofloc system. The CW-PF (2.07E+08) treatment equals the CW-PW (1.71E+08) treatment, both of which are superior and statistically differ from the CW-CTL (0.48E+08) treatment, which presents the lowest average count of bacteria in the gut of shrimp (Figure 6b).
In the specific count for Bacillus subtilis in the culture water, the highest mean value was found in the BFT-PFW treatment (2.01E+07), followed by the other treatments in the biofloc system, BFT-PF (1.43E+07), BFT-PW (1.16E+07), and BFT-CTL (0.77E+07). In the clear water system, the treatment with the highest mean value was observed in the CW-PFW treatment (0.46E+07), followed by the CW-PF (0.41E+07) and CW-PW (0.25E+07) treatments. The CW-CTL treatment (0.09E+07) showed the lowest count of bacilli (Figure 6c). In the gut of shrimp, the highest count of bacilli was found in the BFT-PFW treatment (0.87E+07), as well as in the BFT-PW (0.43E+07) and BFT-PF (0.42E+07) treatments. The CW-PFW treatment (0.26E+07) showed the highest count of bacilli among the treatments in clear water and was equally significant to the BFT-CTL treatment (0.19E+07), followed by the CW-PW (0.20E+07) and CW-PF (0.12E+07) treatments. The control treatment in clear water, CW-CTL (0.03E+07), showed the lowest count of bacilli among all treatments (Figure 6d).
Lactic acid bacteria were not found in the control treatments in clear water (CW-CTL) and in the biofloc system (BFT-CTL) in both samplings, both in the culture water and in the gut of the animals. In the specific count of lactic acid bacteria in the water, the highest mean value was found in the BFT-PFW treatment (0.92E+07), followed by the BFT-PW (0.64E+07), BFT-PF (0.53E+07), CW-PFW (0.29E+07), and CW-PF (0.26E+07) treatments, with the lowest mean value found in the CW-PW treatment (0.12E+07) (Figure 6e). In the gut of the shrimp, the bacterial colonization pattern showed the highest mean value in the biofloc system treatments BFT-PFW (0.17E+07), BFT-PF, and BFT-PW (0.13E+07), differing statistically from the clear water treatments CW-PFW (0.07E+07), CW-PF (0.06E+07), and CW-PW (0.05E+07) (Figure 6f).
Figure 6: Means (± standard deviation) of total bacterial abundance in water (a), total bacterial abundance in the gut (b), Bacillus subtilis in water (c), Bacillus subtilis in the gut (d), lactic acid bacteria in water (e), and lactic acid bacteria in the gut (f) at the end of the 35-day nursery period.
Discussion
The application of the probiotic mix did not affect water quality, as physical and chemical parameters such as temperature, dissolved oxygen, pH, alkalinity, salinity, ammonia, nitrite, nitrate, phosphate, and total suspended solids remained within the ideal and recommended range for P. vannamei [73–80]. However, some differences were found related to the different production systems, clear water and biofloc systems, as expected for this experimental design. Only nitrite concentrations and total suspended solids showed significant differences between the treatments tested in both systems. The average nitrite concentrations were higher in the clear water system due to the nitrification process, with higher values found in treatments CW-PFW and CW-PF, and the lowest concentrations were found in treatments BFT-PF, BFT-CTL, and BFT-PW. Similar behavior was observed in clear water conditions and biofloc system with the use of probiotics, where the absence of nitrification process resulted in higher concentrations in the clear water treatments [19].
In the biofloc system, in all evaluated treatments, the concentrations of total suspended solids remained below the recommended levels for the species. However, with the use of the initial biofloc inoculum, the nitrification process occurred efficiently and without peaks throughout the experiment [55,80].
The addition of probiotics is considered a management practice of extreme importance for the improved zootechnical performance of cultivated animals, contributing to better performance, immunity, and control of potential pathogenic bacteria [31,81). The treatment with the best zootechnical performance was BFT-PFW, where growth and production indices were superior, with average weight 50% higher compared to the control. The addition of probiotic bacteria in biofloc systems (with inoculum) provides better conditions for bacterial growth, where nutrients present in the biofloc system contribute to the better development of the microbial community [55,82,83]. When probiotics were added to both feed and water even in clear water conditions (CW-PFW), significant improvements were observed, such as higher final weight, specific growth rate, and yield, without differing from the biofloc system. This was observed in a study with continuous addition of the probiotic mix (3g/kg feed), where they achieved an average weight 20% higher compared to the control [84].
High stocking densities are common in nurseries in super-intensive systems. When the system is not efficiently controlled, it can lead to reduced survival rates and production losses. However, when management is properly outlined, it can result in better cultivation conditions and animal control [2]. The high stocking density used in this study negatively affected the zootechnical performance in the clear water system without the use of probiotics (CW-CTL), resulting in inferior performance in the evaluated zootechnical parameters. The non-use of probiotics can lead to various problems throughout the production cycle, such as stress and cannibalism, contributing to the unsatisfactory zootechnical performance as observed in studies comparing the addition of bacilli versus control without probiotics [85,86]. Survival rates were high, above 89%, in all treatments except the clear water control (CW-CTL) treatment. The application of probiotics is described in various studies where they are effective in improving different zootechnical parameters, including high survival rates at the end of the experiments [13,87,88].
