Publication Information
ISSN 2691-8803
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
Are There Other Hormones Connected to Lipedema Besides Estrogen? Clinical Experience and Preliminary Findings
Laura Patton1* and Lorenzo Ricolfi2
1Medical clinic of endocrinology – Trento, Italy
2Department of Medical Biotechnology – University of Siena, Italy
Received Date: July 19, 2023; Accepted Date: July 25, 2023; Published Date: July 31, 2023;
Citation: Patton L, Ricolfi L, (2023) Are There Other Hormones Connected to Lipedema Besides Estrogen? Clinical Experience and Preliminary Findings. Adv Pub Health Com Trop Med: APCTM-184.
*Corresponding author: Laura Patton, Medical clinic of endocrinology – Trento, Italy. Email: lauraptt77@gmail.com
DOI: 10.37722/APHCTM.2023205
The term “lipedema” identifies a pathology defined for the first time by Allen and Hines in 1940 and characterized by an increase in subcutaneous adipose tissue associated with transient edema aggravated by prolonged standing, especially in the summer season [1]. Lipedema is a chronic condition characterized by a disproportionate increase in adipose tissue and pain. The disease involves more frequently the lower and the uppers limbs, generally sparing the hands and feet, resulting in pronounced disproportion between extremities and trunk, but potentially could involve any part of the body. Lipedematous adipose tissue is a tissue that shows specific characteristics of chronic inflammation and fibrosis, which does not only involve the adipocytes but in general, the subcutaneous loose connective tissue of the affected areas [2-4]. There are three main clinical stages of lipedema based on the clinical feature of the subcutaneous tissue and skin changes (Figure 1). The severity of the disease increases with the clinical stage [3]:
- Stage 1 skin has a smooth texture with subdermal pebblelike feel due to underlying loose connective tissue fibrosis.
- Stage 2 women have more lipedema tissue than women with Stage 1 and skin dimpling due to progressed fibrotic changes and excess tissue. Palpable nodules may be more numerous and larger.
- Stage 3 features increased lipedema tissue more fibrotic in texture with numerous large subdermal nodules and overhanding lobules of tissue.
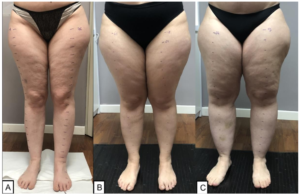
Figure 1: The figure shows the 3 clinical stages of lipedema: Stage 1 (A), Stage 2 (B), Stage 3 (C).
If lymphological alterations arise in a particularly advanced stage 3, some authors define this clinical picture with the term lipo-lymphedema or Stage 4, while otherwise, when the alterations of the lymphatic system arise and remain parallel to the lipedema and therefore can be also present in the initial stages, some authors define the clinical picture with the term lympho-lipedema, a clinical condition in which two pathologies coexist in the same patient [5].
Until a few years ago, the adipose tissue has been considered to be only a passive tissue for the storage of excess energy in the form of fat. However, there is now compelling evidence that adipose tissue acts as endocrine secretory organ being actively involved in cell function regulation through a complex network of endocrine, paracrine, and autocrine signals and that influence the response of many organs, such as hypothalamus, pancreas, liver, skeletal muscle, kidneys, endothelium and immune system [6].
On the other hand, since the disease often occurs with puberty, oral contraceptive use, pregnancy, and the menopause it is hypothesized that a hormonal change may be involved in initiating and in the evolution of the disease [7].
It is thought that there is an involvement of estrogens, also due to the evidence of the disease in male subjects in conditions of sex steroid imbalance.
But another very interesting aspect, in our opinion, is also simply linked to the effect that sex steroids have on the vascular system, both arterial and venous. For example, a vasodilatation effect has been demonstrated which involves multiple mechanisms and signaling pathways of estrogen and progesterone receptors during the physiological changes in the menstrual cycle or in pregnancy with effects on the valve closure time and on the vein diameter, which could at least explain the worsening of symptoms during the luteal phase of the menstrual cycle and in pregnancy. But this aspect could also be the trigger that leads to the onset of the disease [8-11]. In any case, what we are observing in our population of over 200 patients, is that this close relationship between estrogens and lipedema may not always be so close, given that, the disease arises with menarche in the most cases (about 60%), but in the remaining cases (about 40%) before (most of cases) or after puberty, and in sporadic case (about 3%) with the menopause; at the same time, a clinical deterioration following the intake of oestrogens or a pregnancy is observed in about 50% of cases, while in over 60% the disease worsened with menopause. Thus these observations could raise the realistic doubt that there are different clinical or pathogenic phenotypes of this disease, for example, phenotypes with a different sensitivity to estrogens.
