Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Change of Population and Characteristics of Non-Symbiotic Bacteria in Tropical Peat soil by Application of Soil Ameliorant and Nitrogen Fertilizer
Joko Tandiono1, 2*, Thamrin2, Hapsoh2, Trisla Warningsih2
1Minamas Research Centre, The Plaza, Jl MH Thamrin no 28-30, Jakarta, Indonesia
2Universitas Riau, Dept Environmental Science, Jl Pattimura No 9, Pekanbaru. Indonesia
Received Date: July 17, 2024; Accepted Date: July 31, 2024; Published Date: August 06, 2024;
*Corresponding author: Joko Tandiono, Minamas Research Centre, The Plaza, Jl MH Thamrin no 28-30, Jakarta, Indonesia Email: joko.tandiono@sdguthrie.com
Citation: Tandiono J, Thamrin, Hapsoh, Warningsih T (2024) Change of Population and Characteristics of Non-Symbiotic Bacteria in Tropical Peat soil by Application of Soil Ameliorant and Nitrogen Fertilizer. Adv Agri Horti and Ento: AAHE-208.
DOI: 10.37722/AAHAE.2024303
Abstract
Peatlands in Indonesia are widely used to develop oil palm plantation. Peatland development is faced with the problem of low peat soil fertility levels. Efforts made to increase the fertility of peat soil are by applying fertilizer. However, the high price of fertilizer requires other alternatives to support a more efficient supply of nutrients, one of which is the use of bacteria that are able to provide nutrients for plants, such as non-symbiotic nitrogen-fixing bacteria. Non-symbiotic N-fixing bacteria are one of the biological potentials found around plant cultivation areas. This bacterium is used as an agent for fixing free N in the air. This research aims to isolate and characterize non-symbiotic N-fixing bacteria and nitrifying bacteria from peat soil. The experiment used a completely randomized factorial design with nitrogen fertilizer and soil ameliorant each at 3 levels and 3 replicates. Soil sampling was taken by using purposive sampling. The medium used in this study was Ashby's, NFB, TSIA and Burk's medium. The criteria used as observation parameters in identifying bacterial isolates are the morphological characteristics of bacteria including macroscopic characteristics and microscopic characteristics of bacterial isolates, biochemical tests and physiological tests. The data on the number of bacteria obtained was analyzed statistically using variance, then continued with the Least Significant Difference (LSD) test at the 5% level. A total of 36 isolates of non-symbiotic N-fixing bacteria were successfully isolated on Ashby's and NFb medium. Also obtained were 36 isolates of nitrifying bacteria from TSIA and Burk's medium. The results of the potential test for non-symbiotic N-fixing bacteria showed that 18 isolates were able to form pellicle on Ashby's medium and 18 isolates were able to change the color of the medium on NFb medium. The potential test to produce ammonium and nitrate was measured using a spectrophotometer. Isolates no 4, 9, 11 and 18 are isolates that have a low ability to produce ammonium and nitrate so they can be used in peat soil. The results of Gram staining of isolates of non-symbiotic N-fixing bacteria and nitrifying bacteria were all Gram negative. The catalase test results showed that all isolates of non-symbiotic N-fixing bacteria and nitrifying bacteria were classified as aerobic bacteria.
Keywords: Peat, Bacteria, soil ameliorant
Introduction
Indonesia is a tropical country with the largest distribution of tropical peat in the world, namely 14,905,475 ha. Peat areas in Indonesia are spread across Sumatra, Kalimantan and Papua. Riau Province itself has peat covering an area of 2,858,700 ha or 19.18% of the total peat land area in Indonesia (BPS, 2022). Most of the peatlands in Riau Province are used for oil palm plantations. The area of oil palm plantations in Riau Province that have cultivation rights (HGU) is 390,265.31 ha or 41.11% (Department of Food Crops, Horticulture and Plantations, 2016). Efforts that are usually made to obtain higher oil palm productivity on peatlands are by providing fertilizer and ameliorant (Syahminar, 2020).
Nitrogen fertilizer (N) is one of the fertilizers that is often used in the cultivation of oil palm plants, however nitrogen fertilizer has a fairly high price. Fertilizer is an important factor in palm oil cultivation, and also takes up the largest portion of costs. No less than 60% of oil palm plant maintenance costs are allocated for fertilization (Juliansyah, 2018). Other research results also state that around 40-60% of maintenance costs come from fertilization and 30% of total production costs (Syahminar, 2020). Good fertilizer management will greatly help in efficiency of palm oil production costs and minimize the risk of fertilizer loss due to evaporation or leaching (Adriany et al., 2015). An alternative solution that can be implemented to provide N in oil palm plantations is by utilizing the biological potential of the soil which plays a role in increasing the availability and transformation of nutrients that support plant growth. Microbes in soil are an important part of the decomposition process and are also an indicator of the quality of an ecosystem (Pratiwi et al., 2018). One of the important microbes in peat soil is nitrogen-fixing bacteria and nitrifying bacteria (Rohyani et al., 2014). Apart from that, to help decompose organic matter, microbes in peat soil play an important role in providing nutrients for plants and producing enzymes that enable plants to grow well (Mahdiyah, 2015). Biological potential that can be exploited in oil palm plantation areas is non-symbiotic N-fixing bacteria (Herman, 2013). According to Schlegel (1984) N in the air can be utilized by plants with the help of N2-fixing bacteria (BPN), so that the N can be firmly bound to soil components which means that the N is not easily flushed out of the soil and can be utilized by plants. However, information on the existence of these bacteria is very limited because the peat soil conditions do not support the development of bacteria. Peat soil is classified as marginal soil which has low fertility and is characterized by low pH (3.0–5.0) and high water content. Seeing these conditions, non-symbiotic N-fixing bacteria seem to be underdeveloped. Improvements in soil chemical properties are thought to be able to provide optimal conditions for the growth of non-symbiotic N-fixing bacteria. Application of soil ameliorant is one way that can be done to improve the chemical properties of peat soil. The ameliorant that is often used is boiler ash. Boiler ash is one of the materials that can be used as an ameliorant to improve soil chemical properties, especially soil pH (Hardjowigeno, 2010). Providing boiler ash at 1-2 tons/ha/year can increase a number of alkaline cations and soil pH. The existence of non-symbiotic N-fixing bacteria has previously been studied. Isolation of non-symbiotic N-fixing bacteria in peatlands was carried out by (Islam, 2018) to obtain isolates of non-symbiotic N-fixing bacteria in soil where empty oil palm fruit bunches and palm oil mill wastewater were applied. It is necessary to study changes in soil chemical properties by applying boiler ash and nitrogen fertilizer on the activity of non-symbiotic N-fixing bacteria. The application of boiler ash and nitrogen fertilizer is thought to increase the activity of non-symbiotic N-fixing bacteria in peatlands. Therefore, it is necessary to conduct research on the growth of non-symbiotic N-fixing bacteria in peatlands that have been applied with boiler ash and nitrogen fertilizer.