Different methodologies for probiotic application are commonly used, including direct addition to the water, incorporation into feed, immersion baths, and direct addition to sediments [85,89,90]. The daily application of the probiotic mix provides bacteria continuously to the production system. This continuous stimulation contributed to better zootechnical performance in all treatments with at least one application route, as mentioned in several literature reviews on the use of probiotics [13,25,50]. When probiotic bacteria are added to both feed and water, they provide a greater supply of beneficial bacteria to the animals and the cultivation environment. We can observe through the data obtained for animal performance that the dual application of probiotics had satisfactory effects. Studies support this hypothesis, showing that different application routes and methodologies directly influence the performance of the cultured organisms [51,86,91].
The development of the microbial community plays a crucial role in the metabolism of organic matter, nutrient recycling, and nutritional supplementation provided to cultured organisms. It transforms nitrogen into microbial protein, contributing to the overall nutritional profile of the cultivation system [92,93]. In the present study, microorganisms present in the water were evaluated, classified as protozoa (zooplankton) and microalgae (phytoplankton), including flagellates, ciliates, rotifers, nematodes, and microalgae. A higher number of flagellates and ciliates were found in the biofloc system treatments, with the BFT-PFW treatment showing the highest development of these microorganisms. The BFT-PF, BFT-PW, and BFT-CTL treatments demonstrated microbial community development below that of the BFT-PFW treatment but superior to all treatments in the clear water system. Flagellates, ciliates, and microalgae were found in the clear water system treatments, including the control (CW-CTL); however, their abundance is significantly lower than in all other biofloc system treatments. In the biofloc system, the behavior of the microbial community has been reported in various studies, where diverse protozoa and different microalgae are found in this system, directly impacting microbial composition and the performance of cultured organisms [94,95]. Other protozoa such as rotifers and nematodes were found only in the biofloc system and were present in all treatments. These organisms are indicators of a more developed microbial chain, participating in nutrient cycling and the microbial loop acting as high-quality nutritional supplementation due to the supply of proteins and lipid [9,95,96].
Microalgae are responsible for producing proteins, lipids, and sugars and play a role in the dynamics of dissolved oxygen and carbon dioxide in aquaculture systems. They are considered primary producers and are consumed by zooplankton, transferring nutrients to higher trophic levels [97–99]. In the experimental conditions of this study, microalgae were found in all treatments, with the highest abundance observed in the biofloc system treatments, which were superior to the values found in the clear water system. Bioflocs provide nutrients for the growth of microalgae through the decomposition of organic matter and act as fertilizer (nitrogen and phosphate) under controlled conditions. Otherwise, it may lead to the unwanted dominance of filamentous microalgae and cyanobacteria [100,101].
The Fluorescence in situ hybridization (FISH) molecular biology technique used in this study was effective in quantifying and identifying the diversity of bacteria and the abundance of specific bacteria through the use of probes with target markers, as demonstrated in previous studies [19,45]. The present study obtained values for total bacterial abundance, Bacillus subtilis abundance, and lactic acid bacteria abundance, quantified in both the culture water and the gut of the animals. Both quantifications were significantly higher in the treatments with biofloc systems, especially in the treatment with dual probiotic application (BFT-PFW) in both the water and the gut. The colonization of the gut of the shrimp was evident in this study, where bacilli and lactic acid bacteria were found, except in the control groups, which may explain the low zootechnical indices [102,103].
The microbial community consists of various microorganisms, including a large number of bacteria belonging to different groups, such as heterotrophic, chemoautotrophic, photosynthetic, probiotic, and pathogenic bacteria. They are considered the main organisms in biofloc systems [9,40,57,104]. The authors state that there is an intense interaction between the culture environment and the aquatic organisms produced, i.e. the balance of the bacterial community in the water is directly related to the colonization of the shrimp gut [47,105]. The ability to colonize the gut provides numerous benefits to the host, such as the adhesion, survival and multiplication of bacteria in the gut, competition with pathogenic bacteria, better absorption of nutrients, stimulation of the immune system, the ability to secrete antagonistic substances and bacteriocins [106–109]. In the present study, we observed colonization of the intestinal tract consistent with the conditions found in the cultivation water.
The use of probiotic mix (multi-species) is being investigated by several authors currently, and the concern about the interaction between bacteria is being discovered and evaluated. Bacilli are often found in greater abundance in the cultivation environment than in the gut. However, lactic acid bacteria tend to colonize the gut and are capable of tolerating wide variations in pH, salinity, and anaerobic conditions (gut), facilitating their multiplication [29,39,110–112]. The bioflocs contributed to higher bacterial abundance in all tested treatments, and the present study also shows that higher bacterial abundance is found in the water compared to the gut. However, both are in sync, similar results were found in biofloc systems. The bacilli were able to colonize the tract, as well as the lactic acid bacteria, the concentrations differed in relation to the application method, the production system, and the applied dose [113–115].
Conclusions
The use of a multi-species probiotic mix composed of Bacillus subtilis, Lactobacillus plantarum, and Pediococcus acidilactici was able to maintain water quality in both systems and treatments, providing better zootechnical performance when applied in both feed and water in the biofloc system. When applied in the feed and water in the clear water system, it provided performance similar to the biofloc system. The highest abundance of microorganisms and bacteria was found in the biofloc system when the probiotic mix was added in two application routes. Bacilli and lactic acid bacteria were able to colonize the culture water and the intestinal tract of cultivated shrimp, being essential in super-intensive nurseries for marine shrimp.
Author Contributions
Aline Bezerra: investigation; microbiology analysis, formal analysis, methodology, roles/writing-original draft writing-review and editing, visualization.
Luis Poersch: funding acquisition, project administration, writing-review, and editing.
Dionéia Cesar: methodology, molecular biology analysis.
Dariano Krummenauer: methodology, writing-review, and editing.