On the other hand, as it is true that steroids influence the disposition of adipose tissue in women and men [12] if we study other endocrinopathies, we cannot overlook the fact that the distribution of adipose tissue is very often suggestive of the underlying hormonal imbalance (and of the metabolic consequences), such as, for example, as far as can be observed in the classic appearance of women affected by hyperandrogenism or in cases of hypercortisolism or hypogonadism in men. Indeed, it is particularly curious that the classic android disposition of hyperandrogenism is almost the opposite of what is observed in most patients suffering from lipedema.
But in this case, the difference lies not only in the arrangement of the adipose tissue, but also and above all in the signs and symptoms and in the histological alterations that this tissue shows.
Therefore, although fundamental, perhaps it is not only estrogens that we need to observe, but it is possible that in this disease there is the involvement of more than one hormonal system, theoretically all hormonal systems that can have an effect on the growth and metabolic function of adipose tissue such as, for example, the entire pituitary-ovarian and adrenal axis, including androgens and cortisol, thyroid function, glucose metabolism and the GH-IGF-1. And this, in part, is what is emerging from our daily experience. The mechanisms are probably complex, and the involvement of hormonal system, in turn, could be primary or secondary to the disease.
But trying to simplify things, let’s start from the simple observation that, at least in our series, the prevalence of obesity, defined with BMI (BMI ≥30 kg/h2) was about 40%, but differently from what was expected for the phenotype (gynoid phenotype) of these patients, we found an increase in waist circumference > 80 cm , a cut off considered diagnostic for visceral obesity according to the International Diabetes Federation (IDF) and the European Association for the Study of Obesity (EASO) in over 80% of patients and a waist circumference > 88 cm , a value considered equivalent to the presence of BMI > 30 kg/m2 by the European Society of Endocrinology (ESE), was found in over 60 %.
And this data already supports the importance of an adequate endocrine-metabolic study in these patients, even if the increase in waist circumference could be due to the presence of lipedema also in this site, an aspect that will need to be investigated.
An increased BMI leads to several hormonal changes, the most obvious example being insulin resistance. Additionally, concomitant hormonal diseases can be present in obesity and must be properly diagnosed and the diagnosis might be more difficult due to alterations caused by body fatness itself [13].
The presence of alterations in glucose metabolism is the first aspect that must be considered, and in this case, in our opinion, the determination of fasting blood sugar is not sufficient to avoid this alteration, because what we are seeing most frequently is insulin resistance.
Considering the observed cases of fasting hyperglycemia, impaired glucose tolerance and insulin resistance (assessed with HOMA-IR, a ratio between glycemia and insulin), the overall prevalence of alterations in glucose metabolism is approximately 35% and is about 50% in patients with Stage 3, in case of BMI ≥30 kg/h2 and when in addition to the lower limbs, the disease also affects the upper limbs. No cases of diabetes mellitus were diagnosed, while we found a condition of impaired glucose tolerance in only 5% of cases, despite the high prevalence of obesity. And this aspect is even more important, if we consider that in this population, we are noticing greater evidence of menstrual irregularities, such as oligo-anovulation (present in almost a third of patients) and of polycystic ovarian syndrome (present in over 15% of cases), situation in which a greater prevalence of alterations of glucose metabolism has already been demonstrated [14].
Some characteristics of lipedema, such as the presence of bruising, pain in the lower limbs, fatigue with movement, abdominal obesity, especially if in the presence of sudden weight gain and dorsocervical hump (“buffalo hump”), could be sufficient to require the exclusion of hypercortisolism. In our population, we have never diagnosed hypercortisolism, but it is true that some patients have reported a worsening of the clinical picture following the intake of cortisone and that we have had cases of lipedema diagnosed in patients with a positive history of Cushing’s disease. On the other hand, it is interesting to note that in these patients the presence of a dorsocervical hump would also seem to suggest an involvement of the upper limbs due to lipedema, having been found only in this case.
No significant alterations in the levels of gonadotropins, estradiol and progesterone emerged, results compatible with the phase of the menstrual cycle or with the menopausal state. Overall, considering the levels of total testosterone, Free Androgen Index (FAI), androstenedione and DHEA-S, the prevalence of hyperandrogenemia in our population was comparable to that estimated in the general population.
Finally, as already described in the literature [3], hypothyroidism is also frequently found in our population, in about 30% of cases. The prevalence of hypothyroidism also increases with clinical stage, affecting about 50% of the population in the third stage and as expected, is higher prevalence in obese subjects than in normal weight subjects (more than 55%). The prevalence of autoimmune hypothyroidism and primary hypothyroidism was comparable in our population, but it may be interesting to highlight that in non-obese subjects the main cause of hypothyroidism is primary hypothyroidism, in obese subjects instead the main cause of hypothyroidism is autoimmune.