Methods
The study was conducted using factorial randomized block design with 2 factors, first factor is boiler ash (B) as soil ameliorant with three levels (0, 1.5-ton/ha/yr and 3-ton/ha/yr), the second factor is nitrogen fertilizer (F) with 3 levels (0, 0.45 kg N/palm/yr, and 0.9 kg N/palm/yr) with 3 replications. Each plot consisted of 16 oil palms where 4 palms were used as sampling points and 12 palms as guard palm. Soil samples were taken between oil palm trees using a purposive sampling method and then calculated the total population of nitrogen-fixing bacteria and nitrifying bacteria. Calculation of bacterial density was carried out using the pour plate method. The total population of N-fixing bacteria was calculated on Ashby's medium and NFb medium. This potency testing activity begins with inoculation of 1 ml of bacteria in 9 ml of semi-solid Ashby's medium, then incubation for 72 hours. At the end of incubation, around 0.5 cm from the surface of the medium, pellicle was visible. The formation of pellicle is a characteristic of the growth of non-symbiotic N-fixing bacteria, that these bacteria are able to reduce N sources from the medium as nitrogenase activity. The same thing was also done on NFb medium, but what was observed was a color change in the medium.
Results and Discussion
Total Population of Non-Symbiotic Fixing Bacteria and Nitrifying Bacteria
One indicator that can be used to assess the condition of an ecosystem is through the presence of microbes in the soil. The use of peat forests as agricultural areas and oil palm plantations has an impact on microbial growth. Lisa (2019) stated that peatlands that were converted into oil palm plantations experienced an increase in the number of soil microbes due to fertilizer input which improved the nutrient status of the soil. Pratiwi, et al. (2018) said that the increase in soil microbes on agricultural land on peat soil was also due to the action of making drainage ditches which caused the soil surface to become aerobic, this caused the microbes to be more active in breaking down organic material. The total population of bacteria grown on NFb media ranged from 1.8 – 3.1 x 104 cfu/g soil and on Ashby's media ranged from 2.3 – 4.8 x 104 cfu/g soil. The total population of non-symbiotic N-fixing bacteria was highest in the peat soil location with the application of 1.5 tons/ha/year of boiler ash, both on NFb and Ashby's media. Table 1 shows that the boiler ash treatment had a significant effect on the total population of non-symbiotic N-fixing bacteria. The results of observing the number of non-symbiotic N-fixing bacteria can be seen in Table 1. And Fig 1.
Table 1. Total Population of non-symbiotic N-fixing bacteria in Ashby’s medium and NFb medium
Treatment
level
Medium
Abhy's
NFb
(x 104 cfu/g soil)
(x 104 cfu/g soil)
Soil Ameliorant (t/ha/tahun)
0
2.64 b
1.66 b
1.5
3.77 a
3.82 a
3
2.96 b
1.82 b
Nitrogen Fertilizer (kg N/palm/yr)
0
2.96 b
2.33 a
0.45
3.35 a
2.42 a
0.9
3.06 ab
2.53 a
Remark: Number followed by the same lowercase letter in the same column were not significant different according to LSD test at the 5% level.
Application of boiler ash at 1.5 tons/ha had the highest total population of non-symbiotic N-fixing bacteria compared to other treatments, both on Ashby's media and NFb media. The degree of soil acidity at the sampling location ranged from 4.06–5.09, which indicates that all soil samples were acidic. The average pH in the boiler ash treatment of 1.5 tonnes/ha/year was 4.93 which is classified as acid, but is still within the pH range that can be tolerated by non-symbiotic N-fixing bacteria in an acidic environment. The pH value of the soil greatly influences the activity and development of soil microbes. If the soil pH is low, microbial activity will decrease. According to Ku¨mmerer (2014), low pH also disrupts the population and activity of symbiotic nitrogen-fixing bacteria in the nitrogen fixation process. The results of the research support the number of bacterial populations ranging from <100 cells/gram of soil in this study. The population number of non-symbiotic N-fixing bacteria in this study was slightly different from the results of previous studies. Based on the research results of Agisti et al. (2014), it was found that the population of non-symbiotic N-fixing bacteria was around 1.8 x 106 cfu/g of soil at the LCC (Legume Cover Crop) planting location. Furthermore, Pranoto et al. (2015) also reported that the population of non-symbiotic N-fixing bacteria on the slopes of tea plantations ranged from 7.14–11.75 x 107 cfu/g soil. Meanwhile, Islam (2018), found that the total population of bacteria grown on NFb media ranged from 5.87–8.87 x 105 cfu/g soil and on Ashby's media ranged from 3.62–9.45 x 105 cfu/g soil on soil planted with oil palm.