Wilson Wasielesky Jr: conceptualization, methodology, writing-review, and editing, visualization, resources. All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by ASTRAL Project – H2020 grant Agreement 863034.
Data Availability: All data generated or analyzed during this study are included in this published article.
Acknowledgments: The authors are grateful for the financial support provided by the European Union (ASTRAL Project – H2020 – Grant Agreement 863034) and sponsored by National Council for Scientific and Technological Development (CNPq) and by the Brazilian Federal Foundation for Support and Evaluation of Graduate Education (CAPES) with grant number PQ307741/2022-2. Special thanks to KERA Animal Nutrition, GUABI Nutrition and Animal Health S.A. AQUATEC, TREVISAN and Al Aqua for donating the probiotics, experimental diets, post-larvae, and aeration system, respectively.
Declarations
Ethics approval and consent to participate: The research carried out complies with current animal protection laws in Brazil. All authors agree to participate in this experiment.
Consent for publication: All the authors of this article agree to the publication.
Conflicts of Interest: The authors declare that they have no conflict of interest.
References
- El-Saadony MT, Shehata AM, Alagawany M, Abdel-Moneim A-ME, Selim DA, Abdo M, et al. A review of shrimp aquaculture and factors affecting the gut microbiome. Aquac Int. 2022 Dec 28;30(6):2847–69. Available from: https://link.springer.com/10.1007/s10499-022-00936-1
- Wasielesky W, Froes C, Fóes G, Krummenauer D, Lara G, Poersch L. Nursery ofLitopenaeus vannameiReared in a Biofloc System: The Effect of Stocking Densities and Compensatory Growth. J Shellfish Res. 2013;32(3):799–806. Available from: http://www.bioone.org/doi/abs/10.2983/035.032.0323
- Emerenciano MGC, Rombenso AN, Vieira F d. N, Martins MA, Coman GJ, Truong HH, et al. Intensification of Penaeid Shrimp Culture: An Applied Review of Advances in Production Systems, Nutrition and Breeding. Animals. 2022 Jan 19;12(3):236. Available from: https://www.mdpi.com/2076-2615/12/3/236
- Gatesoupe FJ. Probiotics and Other Microbial Manipulations in Fish Feeds. In: Probiotics, Prebiotics, and Synbiotics. Elsevier; 2016. p. 319–28. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128021897000216
- Moriarty DJW. The role of microorganisms in aquaculture ponds. Aquaculture. 1997;151(1–4):333–49.
- Defoirdt T, Sorgeloos P, Bossier P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol. 2011 Jun;14(3):251–8. Available from:https://linkinghub.elsevier.com/retrieve/pii/S1369527411000440
- Luis-Villaseñor IE, Voltolina D, Audelo-Naranjo JM, Pacheco-Marges MR, Herrera-Espericueta VE, Romero-Beltrán E. Effects of biofloc promotion on water quality, growth, biomass yield and heterotrophic community in litopenaeus vannamei (Boone, 1931) experimental intensive culture. Ital J Anim Sci. 2015;14(3):332–7.
- Emerenciano MGC, Martínez-Córdova LR, Martínez-Porchas M, Miranda-Baeza A. Biofloc Technology (BFT): A Tool for Water Quality Management in Aquaculture. In: Water Quality. InTech; 2017. Available from: http://www.intechopen.com/books/water-quality/biofloc-technology-bft-a-tool-for-water-quality-management-in-aquaculture
- Ray AJ, Seaborn G, Leffler JW, Wilde SB, Lawson A, Browdy CL. Characterization of microbial communities in minimal-exchange, intensive aquaculture systems and the effects of suspended solids management. Aquaculture. 2010;310(1–2):130–8.
- De Schryver P, Crab R, Defoirdt T, Boon N, Verstraete W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture. 2008;277(3–4):125–37.
- Cienfuegos-Martínez K, Monroy-Dosta M del C, Hamdan-Partida A, Hernández-Vergara MP, Becerril-Cortés D, López-García E. A review of the use of probiotics in freshwater prawn (Macrobrachium sp.) culture in biofloc systems. Lat Am J Aquat Res. 2020 Sep 1;48(4):518–28. Available from: http://lajar.ucv.cl/index.php/rlajar/article/view/vol48-issue4-fulltext-2464
- Khanjani MH, Shari M, Emerenciano C. Review Article Bio fl oc Technology ( BFT ) in Aquaculture : What Goes Right , What Goes Wrong ? A Scienti fi c-Based Snapshot. 2024;2024.
- Pandiyan P, Balaraman D, Thirunavukkarasu R, George EGJ, Subaramaniyan K, Manikkam S, et al. Probiotics in aquaculture. Drug Invent Today. 2013 Mar;5(1):55–9. Available from: http://dx.doi.org/10.1016/j.dit.2013.03.003
- Hostins B, Wasielesky W, Decamp O, Bossier P, De Schryver P. Managing input C/N ratio to reduce the risk of Acute Hepatopancreatic Necrosis Disease (AHPND) outbreaks in biofloc systems – A laboratory study. Aquaculture. 2019;508(April):60–5. Available from:https://linkinghub.elsevier.com/retrieve/pii/S0044848618312171
- Lara GR, Poersch LH, Wasielesky W. The Quantity of Artificial Substrates Influences the Nitrogen Cycle in the Biofloc Culture System of Litopenaeus Vannamei. Aquac Eng. 2021;94(February):102171. Available from: https://doi.org/10.1016/j.aquaeng.2021.102171
- Wasielesky W, Atwood H, Stokes A, Browdy CL. Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture. 2006 Aug;258(1–4):396–403. Available from:https://linkinghub.elsevier.com/retrieve/pii/S004484860600281X
- Del’Duca A, Cesar DE, Abreu PC. Bacterial community of pond’s water, sediment and in the guts of tilapia (Oreochromis niloticus) juveniles characterized by fluorescent in situ hybridization technique. Aquac Res. 2015;46(3):707–15.