But even more interesting, how unexpected is the data relating to the prevalence of autoimmune thyroiditis that we have found in more than 30% of our population, with no differences between clinical stages. This data has never been reported in the literature, and could be relevant, considering that an association between autoimmune diseases and other adipose tissue diseases has been described, as in the case of acquired generalized lipodystrophy [15]. Another aspect that could explain this, could be the link between immune and inflammatory processes and adipose tissue, while adipose tissue plays a critical role as an endocrine organ which produces number of active peptides, including leptin and adiponectin, for example, and numerous cytokines.
Among the comorbidities detected in this population, the most prevalent finding is the insufficiency of vitamin D (in more than 85%). Even this data is not surprising for many reasons, among which the high prevalence in the general population, especially in female population and in obese subjects, in case of lipedema, in our opinion, it is a problem that should not be underestimated and should be treated as a possible concomitant and worsening factor of the symptoms of lipedema, being able to favor, for example, the pain in the limbs and the fatigue often described by the patients.
The last aspect concerns the levels of IGF-1, as analyzing our first data, it would seem to be the only hormone (besides fasting insulin) that correlates with the severity of the clinical stage. This data has never been described in the literature. although, from in vitro studies, performed on adipose stem cells obtained from lipoaspirate, demonstrated a difference in the expression of IGF-1 during the proliferative activity and a role of IGF-1 in the pathogenesis of lipedema has been postulated [17]. But this aspect still needs to be studied and evaluated in relation to the clinical characteristics, it could be a suggestive index of clinical severity, if confirmed by what has been revealed by these initial observations.
In conclusion, from what we see in daily practice, the importance of thorough clinical evaluation of the patient suffering from lipedema of the lower limbs is emerging more and more, also from an endocrine-metabolic point of view. This is because, even if the pathogenesis is not yet clearly known, the pathophysiological relationship between the endocrine system and adipose tissue is certainly known and this fact must not be ignored, also for possible pharmacological and/or nutritional interventions aimed at the specific problems of each individual patient. Therefore, from the findings obtained, the clinical and endocrine-metabolic evaluation is inseparable from the overall management of patients affected by lipedema especially in the more severe stages, in the event of a greater extension of the disease or, even more simply, in the case of concomitant obesity according to the guidelines international endocrinological guide.
Endocrinology and lipedema can no longer be considered two separate universes.
References
- Allen EV, Hines EA (1940) Lipoedema of the legs: A syndrome characterized by fat legs and orthostatic oedema. Staff Meetings of the Mayo Clinic 15:184-187.
- Reich-Schupke S, Schmeller W et al. S1 guidelines: Lipedema. J Dtsch Dermatol Ges. 2017 Jul; 15:758-767.
- Herbst KL, Kahn LA et al. Standard of care for lipedema in the United States. Phlebology. 2021 Dec; 36:779-796.
- Bertsch T, Erbacher G et al. Lipoedema: a paradigm shift and consensus. J Wound Care. 2020 Nov 1;29(Sup11b):1-51.
- Reich-Schupke S, Schmeller W,. S1 guidelines: Lipedema. J Dtsch Dermatol Ges. 2017 Jul; 15:758-767.
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004 Jun; 89:2548- 56.
- Wounds UK. Best Practice Guidelines: The management of lipoedema. 2017.
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008 Jun; 60:210-41.
- Tostes RC, Nigro D et al. Effects of estrogen on the vascular system. Braz J Med Biol Res. 2003 Sep; 36:1143-58.
- Asbeutah AM, Al-Enezi M et al. Changes in the diameter and valve closure time of leg veins across the menstrual cycle. J Ultrasound Med. 2014 May; 33:803-9.
- Asbeutah AM, Al-Azemi M et al. Changes in the diameter and valve closure time of leg veins in primigravida women during pregnancy. J Vasc Surg Venous Lymphat Disord. 2015 Apr; 3:147-53.
- Lee MJ. Hormonal Regulation of Adipogenesis. Compr Physiol. 2017 Sep 12; 7:1151-1195.
- Pasquali R, Casanueva F et al European Society of Endocrinology Clinical Practice Guideline: Endocrine work- up in obesity. Eur J Endocrinol. 2020 Jan; 182:G1-G32.
- Conway G, Dewailly D, Diamanti-Kandarakis E, et al.; ESE PCOS Special Interest Group. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014 Oct; 171: P1-29.
- Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore). 2003 Mar; 82:129-46.
- Adami S, Romagnoli E et al. Guidelines on the prevention and treatment of hypovitaminosis D with cholecalciferol. SIOMMMS [Guidelines on prevention and treatment of vitamin D deficiency. Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS)]. Rheumatism. 2011; 63:129-147.
- Bauer AT, von Lukowicz D et al. Adipose Stem Cells from Lipedema and Control Adipose Tissue Respond Differently to Adipogenic Stimulation In Vitro. Plast Reconstr Surg. 2019; 144:623-632.