According to Widawati (2015), N-fixing bacteria can live at pH 5.8–8.0. However, there are some bacteria that are able to grow at pH 3-4, namely low pH soil bacteria. Nugroho (2013) stated that the high or low total microbial population is influenced by the availability of energy sources in the environment and the ability of microbes to compete with other microbes that live and reproduce by depending on the same energy sources. Santoso et al. (2019) added that members of the Azotobacter genus are able to grow at a pH ranging from 4.8 to 8.5, with the optimum pH for growth and nitrogen fixation being around 7.0 to 7.5. Meanwhile, members of the Azospirillum genus grow well at pH 7 and prefer acidic pH, have the ability to ferment sugar, are oxidase positive, are chemoorganotrophic, and are facultative hydrogen autotrophs.
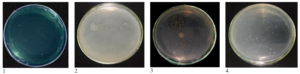
Figure 1. Total population of non-symbiotic N-fixing bacteria on (1) Ashby’s (2) NFb medium (3) medium Triple Sugar Iron Agar (TSIA) and (4) medium Burk’s
Table 2. Total Population of Nitrification bacteria in TSIA medium and Burk’s medium
Treatment
level
Medium
TSIA
Burk’s
(x 104 cfu/g soil)
(x 104 cfu/g soil)
Soil Ameliorant (t/ha/tahun)
0
2.20 a
4.06 a
1.5
2.73 a
3.44 a
3
2.66 a
3.49 a
Nitrogen Fertilizer (kg N/palm/yr)
0
1.76 c
3.52 a
0.45
2.50 b
3.77 a
0.9
3.33 a
3.82 a
Remark: Number followed by the same lowercase letter in the same column were not significant different according to LSD test at the 5% level.
The total population of bacteria grown on specific media Nitrosomonas sp. (Triple Sugar Iron Agar (TSIA)) ranges from 1.8–2.9 x 104 cfu/g soil, while on the specific media Nitrobacter sp. (Burk's medium) ranges from 3.2–4.3 x 104 cfu/g soil. The results of observations of the number of nitrifying bacteria can be seen in Table 2. The total population of nitrifying bacteria grown on TSIA media did not show significant differences, as did Burk's media. This can be seen from the variance results which show that the boiler ash treatment did not have a significant effect on the total population of nitrifying bacteria.
The development of bacteria is influenced by the metabolic activity of the surrounding plant roots. The number of bacteria in soil varies because bacterial development is very dependent on soil conditions (Pratiwi et al., 2018). There are differences in the distribution of non-symbiotic N-fixing bacteria in each rhizosphere. Apart from that, physical and chemical factors in the soil also influence the number of non-symbiotic N-fixing bacteria. Until now, it is difficult to find a type of soil amendment that is able to repair and enhance all soil functions (physical, chemical and biological) with one application and one type. Organic materials have been proven to have many functions (multi-function), but relatively high doses are required, namely around 5–20 tonnes/ha and continuous application is often required (Dariah et al., 2012). Most soil amendments are aimed at improving specific soil properties. For example, lime is mainly intended to increase pH, zeolite to improve CEC, hydrogel to increase the soil's ability to hold water, and so on. Amelioration of peat soil is also needed to reduce the rate of carbon emissions from peat soil (Subiksa et al., 2011).Bacterial activity is more active in conditions of severe nutrient limitation (oligotrophic) compared to mesotrophic conditions. The group of autotrophic bacteria that play a role in the nitrification process is Nitrobacteriaceae, including Nitrosomonas (ammonium oxidizing bacteria) and Nitrobacter sp. (nitrite oxidizing bacteria). Several heterotrophic microorganisms are also reported to be able to oxidize ammonia or organic nitrogen into nitrite or nitrate (Hastuti, 2007).
Characteristics of Non-Symbiotic Nitrogen-Fixing and Nitrifying Bacterial Isolates
The morphological characteristics of colonies and cells of non-symbiotic N-fixing bacterial and nitrifying bacterial isolates from each treatments in this study are presented in Table 3 and Table 4. Observation of colony morphology of non-symbiotic N-fixing bacteria using Ashby's medium for Azotobacter bacteria and solid Azospirillum medium for Azospirillum bacteria seen from colony color, colony edges, colony shape, colony surface, colony elevation, Gram test and cell shape. All isolates of non-symbiotic N-fixing bacteria obtained in this study had round colonies, smooth surfaces, convex elevations, flat colony edges and were classified as Gram-negative bacteria. However, for the color of bacteria, there are 2 different colors, namely cloudy white and off-white. The same thing was also obtained when observing cell shape. Non-symbiotic N-fixing bacterial cells are oval and rod-shaped. Erfin et al. (2016) obtained an isolate of non-symbiotic N-fixing bacteria, namely Azospirillum, the result of isolation and purification, which had a curved or semi-spiral colony shape, a rod cell shape and a pink color, which shows that this bacterium is Gram negative. Hartono and Jumadi (2014) also found that the characteristics of non-symbiotic nitrogen-fixing bacterial isolates were that the colonies were round and irregular, the elevation of the colonies was mostly flat with a smooth shiny surface, and the edges of the colonies were flat and wavy with transparent, transparent white, white and white colors, cloudy. Tarigan et al. (2013) also reported that the colony shape was generally round and irregular, the edges of the colony varied, such as wavy, split, whole, and curly with the most dominant elevation being flat, the color of the colony white and yellow. Based on the research results, bacterial isolates grown on Ashby's medium had a morphology that was almost similar to Azotobacter bacteria. The results of this research are supported by the opinion of Erfin (2016) who found that isolates of non-symbiotic N-fixing bacteria of the Azotobacter type showed that the morphology of the bacteria was cocci or round in shape, pink in color, indicating that these bacteria were Gram negative.