- Krummenauer D, Peixoto S, Cavalli RO, Poersch LH, Wasielesky W. Superintensive culture of white shrimp, Litopenaeus vannamei, in a biofloc technology system in Southern Brazil at different stocking densities. J World Aquac Soc. 2011;42(5):726–33.
- Hostins B, Lara G, Decamp O, Cesar DE, Wasielesky W. Efficacy and variations in bacterial density in the gut of Litopenaeus vannamei reared in a BFT system and in clear water supplemented with a commercial probiotic mixture. Aquaculture. 2017 Nov 1;480:58–64. Available from:https://linkinghub.elsevier.com/retrieve/pii/S0044848617303137
- Widanarni, Yuniasari D, Sukenda, Ekasari J. Nursery Culture Performance of Litopenaeus vannamei with Probiotics Addition and Different C/N Ratio Under Laboratory Condition. HAYATI J Biosci. 2010 Sep 1;17(3):115–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1978301916301875
- Llario, Falco, Sebastiá-Frasquet, Escrivá, Rodilla, Poersch. The Role of Bacillus amyloliquefaciens on Litopenaeus vannamei During the Maturation of a Biofloc System. J Mar Sci Eng. 2019 Jul 18;7(7):228. Available from: https://www.mdpi.com/2077-1312/7/7/228
- Algburi A, Volski A, Cugini C, Walsh EM, Chistyakov VA, Mazanko MS, et al. Safety Properties and Probiotic Potential of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895. Adv Microbiol. 2016;(May):432–52.
- Miranda‐Baeza A, Nolasco‐López M, Rivas‐Vega ME, Huerta‐Rábago JA, Martínez‐Córdova LR, Martínez‐Porchas M. Short‐term effect of the inoculation of probiotics in mature bioflocs: Water quality parameters and abundance of heterotrophic and ammonia‐oxidizing bacteria. Aquac Res. 2020 Jan 23;51(1):255–64. Available from:https://onlinelibrary.wiley.com/doi/10.1111/are.14371
- El‐Sayed AM. Use of biofloc technology in shrimp aquaculture: a comprehensive review, with emphasis on the last decade. Rev Aquac. 2021 Jan 9;13(1):676–705. Available from:https://onlinelibrary.wiley.com/doi/10.1111/raq.12494
- Kumar V, Roy S, Meena DK, Sarkar UK. Application of Probiotics in Shrimp Aquaculture: Importance, Mechanisms of Action, and Methods of Administration. Rev Fish Sci Aquac. 2016;24(4):342–68.
- Das Susmita MK, Haque S. A review on application of probiotic, prebiotic and synbiotic for sustainable development of aquaculture. J Entomol Zool Stud. 2017;5(2):422–9.
- Hancz C. Application of Probiotics for Environmentally Friendly and Sustainable Aquaculture: A Review. Sustainability. 2022 Nov 21;14(22):15479. Available from: https://www.mdpi.com/2071-1050/14/22/15479
- Zan Z, Chen K, Wang H, Han Z, Sun J. Effects of a multistrain probiotic on the growth, immune function and intestinal microbiota of the tongue sole Cynoglossus semilaevis. Aquaculture. 2023;575:739813. Available from: https://www.sciencedirect.com/science/article/pii/S0044848623005872
- Timmerman HM, Koning CJM, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int J Food Microbiol. 2004 Nov;96(3):219–33. Available from:https://linkinghub.elsevier.com/retrieve/pii/S0168160504002855
- Keysami MA, Mohammadpour M. Effect of Bacillus subtilis on Aeromonas hydrophila infection resistance in juvenile freshwater prawn, Macrobrachium rosenbergii (de Man). Aquac Int. 2013 Jun 2;21(3):553–62. Available from: http://link.springer.com/10.1007/s10499-012-9588-3
- Farzanfar A. The use of probiotics in shrimp aquaculture. FEMS Immunol Med Microbiol. 2006 Nov [cited 2018 Aug 27];48(2):149–58. Available from: https://academic.oup.com/femspd/article-lookup/doi/10.1111/j.1574-695X.2006.00116.x
- Giri SS, Sukumaran V, Sen SS, Jena PK. Effects of dietary supplementation of potential probiotic Bacillus subtilis VSG1 singularly or in combination with Lactobacillus plantarum VSG3 or/and Pseudomonas aeruginosa VSG2 on the growth, immunity and disease resistance of Labeo rohita. Aquac Nutr. 2014 Apr;20(2):163–71. Available from: https://onlinelibrary.wiley.com/doi/10.1111/anu.12062
- Aly SM, Abdel-Galil Ahmed Y, Abdel-Aziz Ghareeb A, Mohamed MF. Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol. 2008;25(1–2):128–36.
- Giang T, Shao H, Hu Y, Shia C, Quoc C, Truong P, et al. Bacterial population in intestines of white shrimp , Litopenaeus vannamei fed a synbiotic containing Lactobacillus plantarum and galactooligosaccharide. 2019;(September 2018):1–11.
