Publication Information
Frequency: Continuous
Format: PDF and HTML
Versions: Online (Open Access)
Year first Published: 2019
Language: English
| Journal Menu |
| Editorial Board |
| Reviewer Board |
| Articles |
| Open Access |
| Special Issue Proposals |
| Guidelines for Authors |
| Guidelines for Editors |
| Guidelines for Reviewers |
| Membership |
| Fee and Guidelines |
 |
6-Benzylaminopurineand 2, 4-D Dichlorophenóxyacetic Acid effect on Callogénesis De Brosimumalicastrum.
Domínguez May Angel Virgilio1*, Nah Hau José Augusto2, García Sheseña Israel3, Nahuat Dzib Sara Luz4, Giorgana Figueroa José Luis4
1Tecnológico Nacional de México/Instituto Tecnológico Superior del Sur del Estado de Yucatán. Carretera Muna-Felipe Carrillo Puerto. Tramo Oxkutzcab-Akil Km. 41+400 C.P 97880, Mexico
2Estudiante de Tecnológico Nacional de México/Instituto Tecnológico Superior del Sur del Estado de Yucatán. Carretera Muna-Felipe Carrillo Puerto. Tramo Oxkutzcab-Akil Km. 41+400 C.P 97880, Mexico
3Parque Científico Tecnológico de Yucatán. Km. 5.5 carretera Sierra Papacal-Chuburna C.P. 97302 Sierra Papacal, Yucatán, México
4Tecnológico Nacional de México/Instituto Tecnológico de Mérida. Km 5 Carretera Mérida-Progreso. C.P. 97118, Mexico
Received Date: February 29, 2020; Accepted Date: March 07, 2020; Published Date: March 15, 2020
*Corresponding author: Domínguez May Angel Virgilio, Tecnológico Nacional de México/Instituto Tecnológico Superior del Sur del Estado de Yucatán. Carretera Muna-Felipe Carrillo Puerto. Tramo Oxkutzcab-Akil Km. 41+400 C.P 97880, Mexico. Email: virgiliomay@hotmail.com
Citation: Virgilio DMA, Augusto NHJ, Israel GS, Luz ND, Luis GFJ (2020) 6-Benzylaminopurineand 2, 4-D Dichlorophenóxyacetic Acid effect on Callogénesis De Brosimumalicastrum. Adv Agri Harti and Ento: AAHE-114
Abstract
The Brosimumalicastrum seeds are a source of essential proteins and amino acids that can promote proper nutrition, and they are considered one of the alternatives to replace maize consumption partly. Unfortunately, breadnut trees (Brosimumalicastrum) can be male or female. The disadvantage is that males do not produce seeds. Generally, it is only possible to identify the tree gender, 8 years after it has been planted. Therefore, it has become essential to look for tools to produce plants with the capacity to produce seeds. One of these is the plant tissue culture by somatic embryogenesis. However, to develop the technique, it is required to decontaminate and adapt the tissue or explant under in vitro conditions. In this study, it was possible to decontaminate explants and induce callogenesis from Brosimunalicastrum leaves. It was shown that the concentration of 1.5 mg/L of BAP combined with 1 or 2 mg/L of 2, 4-D allowed callus formation.
Keywords: ANA, BAP, Brosimumalicastrum, Callogenesis, 2, 4-D
Introduction
The Breadnut (Brosimumalicastrum) is a tropical tree distributed in the jungles of Mexico and Central America; its scientific name comes from the Greek Brosimos, which means "edible." In Mexico and Belize, it is commonly known as Breadnut or Oox in the Mayan language [1], and there is both male and female gender [2]. This tree may be able to produce per hectare, 20 tons of seeds to the year; therefore, it would help the crusade against hunger. This tree does not cause soil erosion, and its management does not involve the use of agrochemicals [3]. It can also be used for the preparation of products for human and livestock consumption [4], because of its high protein and other nutrients [5]. Currently, the Breadnut has become necessary for research on the benefits offered, including may be an alternative to replace the excess consumption of the Zea mays in the country. With the importance that the fruits of the tree breadnut represent, the Academy of Sciences of the United States considered it as one of the underexploited species with promising economic value [6]. The genetic variability that exists regarding sexuality in this species is the reason why commercial plantations have not been obtained [2]. Through the use of biotechnology, you can establish clones with desirable characteristics able to produce enough biological material of this tree, since it is considered a recalcitrant timber species, with a high added value [7]. The process of somatic embryogenesis has become an alternative for the clonal propagation of elite individuals to be phenotypically identical or simply germplasm to preserve the genetic material of the species [8].To achieve somatic embryogenesis of any plant species, the in vitro biological material must be established previously.