Tabel 3. Characteristics of Non-Symbiotic Nitrogen Fixing Bacterial Isolates in NFb Medium and Ashby's Medium
No
NFb Medium
Ashby’s Medium
Color
Cell Form
Color
Cell Form
1
Cloudy white
Oval
Cloudy white
Oval
2
Cloudy white
Oval
White
Oval
3
Cloudy white
Oval
White
Oval
4
Cloudy white
Oval
White
Oval
5
Cloudy white
Oval
White
Oval
6
White
Basil
White
Basil
7
White
Basil
Cloudy white
Basil
8
White
Basil
Cloudy white
Basil
9
White
Basil
White
Basil
10
White
Basil
White
Basil
11
White
Basil
White
Basil
12
White
Basil
White
Basil
13
White
Basil
White
Basil
14
White
Basil
White
Basil
15
White
Oval
Cloudy white
Oval
16
White
Oval
Cloudy white
Oval
17
White
Basil
White
Basil
18
Cloudy white
Oval
Cloudy white
Oval
According to Cowan et al. (1993), stated that the Azotobacter genus includes Gram-negative, rod-shaped and cocus bacteria. Based on the research results of Erfin (2016), Azotobacter bacteria show a rod shape and cysts. This is in accordance with the opinion of Brock et al. (1994), these bacteria have special structures called cysts. These cysts are like endospores, namely thick-walled bodies, very reactive and resistant, resistant to the drying process, mechanical breakdown, ultraviolet and ionic radiation. Puspitasari et al. (2012) also added that cysts in Azotobacter bacteria function to protect against extreme environmental conditions, for example drought, ultraviolet light and ionizing radiation. The ability of Azotobacter bacteria to fix N varies from + 2-15 mg nitrogen/gram of carbon source used (Rao, 1994). Nitrifying bacteria isolates are generally round in shape, the surface of the colony is predominantly smooth, although some are dry and shiny with convex elevations and some are flat, having flat edges. Colors are white, yellowish white, and yellow. Cell morphology is generally rod-shaped and oval. There were 18 isolates that were considered Gram negative. The characteristics of nitrifying bacteria obtained are in accordance with research by Islam (2018) which found that isolates of nitrifying bacteria were generally round and irregular in shape, the surface of the colonies was predominantly smooth, although some were dry and smooth with convex elevations, flat and high, had wavy and even edges. Colors include white, milky white, cloudy white, yellow and cream. Cell morphology was generally rod-shaped and round, and one isolate was spiral-shaped. All isolates were Gram negative. Kiding et al., (2015) obtained the characteristics of nitrifying bacteria in the form of round colonies with smooth edges, convex and flat elevations, white, yellow and clear white in color. Microscopic morphological characteristics of bacteria were observed through Gram staining. Gram staining is one of the procedures most widely used to classify various bacteria (Anuar et al., 2014). According to Rostinawati (2008), Gram staining is used to determine the morphology of bacterial cells and to differentiate Gram positive and Gram negative bacteria. Dwijoseputro (2005) added that bacteria generally have rod (coccus), round (bacil) and bent (spiral) cell shapes.
Tabel 4. Characteristics of Nitrifying Bacterial Isolates in TSIA Medium and Burk’s Medium
No
Medium TSIA
Burk’s medium
Elevation
Color
Cell Form
Color
Cell Form
1
Convex
White
Basil
Cloudy white
Basil
2
Convex
White
Basil
White
Basil
3
Convex
White
Basil
White
Basil
4
Flat
Yellowish white
Basil
White
Basil
5
Convex
White
Basil
White
Basil
6
Convex
White
Basil
White
Basil
7
Flat
Yellow
Oval
Cloudy white
Oval
8
Convex
Milky white
Oval
Cloudy white
Oval
9
Convex
White
Oval
White
Oval
10
Convex
White
Basil
White
Basil
11
Flat
Yellowish white
Basil
White
Basil
12
Flat
Yellowish white
Oval
White
Oval
13
Convex
White
Oval
White
Oval
14
Convex
White
Oval
White
Oval
15
Flat
Yellowish white
Basil
Cloudy white
Basil
16
Convex
White
Basil
Cloudy white
Basil
17
Convex
White
Basil
White
Basil
18
Convex
White
Basil
Cloudy white
Basil
Microscopic morphological characteristics of bacteria were observed through Gram staining. Gram staining is one of the most widely used procedures to classify various bacteria. Gram staining is used to determine the morphology of bacterial cells and to differentiate Gram positive and Gram-negative bacteria. The grouping of Gram characteristics is based on the color bound by the bacterial cell wall. This is in accordance with Pelczar and Chan (2010) who said that the difference in bacterial color during Gram staining is due to differences in the structure and chemical composition of the cell wall between Gram-negative bacteria and Gram-positive bacteria. Gram-negative bacteria contain lipids, fats or fat-like substances in higher percentages than those contained in Gram-positive bacteria. Gram negative bacteria have a thin cell wall structure, namely 10–15 nm and are three (multi) layered. Gram positive bacteria have a thick cell wall structure of 15–80 nm and are single layered. The composition of the cell wall contains a small amount of lipids, peptidoglycan as a single layer amounts to around 90% of the weight of the cell wall and contains techoic acid (Madigan et al., 2012).
Potential Test of Non-Symbiotic N-Fixing Bacterial Isolates
Based on the results of population calculations that had been carried out, 36 isolates were obtained, namely 18 isolates from NFb medium and 18 isolates from Ashby's medium. Next, a N-fixing potential test was carried out on each isolate grown in NFb and Ashby's medium. Potential test data for non-symbiotic N-fixing bacteria are presented in Table 5 and Figure 2. The isolate obtained was a non-symbiotic N-fixing bacterium which was characterized by the isolate's ability to form a pellicle on Ashby's medium. Isolates B12 and B22 had the highest ability to produce pellicle compared to other isolates. The formation of pellicle proves that the bacteria are able to reduce N sources from the medium as nitrogenase activity. In Ashby's medium, bacteria use mannitol as a carbon source and KH2PO4 as a phosphate source for bacterial growth. Bacteria produce pellicle in Ashby's medium because there is no excess oxygen in the medium, the oxygen diffusion rate is the same as the organism's respiration rate, which is a good condition for the activity of the nitrogenase enzyme which helps reduce acetylene to ethylene.