- Kongnum K, Hongpattarakere T. Effect of Lactobacillus plantarum isolated from digestive tract of wild shrimp on growth and survival of white shrimp (Litopenaeus vannamei) challenged with Vibrio harveyi. Fish Shellfish Immunol. 2012;32(1):170–7. Available from:http://dx.doi.org/10.1016/j.fsi.2011.11.008
- Guimarães MC, Cerezo IM, Fernandez-Alarcon MF, Natori MM, Sato LY, Kato CAT, et al. Oral Administration of Probiotics (Bacillus subtilis and Lactobacillus plantarum) in Nile Tilapia (Oreochromis niloticus) Vaccinated and Challenged with Streptococcus agalactiae. Fishes. 2022 Aug 1;7(4).
- Hendam BM, Munir MB, Eissa MEH, El-Haroun E, Doan H van, Chung TH, et al. Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against Aspergillus flavus of Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol. 2023 Sep;303:115696. Available from: https://linkinghub.elsevier.com/retrieve/pii/S037784012300130X
- Jastaniah SD, Alaidaroos BA, Shafi ME, Aljarari RM, Abd El-Aziz YM, Munir MB, et al. Dietary Pediococcus acidilactici improved the growth performance, feed utilization, gut microbiota, and disease resistance against Fusarium solani in Pacific white shrimp, Litopenaeus vannamei. Aquac Int. 2023 Nov 13;(0123456789). Available from: https://link.springer.com/10.1007/s10499-023-01318-x
- Won S, Hamidoghli A, Choi W, Bae J, Jang WJ, Lee S, et al. Evaluation of Potential Probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on Growth Performance, Immune Response, Gut Histology and Immune-Related Genes in Whiteleg Shrimp, Litopenaeus vannamei. Microorganisms. 2020 Feb 19;8(2):281. Available from:ttps://www.mdpi.com/2076-2607/8/2/281
- Cardona E, Lorgeoux B, Chim L, Goguenheim J, Le Delliou H, Cahu C. Biofloc contribution to antioxidant defence status, lipid nutrition and reproductive performance of broodstock of the shrimp Litopenaeus stylirostris: Consequences for the quality of eggs and larvae. Aquaculture. 2016 Feb;452:252–62. Available from:http://dx.doi.org/10.1016/j.aquaculture.2015.08.003
- Panigrahi A, Das RR, Sivakumar MR, Saravanan A, Saranya C, Sudheer NS, et al. Bio-augmentation of heterotrophic bacteria in biofloc system improves growth, survival, and immunity of Indian white shrimp Penaeus indicus. Fish Shellfish Immunol. 2020 Mar;98:477–87. Available from:https://linkinghub.elsevier.com/retrieve/pii/S1050464820300218
- Matturro B, Rossetti S, Leitão P. CAtalyzed Reporter Deposition Fluorescence In Situ Hybridization (CARD-FISH) for Complex Environmental Samples. In 2021. p. 129–40. Available from: http://link.springer.com/10.1007/978-1-0716-1115-9_9
- Glöckner FO, Amann R, Alfreider A, Pernthaler J, Psenner R, Trebesius K, et al. An in situ hybridization protocol for detection and identification of planktonic bacteria. Syst Appl Microbiol. 1996;19(3):403–6.
- Cottrell MT, Kirchman DL. Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware estuary. Limnol Oceanogr. 2003;48(1 I):168–78.
- Del’Duca A, Cesar DE, Diniz CG, Abreu PC. Evaluation of the presence and efficiency of potential probiotic bacteria in the gut of tilapia (Oreochromis niloticus) using the fluorescent in situ hybridization technique. Aquaculture. 2013;388–391(1):115–21. Available from:http://dx.doi.org/10.1016/j.aquaculture.2013.01.019
- Holt CC, Bass D, Stentiford GD, van der Giezen M. Understanding the role of the shrimp gut microbiome in health and disease. J Invertebr Pathol. 2021 Nov 1;186:107387. Available from:https://linkinghub.elsevier.com/retrieve/pii/S0022201120300938
- Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. Probiotic Bacteria as Biological Control Agents in Aquaculture. Microbiol Mol Biol Rev. 2000;64(4):655–71.
- Cienfuegos-Martínez K, Monroy-Dosta M del C, Hamdan-Partida A, Hernández-Vergara MP, Aguirre-Garrido JF, Bustos-Martínez J. Effect of the probiotic Lactococcus lactis on the microbial composition in the water and the gut of freshwater prawn (Macrobrachium rosenbergii) cultivate in biofloc. Aquac Res. 2022 Aug 1;53(11):3877–89.
- Ninawe AS, Selvin J. Probiotics in shrimp aquaculture: Avenues and challenges. Crit Rev Microbiol. 2009 Feb;35(1):43–66. Available from: https://www.tandfonline.com/doi/full/10.1080/10408410802667202
- Nayak SK. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Vol. 13, Reviews in Aquaculture. John Wiley and Sons Inc; 2021. p. 862–906.
- Wee W, Abdul Hamid NK, Mat K, Khalif RIAR, Rusli ND, Rahman MM, et al. The effects of mixed prebiotics in aquaculture: A review. Aquac Fish. 2024 Jan;9(1):28–34. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2468550X22000508
- El-Saadony MT, Shehata AM, Alagawany M, Abdel-Moneim AME, Selim DA, Abdo M, et al. A review of shrimp aquaculture and factors affecting the gut microbiome. Vol. 30, Aquaculture International. Springer Science and Business Media Deutschland GmbH; 2022. p. 2847–69.