The purpose of this work was to decontaminate and evaluate the response of leaf explants of Brosimumalicastrum to callogenesis in the presence of different concentrations and combinations of 6-Benzylaminopurine (BAP) and 2, 4-Dichlorophenoxyacetic acid (2, 4-D).
Materials and Methods
Plant Material
The leaf explants of Brosimumalicastrum were collected from a tree specimen found in the science and technology Yucatan Park, unit of the CICY Germ plasm Bank.
Preparation of Treatments for the Callogenesis Induction
Murashige and Skoog base medium was used to evaluate the callogenesis [9], testing 20 treatments, each consisting of 10 ml/L of Gamborg vitamin [10], at 20 X, 2.2 g/L of Gel rite, 30 g/L sucrose, 3 g/L activated carbon, 300 mg/L PVP and 1.5 ml/L PPM (antibiotic).Out of the 20 treatments, 19 of them were supplemented with different concentrations and combinations of BAP and 2, 4-D, while one of the treatments was not added any of the growth regulators. The pH of the medium was adjusted between 5.7-5.8 and sterilized for 20 min at a temperature of 121° C and a pressure of 1.2 kg/cm2.
Decontamination Process
The decontamination process began with the thinning of leaves in branches of Brosimumalicastrum. Subsequently, the younger leaves were selected and placed in water with liquid detergent; with the help of a sponge, they were washed very carefully so as not to damage them and were deposited in a beaker containing citric acid solution (100 mg/L). Later in the laminar flow hood, the decontamination process was continued. Using sterile forceps, the leaves are taken and placed in a 250 ml flask with distilled water, to which was added captan fungicide 50 (3 g/l) plus Tween 20, and then it was shaken for 60 minutes. The solution was removed from the flask and rinsed with distilled water 2 times, each rinse for 1 minute. Then, the leaves were kept in a PPM antibiotic solution (1.5 ml/l) for 40 minutes. A term of that time, the solution of the flask was removed and rinsed with sterile distilled water for 1 minute. Next, the leaves were kept in 70% alcohol for 2 min. At the end of that time, the ethanol was removed and rinsed with distilled water. To finish the decontamination process, the leaves were immersed in a solution of sodium hypochlorite at 30% for 30 minutes. After the decontamination time, three rinses were made with distilled water for 1 minute each. Subsequently, the leaves were cut at the top and bottom of each one, obtaining explants of approximately 1.5 cm2 surface.
Planting of Leaf Explants
Two leaf explants were placed on MS medium [9], each of the 20 treatments with activated carbon added. The 20 were kept in a culture room at a temperature of 25 ± 4° C. At 30 days, and the explants were subculture in fresh medium without activated carbon, maintaining the same chemical and ambient temperature conditions in the laboratory of 29 ± 4° C, and illuminated with natural photoperiod of 16 h light, composition with average brightness of 59 Lumens/m2.
Results and Discussions
Decontamination Process of Leaf Explants with Chlorine
Through the decontamination method described, more than 80% of leaf explants were obtained without being damaged by the chlorine present in each of the repetitions performed (Table 1). It could be said that this method is acceptable; therefore, it can be recommended for the removal of microorganisms from the Brosimumalicastrum leaves, if the idea is to adapt them in vitro conditions. A schematic of the decontamination method used is shown below (Figure 1).
| Repetitions | Number of leafex plants | Number of leaf explants damaged | Number of leaf healthy explants | Healthy explants (%) |
| 1 | 80 | 10 | 70 | 87.5 |
| 2 | 80 | 13 | 67 | 83.8 |
| 3 | 80 | 8 | 72 | 90.0 |

Figure 1: Decontamination Method of Brosimumalicastrum leaf explants A) Brosimumalicastrum Tree. B) Selection of tender leaves. C) Leaves dipped in fungicide Captan. D) PPM antibiotic solution. E) 70% alcohol solution. F) 30% chlorine solution. G) Leaves previously decontaminated. H) Segmented sheets. I) Leaves in treatments enriched with activated carbon. J) Subculture of leaves in treatments without activated carbon.