Figure 2. Test the potential of non-symbiotic N-fixing bacterial isolates in NFb medium. A: color change (1) without inoculation (control), (2) color change to slight blue (+), (3) color change to moderate blue (++), (4) color change to deep blue (+++). B: Ashby's medium pellicle formation. (1) not formed (control), (2) thin pellicle formed (+), (3) medium pellicle formed (++), (4) thick pellicle formed (+++)
According to Santoso et al. (2019), NFb medium is able to provide the nutrients needed by non-symbiotic nitrogen-fixing bacteria. The blue color change in the NFb medium indicates that there is nitrogenase activity carried out by non-symbiotic nitrogen-fixing bacteria. Mallombasi (2018) added that NFb is a selective medium for growing non-symbiotic N-fixing bacteria where the medium does not contain nitrogen elements so that the bacteria that can grow are bacteria that can fix free nitrogen in the atmosphere. According to Ristiati et al. (2008), non-symbiotic N-fixing bacteria that grow on NFb medium use malic acid (C4H6O5) as a carbon source. Color change that occurs in the NFb medium, which was originally yellowish green to blue, indicates bacterial activity in fixing free nitrogen in the atmosphere. This group of bacteria will bind nitrogen and convert it into ammonium (NH4+) which is alkaline, causing the bromothymol blue indicator in the medium to turn blue. These results are supported by the results of research by Islam (2018) which obtained non-symbiotic nitrogen-fixing bacteria characterized by the formation of a pellicle in the form of white fibers or rings on the surface of Ashby's medium and were able to change the color of the NFb medium from green to blue. One type of symbiotic N-fixing bacteria that can grow on NFb medium and Asbhy's medium is Azotobacter and Azospirillum bacteria, which are non-symbiotic N-fixing bacteria or free-living nitrogen-fixing rhizobacteria that are able to live in root areas, fertile soil, marginal soil, saline soil. or in acidic soil (Figueiredo et al., 2010). Azospirillum and Azotobacter are free-living in the root area and are able to fix nitrogen (Sy et al., 2001). Azospirillum bacteria have great potential as a biofertilizer, because Azospirillum is able to produce the growth hormone IAA and also act as a soil aggregate stabilizer (Widawati et al., 2012). These bacteria are often associated with grass-type plants, including several types of cereals, corn, wheat and grass (Kumar, 2014). Meanwhile, Azotobacter bacteria are nitrogen-fixing bacteria that are capable of producing the growth-promoting substances gibberellins, cytokinins and indole acetic acid, so they can stimulate root growth (Nosrati et al., 2014). This is in accordance with the research results of Widiastuti et al. (2012) found that all isolates of Azotobacter sp. isolated from several habitats are capable of producing IAA. Hosseini et al. (2014) stated that bacteria that have this ability belong to the Plant Growth Promoting Rhizobacteria (PGPR) group and according to Singh et al. (2011) these bacteria are useful as biofertilizers.
Table 5. Potential of non-symbiotic N-fixing bacteria based on pellicle formation in Ashby's medium and changes in medium color in NFb medium
Isolat No
Characteristics of non-symbiotic N-fixing bacteria
Pellicle in Ashby’s medium
Color change in NFb medium
1
+
+
2
+
+
3
+
+
4
+
+
5
+
+
6
+
+
7
+
+
8
+
+
9
+
+
10
+
+
11
+
+
12
+
+
13
+
+
14
+
+
15
+
+
16
+
+
17
+
+
18
+
+
The isolate obtained had the ability to change the color of the NFb medium from yellowish green to blue, which was indicated as nitrifying bacteria. It is known that isolates 1, 2, 9, and 15 have the highest ability compared to other isolates. NFb medium is able to provide the nutrients needed by non-symbiotic nitrogen-fixing bacteria. The blue color change in the NFb medium indicates that there is nitrogenase activity carried out by non-symbiotic nitrogen-fixing bacteria. Non-symbiotic N-fixing bacteria that grow on NFb medium use malic acid (C4H6O5) as a carbon source. The color change that occurs in the NFb medium, which was originally yellowish green to blue, indicates bacterial activity in fixing free nitrogen in the atmosphere. This group of bacteria will bind nitrogen and convert it into ammonium (NH4+) which is alkaline, causing the bromothymol blue indicator in the medium to turn blue.
Potential Test of Nitrification Bacterial Isolates
Based on the results of population calculations that had been carried out, 36 isolates were obtained, namely 18 isolates from TSIA medium and 18 isolates from Burk's medium. Next, the potential of nitrifying bacterial isolates was tested by measuring the ability of the isolates to oxidize ammonium (NH4+) and produce nitrate (NO3-) on TSIA and Burk's medium. Each media was given a different concentration, namely 250 ppm and 500 ppm (NH4)2SO4. Figure 3 show that isolate 9 has the lowest potential in oxidizing ammonium in TSIA medium with concentrations of 250 ppm and 500 ppm (NH4)2SO4. This is characterized by a high concentration of ammonium in the TSIA medium. Meanwhile, isolate 1 had the highest ability to oxidize ammonium in TSIA medium with concentrations of 250 ppm and 500 ppm (NH4)2SO4 that indicate by the lowest concentration of ammonium in the medium.
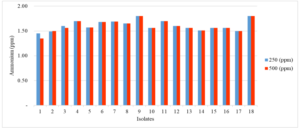
Figure 3. The potential of several bacterial isolates in oxidizing ammonium in TSIA medium with concentrations of 250 ppm and 500 ppm (NH4)2SO4.