- Huang Z, Zeng S, Xiong J, Hou D, Zhou R, Xing C, et al. Microecological Koch’s postulates reveal that intestinal microbiota dysbiosis contributes to shrimp white feces syndrome. Microbiome. 2020 Dec 10;8(1):32. Available from: https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-020-00802-3
- Bentzon-Tilia M, Sonnenschein EC, Gram L. Monitoring and managing microbes in aquaculture – Towards a sustainable industry. Microb Biotechnol. 2016;9(5):576–84.
- Krummenauer D, Samocha T, Poersch L, Lara G, Wasielesky W. The Reuse of Water on the Culture of Pacific White Shrimp, Litopenaeus vannamei , in BFT System. J World Aquac Soc. 2014 Feb 4;45(1):3–14. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jwas.12093
- Avnimelech Y. C / N ratio as a control element in aquaculture systems. 1999;(June 1999):227–35.
- Ebeling JM, Timmons MB, Bisogni JJ. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture. 2006 Jun;257(1–4):346–58. Available from: https://linkinghub.elsevier.com/retrieve/pii/S004484860600216X
- Furtado PS, Poersch LH, Wasielesky W. Effect of calcium hydroxide, carbonate and sodium bicarbonate on water quality and zootechnical performance of shrimp Litopenaeus vannamei reared in bio-flocs technology (BFT) systems. Aquaculture. 2011 Nov;321(1–2):130–5. Available from: http://dx.doi.org/10.1016/j.aquaculture.2011.08.034
- Jory DE, Cabrera TR, Dugger DM, Fegan D, Lee PG, Lawrence L, et al. a Global Review of Shrimp Feed Management : Status and Perspectives. Aquaculture. 2001;104–52.
- Wasielesky W, Bezerra A, Poersch L, Hoffling FB, Krummenauer D. Effect of feeding frequency on the white shrimp Litopenaeus vannamei during the pilot‐scale nursery phase of a superintensive culture in a biofloc system. J World Aquac Soc. 2020 Oct 29;51(5):1175–91. Available from:https://onlinelibrary.wiley.com/doi/10.1111/jwas.12694
- Chemical methods for use in marine enviromental monitoring. Manual and Guides 12. Paris, France; 1983.
- Strickland JDH, Parsons TR. A Practical Handbook of Seawater Analysis. Bull Fish Res Board Canada. 1972;167(2nd edition). Available from: https://repository.oceanbestpractices.org/handle/11329/1994
- Aminot A, Chaussepied M. Manuel des analyses chimiques en milieu marin. CNEXO, Brest. 1983; Available from: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=6411644
- AOAC C. Official methods of analysis of the Association of Analytical Chemists International. Off Methods Gaithersburg, MD, USA. 2005;
- Utermöhl H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int Vereinigung für Theor und Angew Limnol Mitteilungen. 1958 Jan [cited 2024 Feb 9];9(1):1–38. Available from:https://www.tandfonline.com/doi/abs/10.1080/05384680.1958.11904091
- Yokokawa T, Nagata T. Growth and Grazing Mortality Rates of Phylogenetic Groups of Bacterioplankton in Coastal Marine Environments. Appl Environ Microbiol. 2005 Nov;71(11):6799–807. Available from:https://journals.asm.org/doi/10.1128/AEM.71.11.6799-6807.2005
- Ichijo T, Yamaguchi N, Tani K, Nasu M. Oligonucleotide Probes for Phylogenetic Detection of Waterborne Bacteria. J Heal Sci. 2010;56(3):321–5. Available from: http://www.jstage.jst.go.jp/article/jhs/56/3/56_3_321/_article
- Salzman NH, de Jong H, Paterson Y, Harmsen HJM, Welling GW, Bos NA. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria b bThe GenBank accession numbers for the clone sequences reported in this paper can be found in Table 1 T1 ; the accession number for iso. Microbiology. 2002 Nov 1;148(11):3651–60. Available from:https://www.microbiologyresearch.org/content/journal/micro/10.1099/00221287-148-11-3651
- Kyselková M, Kopecký J, Frapolli M, Défago G, Ságová-Marečková M, Grundmann GL, et al. Comparison of rhizobacterial community composition in soil suppressive or conducive to tobacco black root rot disease. ISME J. 2009 Oct 1;3(10):1127–38. Available from:https://academic.oup.com/ismej/article/3/10/1127/7588326
- Demanèche S, Sanguin H, Poté J, Navarro E, Bernillon D, Mavingui P, et al. Antibiotic-resistant soil bacteria in transgenic plant fields. Proc Natl Acad Sci. 2008 Mar 11;105(10):3957–62. Available from:https://pnas.org/doi/full/10.1073/pnas.0800072105
- Meier H, Amann R, Ludwig W, Schleifer KH. Specific Oligonucleotide Probes for in situ Detection of a Major Group of Gram-positive Bacteria with low DNA G+C Content. Syst Appl Microbiol. 1999 May;22(2):186–96. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0723202099800654
- Zar JH. Biostatistical analysis. Pearson Educ India. 1999;
- Ponce-Palafox J, Martinez-Palacios CA, Ross LG. The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp, Penaeus vannamei, Boone, 1931. Aquaculture. 1997 Nov;157(1–2):107–15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0044848697001488
- Wyk P Van, Scarpa J. Water quality requirements and management. Farming Mar Shrimp Recirc Freshw Syst Florida. 1999;141–62.
- Furtado PS, Fugimura MMS, Monserrat JM, Souza DM, Garcia L de O, Wasielesky W. Acute effects of extreme pH and its influences on the survival and biochemical biomarkers of juvenile White Shrimp, Litopenaeus vannamei. Mar Freshw Behav Physiol. 2015;48(6):417–29.