Callus Formation from Brosimumalicastrum Leaf Explants
The induction of the callogenesis process was dependent on the combination of a specific concentration of 6-Benzylaminopurine (BAP) and 2, 4-Dichlorophenoxyacetic (2, 4-D) because it was shown that callus formation does not occur at any concentration of both growth regulators (Table 2).From all treatments evaluated, both are favoring the formation of callus were treatment with 1.5 mg/L of BAP and 1 mg/L of 2,4-D named as TN, and treatment with 1.5 mg/L of BAP and 2 mg/L of 2,4-D designated as TM (Figure 2).It is important to note that the evaluation of the response of leaf explants in the presence of BAP and 2, 4-D was carried out twice, and in both repetitions, the same response was obtained.
| Treatment | BAP(mg/L) | 2,4-D(mg/L) | Number of explants in callus inductions | Number of explants with callogenesis | Callus formations (%) |
| TA | 0 | 0 | 2 | 0 | 0 |
| TB | 0 | 0.5 | 2 | 0 | 0 |
| TC | 0 | 1 | 2 | 0 | 0 |
| TD | 0 | 2 | 2 | 0 | 0 |
| TE | 0 | 3 | 2 | 0 | 0 |
| TF | 1 | 0 | 2 | 0 | 0 |
| TG | 1 | 0.5 | 2 | 0 | 0 |
| TH | 1 | 1 | 2 | 0 | 0 |
| TI | 1 | 2 | 2 | 0 | 0 |
| TJ | 1 | 3 | 2 | 0 | 0 |
| TK | 1.5 | 0 | 2 | 0 | 0 |
| TL | 1.5 | 0.5 | 2 | 0 | 0 |
| TM | 1.5 | 1 | 2 | 2 | 100 |
| TN | 1.5 | 2 | 2 | 2 | 100 |
| TÑ | 1.5 | 3 | 2 | 0 | 0 |
| TO | 2 | 0 | 2 | 0 | 0 |
| TP | 2 | 0.5 | 2 | 0 | 0 |
| TQ | 2 | 1 | 2 | 0 | 0 |
| TR | 2 | 2 | 2 | 0 | 0 |
| TS | 2 | 3 | 2 | 0 | 0 |
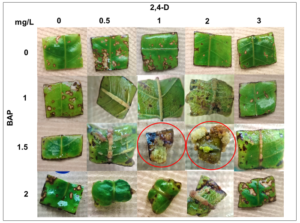
Figure 2: Callus formation of Brosimumalicastrum in the presence of BAP and 2, 4-D. The explants marked with a red circle are those that formed calluses in the presence of specific concentrations, both of BAP and 2, 4-D when combined.
The Subculture of Calluses in the Medium of Fresh Culture
It was noted that callus formation started around 20 days after the leaf explant was in TN and TM enriched with activated carbon; under these conditions, callus growth was slow until 30 days; however, when the calluses were sub cultured in TN and TM without the presence of activated carbon, it was noted that the cell mass started to increase, reaching a remarkable size until six weeks (Figure 3). The types of calluses that were formed are class III and VI; described by Hernández Espinoza, 2009 [11], those of class VI are friable calluses, since these are separated easily, and when they are sub cultured, they multiply in volume, and after their growth, it separates from its original explants (Figure 3, TN).While class III are structures that can become crystalline and hyper hydrated in a globular shape with a pale green color, they can also reproduce and may have pre-embryogenic structures (Figure 3, TM), These characteristics described by this author match with the corns that were obtained in this research study.

Figure 3: The Brosimumalicastrum cells can trigger their proliferation, placing them in a new culture medium.
Different studies have been published about the callogenic response of different plant species, applying auxin and cytokinin growth regulators, to multiplying individuals. Así, Ahmet y Sezai en 2015 [12]. Thus, Ahmet and Sezai in 2015 [12], evaluated the effect of growth regulators on explants of nodal segments and Solanumtuberosum leaf tissue, they found the development of callogenesis in both explants with DM culture medium containing 3 mg/L of benzyl amino purine (BAP) + 2 mg/L naphthalene acetic acid (ANA).However, the best callus production was observed in nodal segments, both explants tested after callogenesis resulted in bud formation when calluses were sub cultured in MS with the presence of BAP or Kinetin. In the current study, there was also callogenesis but using a different auxin. Zhu et al. in 2018 [13] they induced the callus formation and subsequent regeneration of plants from young leaves explants of Paeonia suffruticosa Andr., for callogenesis, the best medium found was the MS added with 0.2 mg/L of 2, 4-dichlorophenoxyacetic acid (2, 4-D) + 3 mg/L of thidiazuron (TDZ). Calluses differentiated outbreaks when they were subcultured in MS + 2 mg/L of BAP *0.2 mg/L of ANA + 0.3 mg/L of TDZ. This work coincides with Zhu et al., 2018 [13], in obtaining callus from young leaves explants in MS medium but with a higher concentration of 2,4-D, 2 mg/L, and with BAP and replacing TDZ.