On the 8th day there was another increase in ammonium levels in the media. It is known that isolate 9 has the lowest potential in oxidizing ammonium in TSIA medium with concentrations of 250 ppm and 500 ppm (NH4)2SO4. Meanwhile, isolate 1 had the highest ability to oxidize ammonium in TSIA medium with concentrations of 250 ppm and 500 ppm (NH4)2SO4. The difference in ammonium levels occurred due to different oxygen levels. Nitrite does not last long and the presence of nitrite is usually a temporary state of the oxidation process between ammonia and nitrate. The presence of nitrite illustrates the ongoing biological process of breakdown with very low oxygen levels. Low oxygen content can inhibit ammonium oxidation, resulting in ammonium accumulation. The same results were reported by Qoriatul (2021) which stated that the ammonium concentration increased on day 6, which means the ammonium oxidation process decreased. Islam (2018) also reported that on day 4 of incubation there was a sharp decrease in ammonium concentration in the specific Nitrosomonas sp medium, while on day 8 the decrease was very slight.
The decrease in the ammonium oxidation process due to the continuous formation of ammonium compounds while the rate of ammonium degradation or oxidation is slow. The ammonium concentration will increase when some bacteria die during the oxidation process. Zhang et al. (2014) explained that when bacteria die, the ammonium that has been absorbed in the bacterial cells will be released and increase the ammonium concentration in the experimental medium. Based on the research results, bacterial isolates that can be used in peat soil are isolates that have a low ability to oxidize ammonium, such as isolates 13, 15, 7, and 9. This is because the state of nitrogen in peat soil is very dynamic and easy to change.
Figure 4 show that isolate 16 had the highest potential in producing nitrate in Burk's medium with a concentration of 250 ppm and isolate 5 at a concentration of 500 ppm (NH4)2SO4. Meanwhile, isolate 1 had the lowest ability to produce nitrate in TSIA medium with a concentration of 250 ppm and isolate 15 in a medium concentration of 500 ppm (NH4)2SO4. So, it is known that isolates 5, 16, 13, 1, and 15 can be used as biological agents in peat soil because they produce small amounts of nitrate when a potency test is carried out.
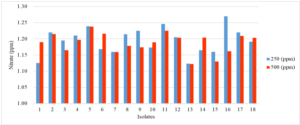
Figure 4. The potential of several bacterial isolates in oxidizing nitrate in TSIA medium with concentrations of 250 ppm and 500 ppm (NH4)2SO4.
On the 8th day it was discovered that isolate 16 had the highest potential in producing nitrate in Burk's medium with a concentration of 250 ppm and isolate 5 at a concentration of 500 ppm (NH4)2SO4. Meanwhile, isolate 1 had the lowest ability to produce nitrate in TSIA medium with a concentration of 250 ppm and isolate 15 in a medium concentration of 500 ppm (NH4)2SO4. So it is known that isolates 5. 16, 13, 1, and 15 can be used as biological agents in peat soil because they produce small amounts of nitrate when a potency test is carried out. The results of this study are the same as Qoriatul (2021) who found that an increase in nitrate concentration was observed on day 6, indicating low bacterial activity in converting nitrite to nitrate. However, it is different from several previous research results. It has been reported by Islam (2018), on the 4th day of incubation there was a sharp increase in nitrate concentration in the specific medium Nitrobacter sp. at concentrations of 250 ppm and 500 ppm (NH4)2SO4, while on day 8 the nitrate production was less. Furyanti (2009) also reported that the formation of nitrate (NO3-) after adding Tephrosia candida litter increased in the first week to the 4th week and began to decrease in the 7th week. According to Dahiya et al. (2016), each bacteria has a different ability to metabolize nitrite, nitrate and ammonium compounds. The process of oxidation of ammonium to nitrite (nitrification) by bacteria involves the enzyme ammonia monooxygenase. According to Zhang et al. (2014), differences in nitrate levels are caused by differences in nitrite accumulation. The nitrite conversion process is hampered due to the high carbon content. This activity is influenced by the reductase enzyme. Li et al. (2016) added that low nitrate levels also occur due to disruption of the balance of enzymatic reactions. Nitrate concentrations will increase if sufficient oxygen is available. Aerobic conditions cause nitrification to occur and produce nitrate using the raw material ammonium in the soil and the availability of water as a medium for nitrifying bacteria. If these two things are optimal, the nitrification process will run smoothly.
Conclusions
There were 72 bacterial isolates including 18 isolates of non-symbiotic N-fixing bacteria grown on Ashby's medium, 18 isolates of non-symbiotic N-fixing bacteria on NFb medium, 18 isolates of nitrifying bacteria on TSIA medium and 18 isolates of nitrifying bacteria on Burk's medium. Isolates no 3 and 4 have the highest ability to produce pellicle on Ashby's medium. Isolates no 1, 2, 9, and 10 have the highest ability to change the color of the NFb medium from green to blue. Bacterial isolates that can be used in peat soil are isolates that have a low ability to oxidize ammonium, such as isolates no 4, 9, 11 and 18. This is because the state of nitrogen in peat soil is very dynamic and easy to change. Isolates no 1, 7, 13, and 15 can be used as biological agents in peat soil because they produce small amounts of nitrate when tested for potency.
References
- Adriany, T.A., A. Wihardjaka, Setyanto, P., Salwati. 2015. Pengaruh Pemberian Amelioran pada Perkebunan Kelapa Sawit di Lahan Gambut Provinsi Jambi Terhadap Emisi CO2. Balai Penelitian Lingkungan Pertanian. Jambi.
- Agisti, A., Alami, N.H., Hidayati, T.N. 2014. Isolasi dan Dentifiasi Bakteri Penambat Nitrogen Non Simbiotik pada Lahan Restorasi dengan Metode Legume Cover Crop (LCC) di Daerah Pasirian Lumajang Jawa Timur. Jurnal Sains dan Seni POMITS. Vol. 3 (2).
- Anuar, W.D. Andi dan C. Jose. 2014. Isolasi bakteri selulolitik dari perairan Rumai. Jurnal Online Mahasiswa. 1(2).