- Furtado PS, Poersch LH, Wasielesky W. The effect of different alkalinity levels on Litopenaeus vannamei reared with biofloc technology (BFT). Aquac Int. 2014;23(1):345–58.
- Lin YC, Chen JC. Acute toxicity of ammonia on Litopenaeus vannamei boone juveniles at different salinity levels. J Exp Mar Bio Ecol. 2001;259(1):109–19.
- Lin Y-C, Chen J-C. Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture. 2003 Jun;224(1–4):193–201. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0044848603002205
- Burford MA, Thompson PJ, McIntosh RP, Bauman RH, Pearson DC. Nutrient and microbial dynamics in high-intensity, zero-exchange shrimp ponds in Belize. Aquaculture. 2003 Apr;219(1–4):393–411. Available from:https://linkinghub.elsevier.com/retrieve/pii/S0044848602005756
- Gaona C a P, Poersch LH, Krummenauer D, Foes GK. The Effect of Solids Removal on Water Quality , Growth and Survival of Litopenaeus vannamei in a Biofloc Technology Culture System. Int J Recirc Aquac. 2011;12(June 2011):54–73.
- de Souza DM, Suita SM, Leite FPL, Romano LA, Wasielesky W, Ballester ELC. The use of probiotics during the nursery rearing of the pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817) in a zero exchange system. Aquac Res. 2012 Nov;43(12):1828–37. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2109.2011.02992.x
- Kuhn DD, Lawrence AL, Boardman GD, Patnaik S, Marsh L, Flick GJ. Evaluation of two types of bioflocs derived from biological treatment of fish effluent as feed ingredients for Pacific white shrimp, Litopenaeus vannamei. Aquaculture. 2010;303(1–4):28–33.
- Ekasari J, Angela D, Waluyo SH, Bachtiar T, Surawidjaja EH, Bossier P, et al. The size of biofloc determines the nutritional composition and the nitrogen recovery by aquaculture animals. Aquaculture. 2014;426–427:105–11.
- Kesselring JC, Gruber C, Standen B, Wein S. Continuous and pulse-feeding application of multispecies probiotic bacteria in whiteleg shrimp, Litopenaeus vannamei. J World Aquac Soc. 2019;50(6):1123–32.
- Rengpipat S, Phianphak W, Piyatiratitivorakul S, Menasveta P. Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture. 1998;167(3–4):301–13.
- Zokaeifar H, Balcázar JL, Saad CR, Kamarudin MS, Sijam K, Arshad A, et al. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2012;33(4):683–9. Available from: http://dx.doi.org/10.1016/j.fsi.2012.05.027
- Ramu D, Sigamani S, Venkatachalam H, Bommannan P, Ramamurthy D. The role of probiotics in the control of bacterial diseases and biodegradation of organic matter in shrimp (Penaeus vannamei) culture ponds of South India. J Coast Life Med. 2017 Jul 13;5(7):293–8. Available from: http://www.jclmm.com/qk/20159/2.pdf
- Abumourad I, Authman MMN, Sharaf O. Evaluation of Lactobacillus plantarum as a probiotic in aquaculture: Emphasis on growth performance and innate immunity. Vol. 1, Article in Journal of Applied Sciences Research. 2013. Available from: https://www.researchgate.net/publication/257958772
- Lalloo R, Ramchuran S, Ramduth D, Görgens J, Gardiner N. Isolation and selection of Bacillus spp. as potential biological agents for enhancement of water quality in culture of ornamental fish. J Appl Microbiol. 2007 Nov;103(5):1471–9. Available from:https://academic.oup.com/jambio/article/103/5/1471/6718846
- Gullian M, Thompson F, Rodriguez J. Selection of probiotic bacteria and study of their immunostimulatory effect in Penaeus vannamei. Aquaculture. 2004;233(1–4):1–14.
- Bidhan C De, Meena DK, Behera BK, Das P, Das Mohapatra PK, Sharma AP. Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses. Fish Physiol Biochem. 2014 Jan 14;40(3):921–71. Available from: http://link.springer.com/10.1007/s10695-013-9897-0
- Amjad K, Dahms H-U, Ho C-H, Wu Y-C, Lin F-Y, Lai H-T. Probiotic additions affect the biofloc nursery culture of white shrimp (Litopenaeus vannamei). Aquaculture. 2022;560:738475. Available from:https://www.sciencedirect.com/science/article/pii/S0044848622005919
- Huerta-Rábago JA, Martínez-Porchas M, Miranda-Baeza A, Nieves-Soto M, Rivas-Vega ME, Martínez-Córdova LR. Addition of commercial probiotic in a biofloc shrimp farm of Litopenaeus vannamei during the nursery phase: Effect on bacterial diversity using massive sequencing 16S rRNA. Aquaculture. 2019 Mar 15;502:391–9.
- Reis WG, Wasielesky W, Abreu PC, Brandão H, Krummenauer D. Rearing of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) in BFT system with different photoperiods: Effects on the microbial community, water quality and zootechnical performance. Aquaculture. 2019 Jun;508(April):19–29. Available from:https://linkinghub.elsevier.com/retrieve/pii/S0044848619302959
- Khanjani MH, Mohammadi A, Emerenciano MGC. Microorganisms in biofloc aquaculture system. Aquac Reports. 2022 Oct;26(May):101300. Available from: https://doi.org/10.1016/j.aqrep.2022.101300
- Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F. The Ecological Role of Water-Column Microbes in the Sea. Mar Ecol. 1983 Jul 1;10:257–63. Available from:https://www.degruyter.com/document/doi/10.7208/chicago/9780226125534-024/pdf
- Martins TG, Odebrecht C, Jensen L V., D’Oca MG, Wasielesky W. The contribution of diatoms to bioflocs lipid content and the performance of juvenile Litopenaeus vannamei (Boone, 1931) in a BFT culture system. Aquac Res. 2016;47(4):1315–26.