Conclusion
The clonal propagation of woody and recalcitrant species has faced a challenge in the biotechnology area due to its high content of pathogens, doing the work of establishing in vitro explants of these species, titanic; once the decontamination protocol is established, the somatic embryogenesis pathway is a prominent technique for the micro-propagation of Brosimumalicastrum trees. The phenotypic variety of calluses that were formed from Brosimumalicastrum in this research gives us the pattern of the possibility of creating somatic embryos from them. Getting these calluses has been a significant breakthrough, which allows us to lay the foundations for a new study that is the ultrastructural analysis, which would give us the certainty if there is a pre-embryogenic development. If somatic embryogenesis is achieved in future research, it would allow the regeneration of genotypically identical seedlings with desirable characteristics, which has not been possible so far, because there is no way to know in the early years, phenotypically if the tree will bear fruit or not, since usually the tree gender is identified when the formation of fruits begins, and this is generally detected up to 8 years of age of the tree.
By molecular techniques, the gender of the plant and the possibility of producing fruits could be established; based on this assessment, if the process for clonal multiplication of promising individuals will be available, biotechnological development could be set for the production and sustainable use of this species. It is in this sense that the in vitro establishment and the callogenic production of B. alicastrum, a result obtained in the current study, generates an essential advance for subsequent researches in somatic organogenesis or embryogenesis for the production of plants and the sustainable use of B. alicastrum as a food alternative.
References
- Meiners M, Sánchez C, Sylvie DB (2009) El Ramon:Fruto de nuestra cultura y raíz para la conservacion. CONABIO,Biodiversitas 87: 7-10.
- Berg C (1972). Brosimum alicastrum Flora Neotropica 7: 170-171.
- Hernández-Santos V, Munguía-Gil A, Monforte –Méndez MA (2015) Caracterización de la producción con árboles de ramón (w) y sus derivados para el desarrollo sustentable de la región sur del Estado de Yucatán. AMECIDER-CRIM, UNAM 1-24.
- Ramirez-SanchezS, Ibáñez-Vázquez D, Gutierrez-Peña M, Ortega-Fuentes MS, García Ponce LL, et al. (2017) El ramón (BrosimumalicastrumSwartz) una alternativa para la seguridad alimentaria en México.Agroproductividad 10: 80-83.
- Kishur SD (5 de 10 de 2019) Kishur. Obtenido de Kishur: http://www.kishur.com.mx/
- Saavedra AL (2013) El árbol Ramón, opción viable de alimentación; complementa al maíz. En La Jornada Mexico,D.F P: 24-31.
- Sheseña IG (2011) Tesis de licenciatura; Analisis ultraestructural de un sistema de embriogenesis somatica de cedro rojo (Cedrela Odorata ) Acayucan, Veracruz.
- Thorpe T, Harry I, Kumar PP (1991) Aplication of micropropagation to forestry. In: Micropropagation: Tecnology and Aplication. Instituto de Biotecnologia de las plantas. Santa Clara-Cuba P 134.
- Murashige, T. Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plantar 15: 473-497.
- Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirement of suspensions cultures of soybean root cells. Cell Res 50: 151-158.
- Hernández Espinoza A (2009) Multiplicación, Maduración, Germinación y Conversión de Embriones Somáticos en Plantas de Cedro Rojo (Cedrelaodorata). Acayucan, México: Instituto Tecnológico Superior de Acayucan.
- Kumlay AM, Ercisli S (2015) Callus induction, shoot proliferation and root regeneration of potato (Solanumtuberosum) stem node and leaf explants under long-day conditions, Biotechnology & Biotechnological Equipment 29: 1075-1084.
- Zhu X, Li X, Ding W, Jin S, Wang Y (2018) Callus induction and plant regeneration from leaves of peony. Hortic. Environ. Biotechnol 9: 575-582.