- Badan Pusat Statistik. 2022. Luas Areal Perkebunan Kelapa Sawit. https://bps.go.id. Diakses pada 30 Mei 2023.
- Brock T.D., dkk. 1994. Biology of Microorganism, seventh edition. Prentice – Hall.New Jersey.
- Cowan and Steel. 1993. Manual for the Identification of Medical Bacteria. 3rd edn. Ed GI Barrow, RKA Feltham. Cambridge University Press.
- Dahiyah, S., Jaggi, S., Chaturvedi, K.K., Bhardwaj, A., Goyal, R.C., and Vagese, C. 2016. An eLearning System for Agricultural Education. Indian Research Journal of Extension Education. Vol. 12(3): 132–135.
- Dariah, A. dan Nurida, N.L., 2012, Penggunaan pembenah tanah organik dan hayati untuk meningkatkan produktivitas lahan kering di Ciampea, Bogor, dalam Prosiding Seminar Nasional Peran Teknologi untuk Mewujudkan Kedaulatan Pangan dan Peningkatan Ekonomi Rakyat, Fakultas Pertanian UPN Veteran, Yogyakarta.
- Department of Food Crops, Horticulture and Plantations. 2016. Statistik Perkebunan Indonesia. Direktorat Jenderal Perkebunan. Jakarta.
- Dwidjoseputro, D. 2005. Dasar-Dasar Mikrobiologi. Djambatan. Jakarta.
- Erfin, Sandiah, N., dan Males, L. 2016. Identifikasi Bakteri Azospirillum dan Azotobacter pada Rhizosfer Asal Komba-Komba (Chromolaena odorata). JITRO. Vol. 3(2).
- Figueiredo Ma´rcia do V.B., L. Seldin, F.F. de Araujo, and Mariano, 2010, Plant Growth Promoting Rhizobacteria: Fundamentals and Applications, Plant Growth and Health Promoting Bacteria, Microbiology. Monographs 18, Verlag Berlin Heidelberg.
- Furyanti D., 2009, Pengaruh Kualitas Serasah Pengkas Tephrosia candida dan Acacia auriculiformis Terhadap Pembentukan Nitrat (NO3) dan Potensial Nitrifikasi di Alfisol, Jumantono. Fakultas Pertanian Universitas Sebelas Maret, Surakarta.
- Hardjowigeno, S. 2010. Ilmu Tanah. Akademi Pessindo. Jakarta. 288 hal
- Hartono dan Jumadi O., 2014, Seleksi dan Karakterisasi Bakteri Penambat Nitrogen Non Simbiotik Pengekskresi Amonium Pada Tanah Pertanaman Jagung (Zea mays) dan Padi (Oryza sativa L.) Asal Kabupaten Barru, Sulawesi Selatan Indonesia, Jurnal Sainsmat Vol.3. No. 2: 143-153.
- Hastuti R.D., 2007, Bakter Penambat Nitrogen Bebas, dalam R.D.M. Simanungkit, Suriadikarta, Saraswati, E. Husein, Metode Biologi Tanah. Balitbang Sumberdaya Lahan Pertanian, Departemen Pertanian, Jakarta.
- Herman, M. dan D.P (2013). Pengaruh Mikroba Pelarut Fosfat Terhadap Pertumbuhan Dan Serapan Hara P Benih Kakao ( Theobroma Cacao L .) Effect Of Phosphate Solubilizing Microbes On The Growth And. Balittri: 129–138.
- Hosseini, A., A. Maleki, K. Fasihi & R. Naseri. 2014. The Co-application of Plant Growth Promoting Rhizobacteria and Inoculation with Rhizobium Bacteria on Grain Yield and Its Components of Mungbean (Vigna radiate L.) in Ilam Province, Iran World Academy of Science, Engineering and Technology. International Journal of Biological, Food, Veterinary and Agricultural Engineering. 8 (7).
- Islam, H. 2018. Isolasi dan Uji Potensi Bakteri Penambat N Non Simbiotik dan Bakteri Nitrifikasi Asal Tanah Kebun Kelapa Sawit dengan Aplikasi Tandan Kosong dan Limbah Cair Kelapa Sawit. Tesis. Universitas Riau.
- Juliansyah, G. dan Supijatno. 2018. Manajemen Pemupukan Organik dan Anorganik Kelapa Sawit di Sekuinyir Estate, Kalimatan Tengah. Bul. Agrohorti. Vol. 6 (1): 32–41.
- Kiding A., Khotimah S., Linda R., 2015, Karakterisasi dan Kepadatan Bakteri Nitrifikasi pada Tingkat Kematangan Tanah Gambut yang Berbeda di Kawasan Hutan Lindung Gunung Ambawang Kab. Kubu Raya, dalamJurnal Protobiont, 4(1): 17-21.
- Ku¨mmerer, K. 2014. Resistance in the environment. Journal of Antimicrobial Chemotherapy. Vol 54.
- Kumar M. 2014. Bacteria involving in nitrogen fixation and their evolutionary correlation. International Journal of Current Microbiology and Applied Sciences 3(3): 824- 830.
- Li, X.N. Song, H.L., Li, W., Lu, X.W. and Nishimura, O. 2016. An Integrated Ecological Foating-Bed Employing palnt, freshwater calm and biofilm carrier for purification of eutropic water. Ecol. Eng. Vol. 36:382-390.
- Lisa, M. 2019. Isolasi dan Karakterisasi Bakteri Pengikat Nitrogen dari Tanah Gambut Kecamatan Trumon, Aceh Selatan. Universitas Islam Negeri Ar-Raniry Darussalam-Banda Aceh
- Madigan M.T, Martinko J.M, Stahl D.A, Clark D.P., 2012, Brock Biology of microorganism. Edisi ke-13. San Francisco (US): Benjamin Cummings.