- Brown MR, Jeffrey SW, Volkman JK, Dunstan G. Nutritional properties of microalgae for mariculture. Aquaculture. 1997 May;151(1–4):315–31. Available from:https://linkinghub.elsevier.com/retrieve/pii/S0044848696015013
- Ju ZY, Forster I, Conquest L, Dominy W, Kuo WC, David Horgen F. Determination of microbial community structures of shrimp floc cultures by biomarkers and analysis of floc amino acid profiles. Aquac Res. 2008 Jan 8;39(2):118–33. Available from:https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2109.2007.01856.x
- Hargreaves JA. Biofloc Production Systems for Aquaculture. 2013;(4503):1–12.
- Holanda M, Besold C, Sempere FL, Abreu PC, Poersch L. Treatment of effluents from marine shrimp culture with biofloc technology: Production of Arthrospira ( Spirulina ) platensis (cyanobacteria) and nutrient removal. J World Aquac Soc. 2021 Aug 9;(June):jwas.12840. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jwas.12840
- Knipe H, Temperton B, Lange A, Bass D, Tyler CR. Probiotics and competitive exclusion of pathogens in shrimp aquaculture. Rev Aquac. 2021 Jan 24;13(1):324–52. Available from: https://onlinelibrary.wiley.com/doi/10.1111/raq.12477
- Huyghebaert G, Ducatelle R, Immerseel F Van. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 2011;187(2):182–8. Available from: http://dx.doi.org/10.1016/j.tvjl.2010.03.003
- Luis-Villaseñor IE, Campa-Córdova ÁI, Huerta-Aldaz N, Luna-González A, Mazón-Suástegui JM, Flores-Higuera F. Effect of beneficial bacteria on larval culture of Pacific whiteleg shrimp, Litopenaeus vannamei. African J Microbiol Res. 2013;7(27):3471–8. Available from:http://www.academicjournals.org/AJMR
- Moriarty DJW. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture. 1998;164(1–4):351–8.
- González-Félix ML, Gatlin DM, Urquidez-Bejarano P, de la Reé-Rodríguez C, Duarte-Rodríguez L, Sánchez F, et al. Effects of commercial dietary prebiotic and probiotic supplements on growth, innate immune responses, and intestinal microbiota and histology of Totoaba macdonaldi. Aquaculture. 2018;491:239–51. Available from: https://doi.org/10.1016/j.aquaculture.2018.03.031
- Wang J, Wu Z, Wang S, Wang X, Zhang D, Wang Q, et al. Inhibitory effect of probiotic Bacillus spp. isolated from the digestive tract of Rhynchocypris Lagowskii on the adhesion of common pathogenic bacteria in the intestinal model. Microb Pathog. 2022;169:105623. Available from:https://www.sciencedirect.com/science/article/pii/S0882401022002364
- Merrifield DL, Bradley G, Harper GM, Baker RTM, Munn CB, Davies SJ. Assessment of the effects of vegetative and lyophilized Pediococcus
- acidilactici on growth, feed utilization, intestinal colonization and health parameters of rainbow trout (Oncorhynchus mykiss Walbaum). Aquac Nutr. 2011;17(1):73–9.
- Amoah K, Huang Q, Tan B, Zhang S, Chi S, Yang Q, et al. Dietary supplementation of probiotic bacteria, Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019; Available from: https://doi.org/10.1016/j.fsi.2019.02.029
- Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L. Probiotics in aquaculture: The need, principles and mechanisms of action and screening processes. Aquaculture. 2008 Jan 31;274(1):1–14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0044848607010903
- Decamp O, Moriarty DJW, Lavens P. Probiotics for shrimp larviculture: review of field data from Asia and Latin America. Aquac Res. 2008 Mar [cited 2018 Aug 27];39(4):334–8. Available from: http://doi.wiley.com/10.1111/j.1365-2109.2007.01664.x
- Mohapatra S, Chakraborty T, Prusty AK, Das P, Paniprasad K, Mohanta KN. Use of different microbial probiotics in the diet of rohu, Labeo rohita fingerlings: effects on growth, nutrient digestibility and retention, digestive enzyme activities and intestinal microflora. Aquac Nutr. 2012 Feb;18(1):1–11. Available from:https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2095.2011.00866.x
- Hu X, Cao Y, Wen G, Zhang X, Xu Y, Xu W, et al. Effect of combined use of Bacillus and molasses on microbial communities in shrimp cultural enclosure systems. Aquac Res. 2017;48(6):2691–705.
- Nguyen Thi Truc L, Nguyen Thanh T, Tran Thi Hong T, Pham Van D, Vo Thi Tuyet M, Nguyen Trong N, et al. Effects of Feed Mixed with Lactic Acid Bacteria and Carbon, Nitrogen, Phosphorus Supplied to the Water on the Growth and Survival Rate of White Leg Shrimp (Penaeus vannamei) Infected with Acute Hepatopancreatic Necrosis Disease Caused by Vibrio parahaemol. Biology (Basel). 2021 Mar 30;10(4):280. Available from:https://www.mdpi.com/2079-7737/10/4/280
- Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12(1–3):39–85.