- D. 2015. Isolasi Bakteri dari Tanah Gambut Penghasil Enzim Protease. Pharmascience. 2 (2): 71–79
- Mallombasi, N.A. 2018. Isolasi dan Identifikasi Bakteri Penambat Nitrogen Non Simbiotik Daerah Perakaran Padi (Oryza sativa) di Kelurahan Balang Kecamatan Binamu Kabupaten Jeneponto. UIN Alauddin Makassar: Fakultas Sains dan Teknologi.
- Nosrati, R., P. Owlia, H. Saderi, I. Rasooli & MA. Malboobi. 2014. Phosphate solubi lization characteristics of efficient nitro gen fixing soil Azotobacter strains Iran. Journal Microbiology 6 : 285-295.
- Nugroho, K. dan & Sarwani, M. (2013). Characterizing the cultivated lowland peat soils in two physiography positions in Kalimantan , Indonesia. [Online] 3 (7), 246–255. Available from: http://www.interesjournals. org/IRJAS
- Pelczar M. J dan Chan E. C. S., 2010, Dasar-Dasar Mikrobiologi, Edisi 2, Terjemahan Hadioetomo, R.S., Imas, T., Tjitrosomo, S.S dan Angka, S.L, UI-Press.
- Pratiwi, E., Satwika, T.D., dan Agus, F. 2018. Keanekaragaman Mikroba Tanah Gambut di Bawah Hutan dan di Bawah Perkebunan Kelapa Sawit di Provinsi Jambi. Jurnal Tanah dan Iklim. Vol. 42 (1).
- E., Pratiwi. S., Wachyuni. H., and Anindita. S. 2015. Population Distribution Pattern of Azotobacter sp and Organic Material on Variety Slope Classification of Tea Highland Plantation at PPTK Gambung. Biospecies Vol. 8 No.1, January 2015, pp. 33-41.
- Puspitasari, F.D., Maya, S., dan Nengah, D.K. 2012. Isolasi dan Karakterisasi Bakteri Aerob Proteolitik dari Tangki Septik. Jurnal Sains dan Seni ITS. Vol. 1 (1)
- Qoriatul, I. 2021. Isolasi dan Uji Kemampuan Bakteri dari Biofilm Akuakultur Air Tawar yang Berperan dalam Penurunan Kadar Nitrit, Nitrat dan Amonium. Fakultas Sains dan Teknologi UIN Syarif Hidayatullah. Jakarta.
- Rao, N.B., dan Subba. 1994. Mikroorganisme Tanah Dan Pertumbuhan Tanaman. Terjemahan oleh HerawatiSusilo UI- Pres, Jakarta.
- Ristiati, N.P., Muliadihardja, S., dan Nurlita, F. 2008. Isolasi dan Identifikasi Bakteri Penambat Nitrogen Non Simbiosis dari dalam Tanah. Jurnal Penelitian dan Pengembangan Sains & Humaniora. Vol. 2: 68-80.
- , Delita, Z., dan Fibrianti, B.L. 2014. Isolasi Bakteri Indigenus yang Potensial Sebagai Agen Biofertilizer Asal Tanah Gambut di Kawasan Zamrud dan Taman Nasional Tesso Nilo, Riau. Jurnal Online Mahasiswa FMIPA. Vol. 1 (2).
- 2008. Skrining dan Identifikasi Bakteri Penghasil Enzim Kitinase dari Air Laut di Perairan Pantai Pondok Bali. Universitas Padjadjaran. Jatinangor.
- Santoso, K., Rahmawati, dan Rafdinal. 2019. Eksplorasi Bakteri Penambat Nitrogen dari Tanah Hutan Mangrove Sungai Peniti, Kabupaten Mampawah. Jurnal Protobiont. Vol. 8 (1).
- Schlegel, H.G. 1984. Mikrobiologi Umum. Gadja Mada University Press. Yogyakarta.
- Singh J. S., VC. Pandey, & DP. Singh. 2011. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agriculture, Ecosystems and Environment. 140: 339– 353.
- Subiksa, I.G.M., Hartatik, W., dan Agus, F., 2011. Pengelolaan Gambut Secara Berkelanjutan, dalam Pengelolaan Gambut Berkelanjutan (Edisi Nurida et al.), Badan Litbang Pertanian, Bogor.
- Sy, A., E., Giraud, P., Jourand, N. Garcia, A., Willem, P. de Lajudie, Y. Prin, M. Neyra, M., Gillis, B., Boivin-Masson and B. Dreyfus, 2001, Methylotrophic Methylobacterium Bacteria Nodulate and Fix Nitrogen In Symbiosis with Legumes, Journal of Bacteriology, Vol 1 No 83, Hal 214- 220.
- 2020. Ameliorasi dan Pemupukan pada Lahan Gambut untuk Peningkatan Produktivitas Kelapa Sawit. Disertasi. Universitas Sumatera Utara
- Tarigan, R.S., It, j., dan Elimasni. 2013. Seleksi Bakteri Penambat Nitrogen dan Penghasil Hormon IAA (Indole Acetic Acid) dari Rizosfer Tanah Perkebunan Kedelai (Glycine max). Jurnal Saintia Biologi. Vol. 1 (2): 42-48.
- Widawati S dan Muharam, 2012, Uji Laboratorium Azospirillum yang diisolasi dari beberapa ekosistem, Jurnal Hortikultura, 22(3): 258-267.
- Widawati S. 2015. Uji Bakteri Simbiotik dan Non simbiotik Pelarut Ca vs P dan Efek Inokulasi Bakteri pada Anakan Turi (Sesbania grandiflora Pers.). Jurnal Biologi Indonesia. Vol 11 No 2, hal. 295-307.
- Zhang D., Shouguan Ma., Zhang W dan Wang Y., 2014, Ammonia Stimulates Growth and Nitrite-Oxidizing Activity of Nitrobacter winogradskyi, Biotechnology & Biotechnologycal Equipment, Vol.28.N0.1, 27-32.